Abstract
Prion infection relies on a continuous chain of PrPc-expressing tissues to spread from peripheral sites to the central nervous system (CNS). Direct neuroinvasion via peripheral nerves has long been considered likely. However, the speed of axonal flow is incompatible with the lengthy delay prior to the detection of PrPSc in the brain. We hypothesized that Schwann cells could be the candidate implicated in this mechanism; for that, it has to express PrPc and to allow PrPSc conversion. We investigated in vivo localization of PrPc in sciatic nerve samples from different strains of mice. We demonstrated that PrPc is mainly localized at the cell membrane of the Schwann cell. We also studied in vitro expression of PrPc in the Schwann cell line MSC-80 and demonstrated that it expresses PrPc at the same location. More specifically, we demonstrated that this glial cell line, when infected in vitro with the mouse Chandler prion strain, both produces the PrPSc till after 18 passages and is able to transmit disease to mice, which then develop the typical signs of prion diseases. It is the first time that infection and replication of PrPSc are shown in a peripheral glial cell line.
Prion diseases, such as scrapie, kuru, Creutzfeldt-Jacob diseases, and bovine spongiform encephalopathy, are transmissible fatal neurodegenerative disorders associated with the conversion of the membrane glycoprotein PrPc to PrPSc (27). Acquired forms transmitted mostly through oral absorption (4, 6, 13) or peripheral inoculation of infected tissues raise the question of how prions are transported to the central nervous system (CNS). However, the transfer of infectivity from the spleen to the CNS has been shown to be dependent on the expression of PrPc (3). Using a mathematical model, Payne (26) has suggested a role for a continuous chain of PrP-expressing tissue linking peripheral sites to the brain. Both the lymphoreticular system and the peripheral nervous system (PNS) are involved in neuroinvasion (17). Direct neuroinvasion via peripheral nerves might occur after a high-dose peripheral infection, whereas after a low-dose infection amplification in follicular dendritic cells in lymphoid tissue might be necessary prior to neuroinvasion via peripheral nerves (20). Several reports evidence the role of the lymphoreticular system in the initial step of scrapie replication (3); however, PrPSc accumulation occurs in the brain of severe combined immunodeficient mice (20), indicating that neuroinvasion can use another pathway. After intraperitoneal or oral infection, the initial sites of infectivity are consistent with entry via the vagus or other peripheral nerves (1). However, the speed of axonal flow is incompatible with the transport of infectivity in peripheral nerves (14) (0.7 mm per day). We suggest a more important role of the Schwann cell in the cellular mechanism of prion propagation.
MATERIALS AND METHODS
Cell culture.
MSC-80 (5), N2a (ATCC CCL131), and N2aCh (25) cells were maintained in a Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and 2 mM l-glutamine at 37°C in a 5% CO2 atmosphere. Cells were split (1:10; vol/vol) every 7 days.
Immunohistochemistry.
Sciatic nerves were fixed in 4% formaldehyde-phosphate-buffered saline (PBS) overnight and then placed in 20% sucrose-PBS (18 h). Frozen samples (−80°C) were embedded in Tissue-Tek OCT-Compound (Sakura Finetechnical, Tokyo, Japan). Cryosections (6 nm) were dried, fixed in cold acetone, and incubated for 1 h with the biotinylated anti-PrP 3B5 monoclonal antibody (MAb) (19) (15 μg/ml). Immunoreactions were revealed using the streptavidin-biotin and the streptavidin-peroxidase complexes (Dako, Carpinteria, Calif.). An anti-trinitrophenol IgG2a MAb was used as control (30 μg/ml; Pharmingen, San Diego, Calif.).
Electron microscopy.
Immunogold electron microscopy was processed according to a method described by Berryman (2), and immunostaining was performed with the 3B5 MAb (15 μg/ml). An irrelevant immunoglobulin G2a (IgG2a) isotypic MAb (15 μg/ml) was used as control (data not shown).
Fluorescence-activated cell sorter (FACS) analysis.
Cells were washed and resuspended in PBS-0.5% bovine serum albumin buffer. Samples were incubated for 1 h at 4°C with the primary biotinylated anti-PrP MAb 3B5 (15 μg/ml) (19). After washing, cells were incubated with a streptavidin fluorescein isothiocyanate solution at 4°C for 30 min. Cell preparations were then washed in PBS and analyzed by flow cytometry using a FACScan (Becton Dickinson, Sunnyvale, Calif). An IgG2a MAb was used as an isotypic control (15 μg/ml) (Pharmingen.).
PrPc detection.
Brain homogenates (10% [wt/vol]) and cell lysates were prepared by extrusion in a potter homogenizer followed by an extrusion through a 16-gauge and then a 22-gauge syringe in a lysis buffer (150 mM NaCl, 0.5% Triton X-100, 0.5% sodium deoxycholate, and 50 mM Tris-HCl, pH 7.5) completed with protease inhibitors (1 mg [each] of pepstatin and leupeptin per ml and 2 mM EDTA). After 15 min of incubation at 4°C and 4 min of centrifugation at 10,000 × g, supernatants were collected and total protein concentration was measured by bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Samples (12.5 μg of total protein per lane) subjected to electrophoresis on sodium dodecyl sulfate (SDS)-polyacrylamide gel (12% polyacrylamide) were transferred to nitrocellulose membrane. The blots were then blocked for 1 h in 5% (wt/vol) nonfat dry milk in PBS-1% Tween 20 and 0.001% azide. After incubation for 1 h with the 3B5 MAb (19) (300 ng/ml) and 30 min with the secondary mouse peroxidase-conjugated antibody at 1:2,500 (Sigma, St. Louis, Mo.). Immunoreactive bands were visualized using the Enhanced Chemiluminescence method (ECL; Amersham Pharmacia, Piscataway, N.J.).
Indirect immunofluorescence assay.
After incubation at 4°C in 1% bovine serum albumin (BSA)-PBS buffer for 30 min, cells were incubated in the same buffer, with or without 0.5% saponin (Sigma). After cell washing, the biotinylated 3B5 MAb (4.5 μg/ml) was added for 1 h at 4°C and incubated with strepatvidin fluorescein isothiocyanate (1:100; Becton Dickinson). Cells were then fixed at 4°C for 20 min with 4% paraformaldehyde. The IgG2a antibody was used as an isotypic control.
Cell infection.
Brains infected with Chandler strain were homogenized to 10% (wt/vol) in cold culture medium. Cells were grown in six-well plates at 2 × 105 cells/well 2 days before inoculation. They were then incubated for 72 h with 1 ml of 2% brain homogenate diluted in culture medium (5.9 log mean lethal doses [50% lethal doses {LD50s}] units of mouse-adapted Chandler scrapie strain [9]). The inoculum was changed every 24 h. Then, the supernatant was replaced by 2 ml of regular culture medium and the cells were incubated until confluence. Finally, they were cultured in flasks with 150 cm2 of surface and were split (1:10; vol/vol) every 7 days.
PrPSc detection.
Cells (8 × 106) were lysed in 200 μl of the same lysis buffer as was described for PrPc detection, at 4°C for 15 min, and then samples were centrifuged 10 min at 10,000 × g. The protein concentration was adjusted to 1 mg/ml and treated with proteinase K (PK) (Sigma) (20 μg/ml) at 37°C for 30 min. The reaction was stopped with 2 mM phenylmethylsulfonyl fluoride (Sigma) for 5 min at 4°C and centrifuged for 45 min at 14,000 × g. Brain homogenates (10% [wt/vol]) from frozen tissues were prepared in lysis buffer and adjusted to 3 mg of protein/ml. Each sample (300 mg of total protein) was treated with PK (10 μg/ml) at 37°C for 30 min. Samples (37.5 pg of total protein) were then mixed with a volume of 2× Laemmli buffer (4% SDS, 2% β-mercaptoethanol, Tris-glycine [pH 8.2], 5% sucrose), heated at 100°C for 3 min, and then loaded onto a 12% polyacrylamide gel just after boiling. They were then analyzed by Western blotting as described above with a mixture of three MAbs: SAF 60, SAF 69, and SAF 70 (10).
Metabolic pulse-chase radiolabeling.
Cells (80% confluency in 25-cm2 flask) were labeled with 1 mCi of l-[35S]methionine (Amersham Pharmacia) per ml in methionine/cysteine-free DMEM supplemented with 1/40 (vol/vol) culture medium, 1% fetal calf serum (FCS), and 2 mM l-glutamine for 24 h. Cells were chased for 24 h in culture medium and then lysed in 1 ml of lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 5 mM EDTA; 0.5% NP-40; 0.5% deoxycholate [DOC]) on ice for 5 min. After centrifugation at 4,000 × g for 1 min and addition of Sarkosyl to 0.4%, cell lysates (500 μl) were incubated with 5 μg of PK for 30 min at 37°C. For immunoprecipitation, the MAb 3B5 (10 μg) was coupled to protein A-Sepharose CL-4B (Amersham Pharmacia). Cell lysates were then incubated with 3B5-Sepharose beads overnight at 4°C. After centrifugation at 10,000 × g for 1 min, Sepharose-absorbed proteins were washed twice with lysis buffer containing 0.4% Sarkosyl, followed by two wash steps in high-salt buffer (50 mM Tris-HCl, pH 7.5; 500 mM NaCl; 0.1% NP-40; 0.05% DOC) and one wash step in low-salt buffer (50 mM Tris-HCl, pH 7.5; 0.1% NP-40; 0.05% DOC). Sepharose was resuspended in 3× loading buffer (150 mM Tris-HCl, pH 6.8, 6% SDS; 0.3% bromophenol blue; 30% glycerol) and analyzed by SDS-12% PAGE.
Infectivity bioassay.
After three cycles of freezing-thawing and cell death control by trypan blue microscopy, 105 cells at the seventh passage were injected intracerebrally (i.c.) into four tga20 mice. Five tga20 mice were inoculated i.c. with uninfected MSC-80 or with the Chandler strain (6.5 log LD50) as negative and positive controls, respectively.
Mice were monitored every 2 days, and scrapie was diagnosed according to standard clinical criteria (18).
Histopathological analysis.
Brain sections (5 μm) were deparaffined, and endogenous peroxidase was inhibited with 33% H2O2 for 30 min. After washing and blocking with an unspecific goat serum, samples were incubated overnight with the rabbit anti-cow anti-glial fibrillary acidic protein antibody (Dako, Carpinteria, Calif.). Samples were then washed in PBS and incubated for 1 h at room temperature with the biotinylated anti-rabbit IgG. Colors were developed with peroxidase substrate.
RESULTS
Expression and localization of PrPc on mouse sciatic nerves.
To investigate the localization of PrPc in mouse nerves, we performed immunohistochemistry on sciatic nerves from PrP-overexpressing tga20 mice (11) which were determined to express five to seven times more PrPc in the PNS than wild-type mice (14). We used the anti-PrP MAb 3B5, which recognizes residues 79 to 92 of human PrP (19), as this antibody has already been extensively used to detect murine PrPc. Labeling was observed at the Schwann cell surface and in the cytoplasm (Fig. 1A) but not in the myelin sheath. Both antibody control (IgG2a; Fig. 1B) and antigen control (7) (PrP0/0 mice; data not shown) were negative. A clear labeling could be observed only on sections passing through the nucleus of the Schwann cell and thus through a large area of cytoplasm. We confirmed this result by the analysis of sciatic nerves from three different transgenic mice strains expressing the green fluorescent protein (GFP) reporter gene under the control of the 5" regulatory region of the bovine prp gene (prp-gfp transgenic mice). These mice models have previously been shown to exhibit GFP fluorescence in good correlation with murine endogen PrPc expression (22). The GFP fluorescence clearly observed in Schwann cells provides an additional argument in favor of PrP expression by this cell type (Fig. 1C). However, as one cannot exclude ectopic expression of PrPc or GFP in transgenic animals, we carried out immunogold electron microscopy, with the 3B5 MAb, on sciatic nerves from wild-type BALB/c mice. We observed the immunolabeling at the cell surface of Schwann cells as well as intracellulary (Fig. 1D and E). In contrast, we did not observe any labeling in the myelin sheath. Control staining without the 3B5 MAb was negative (Fig. 1F).
FIG. 1.
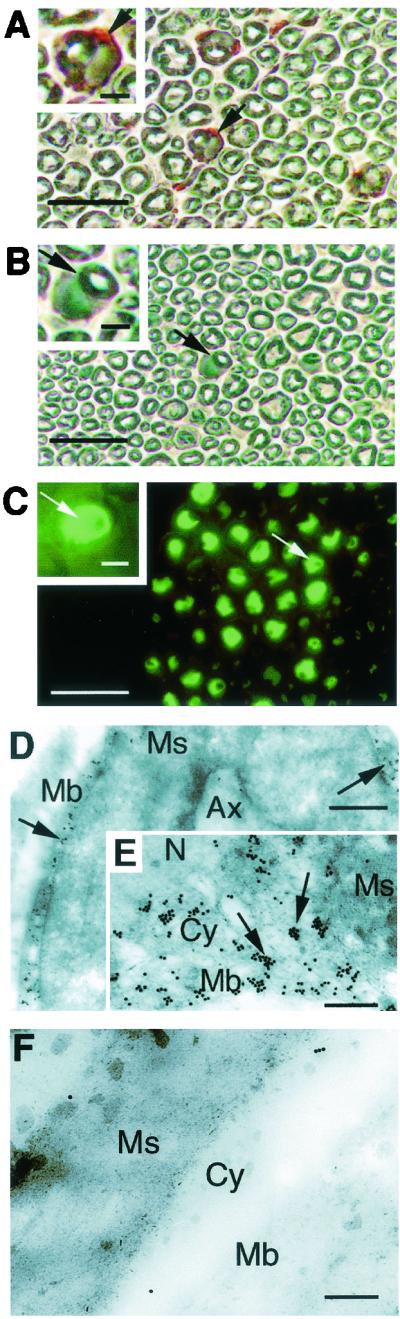
Expression and localization of PrPc on sciatic nerve sections. (A) Transversal sciatic nerve sections from tga20 mice were processed with the 3B5 MAb (19). The arrows point at the labeled cytoplasm of the Schwann cell. (B) The sciatic nerve sections were also processed with an anti-trinitrophenol mouse IgG2a(κ) MAb immunoglobulin isotype standard used as an isotypic control. The arrows point at the unlabeled cytoplasm of the Schwann cell. (C) Sciatic nerves from prp-gfp transgenic mice (22) exhibit widespread expression as evidenced by fluorescence localized to the cytoplasm. (A, B, and C) Shown are a wide view (bar, 50 nm) and an enlarged view (bar, 10 nm). (D) Immunogold electron microscopy of BALB/c sciatic nerve sections. Nerve section assays were performed with the 3B5 MAb. Gold particles (arrows) were localized at the plasma membrane (Mb) of Schwann cells. No labeling was seen within the myelin sheath (Ms). Ax, axon. Bar, 1 nm. (E) Enlarged view of cytoplasmic area of Schwann cell exhibiting a positive labeling at the outer collar cytoplasm (Cy). No staining was seen in the nucleus (N) or the myelin sheath (Ms). (F) Control staining without the 3B5 MAb. Bar, 0.2 nm.
Expression and localization of PrPc in mouse Schwann cell line MSC-80.
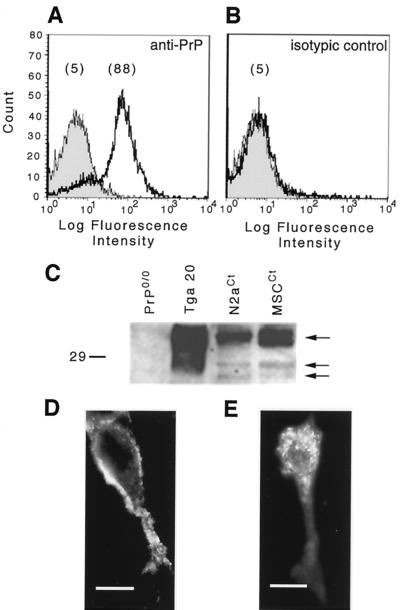
Since prion replication is a PrPc-dependent mechanism (3), we investigated the expression of PrPc in a mouse Schwann cell line, the MSC-80 (5), which is one of the few cell lines isolated from the PNS. After cell membrane staining using the 3B5 MAb (19), MSC-80 displayed positive labeling with a monophasic distribution (Fig. 2A and B) as shown by FACS analysis. We compared the Western blot pattern of PrPc from MSC-80 to that of mouse brain and of the N2a neuroblastoma cell line (8) (Fig. 2C). The three glycoforms of PrPc, the nonglycosylated 25- to 27-kDa protein, and the mono- and diglycosylated forms were revealed. Finally, an indirect immunofluorescence assay showed a well-defined labeling at the cell surface of MSC-80 (Fig. 2D), while after saponin permeabilization we detected intracellular labeling (Fig. 2E). We did not observe a nuclear localization of the protein. No staining was observed with the IgG2a isotype control (data not shown).
FIG. 2.
In vitro PrPc expression and localization in mouse Schwann cells (MSC-80). (A) PrPc was detected at the surface of MSC-80 cells (5) by FACS analysis. (B) An IgG2a MAb was used as an isotypic control. The mean fluorescence intensity of cells is presented within brackets. (C) PrPc was also detected by immunoblot analysis from control MSC-80 (MSCCt) extracts. Murine neuroblastoma cell line extracts (N2aCt) and brain homogenates from tga20 mice (11) were used as positive controls, and brain homogenates from PrP0/0 mice (7) were used as negative controls. The arrows point to the three glycosylated forms of the murine PrPc. The molecular mass marker is in kilodaltons. (D and E) Cellular localization of PrPc was analyzed by immunofluorescence microscopy. (D) The staining was localized on the cell membrane. Bar, 10 nm. (E) After saponin permeabilization, the signal became intracellular. Bar, 5 nm.
Production of PrPSc in MSC-80 after inoculation with Chandler strain.
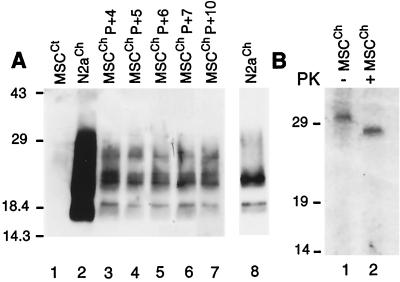
Currently, only a few neuronal cell lines have been persistently infected with a mouse-adapted scrapie strain (N2a [8], mouse PrPc transfected N2a [25], PC12 [29], or GT1 [30]), and more recently, a rabbit epithelial cell line stably transfected with ovine prp gene was infected with scrapie agent (31). In order to investigate the ability of the MSC-80 line to sustain PrP conversion from the cellular to the protease-resistant form, we incubated MSC-80 cells for 3 days with 5.9 log LD50 units of mouse-adapted Chandler scrapie strain (9). PrPSc production was analyzed by Western blotting using a mixture of three MAbs (SAF 60, SAF 69, and SAF 70 [10]) directed against hamster PrP (residues 142 to 160; Fig. 3A and B). This mixture is known to have high sensitivity to PrPSc (25). During the fourth passage (Fig. 3A, lane 3), the typical profile of PrPSc appeared without any loss of signal intensity during passages 5, 6, 7, and 10 (Fig. 3A, lanes 4, 5, 6, and 7, respectively). This PrPSc pattern is also observed after 15 passages (data not shown). Murine PrP-transfected N2a cells (N2aCh subclone 58 [25]), which express five times the PrP level of untransfected N2a cells, were tested on the same Western blot (Fig. 3A, lanes 2 and 8). Control uninfected MSC-80 cells treated by PK digestion did not exhibit any signal (Fig. 3A, lane 1). These results were identical in three independent experiments.
FIG. 3.
Production of PrPSc in MSC-80 cells after inoculation with Chandler strain. (A) Cells were challenged with the mouse-adapted Chandler scrapie strain and were analyzed by Western blotting after PK treatment. Uninfected MSC-80 cells (MSCCt) were used as negative control (lane 1). PrP-overexpressing N2a cells infected with Chandler (N2aCh) were used as the positive control (lane 2 and 8). Infected cells (MSCCh) produce PrPSc after 4, 5, 6, 7, and 10 passages (lanes 3, 4, 5, 6, and 7, respectively). Time exposure, 5 min. Lane 8, N2aCh cells with a shorter exposure time (30 s). (B) Immunoprecipitation of PrP in infected MSC-80 cells after 18 passages. MSCCh cells were 35S metabolically radiolabeled, followed by a chase period. After cell lysis, the extracts were (lane 2) or were not (lane 1) subjected to PK digestion. PrP was immunoprecipitated with 3B5 MAb. Molecular mass markers are in kilodaltons.
Transconformation of PrP at the cellular level was also investigated in MSCCh, using a 35S metabolic pulse-chase radiolabeling followed by an immunoprecipitation with the 3B5 MAb. We performed the experiment with infected MSC-80 cells at the 18th passage. Immunoprecipitation resulted in a clear PrPc-specific signal in the range of 30 kDa (Fig. 3B, lane 1), which represents PrPc synthesized de novo. After PK treatment, (Fig. 3B, lane 2), the PrPSc signal appeared (27 to 30 kDa), demonstrating that the Chandler-infected MSC-80 line converts PrPc into PrPSc. The complete shift down observed between lanes 1 and 2 leads us to consider that the pattern is not due to a partial PK digestion of PrPc. The single band pattern could be due to a lower efficiency of the 3B5 MAb in immunoprecipitation compared to that of Western blotting.
Transmission of infected MSC-80 to mice.
With a view to test the presence of infectivity in PrPSc-positive MSC-80 cells, we inoculated i.c. inoculated MSCCh cells at the seventh passage to four tga20 mice. Mice developed typical signs of spongiform encephalopathies and died at 91.5 ± 5 (mean ± standard deviation) days postinoculation compared to the 66.4 ± 0.5 days for the five control tga20 mice inoculated with the Chandler strain. Five mice inoculated with uninfected MSC-80 cells did not exhibited any signs of transmissible spongiform encephalopathy up to 280 days postinoculation (Table 1). We evaluated the titer of MSCCh to 4.3 log LD50 units per gram of cell lysate. It was decreased by only 2 orders of magnitude in comparison to that estimated in the same brain homogenate as used for cell infection (6.5 log LD50 units per g). The apparent low quantity of PrPSc in MSCCh shown on the Western blot (Fig. 3A, lane 6) does not seem to correlate with the resulting high infectivity titer. However, this has already been described in previous work (21).
TABLE 1.
Scrapie titers of challenged Schwann cells and control cells
| Inoculation | Incubation period
|
Titer (log LD50 units/g)a | |
|---|---|---|---|
| No. of days | Mean ± SD | ||
| Chandler (2%) | 66, 66, 67, 67, 67 | 66.4 ± 0.5 | 6.6 ± 0.05 |
| Uninfected MSCCt | >280, >280, >280, >280, >280 | ||
| Infected MSCCh | 89, 89, 89, 99 | 91.5 ± 5 | 4.39 ± 0.50 |
Titers in the experimental samples were determined using the infectivity bioassay with an equation determined previously (23) for mouse scrapie strain: y = 11.45 − 0.088 x, where y is the titer in log LD50 units per milliliter of 10% original inoculum and x is the average of incubation days in tga20 mice.
We confirmed transmissible spongiform encephalopathies by PrPSc accumulation in brain extracts from Chandler-inoculated (Fig. 4A, lane 1) and from MSCCh-inoculated (Fig. 4A, lane 3) mice. Mice inoculated with uninfected MSC-80 cells (MSCCt) did not develop scrapie and did not show any PrPSc in their brain (Fig. 4A, lane 2). The apparent larger amount of PrPSc in brains of mice inoculated with MSCCh could be a consequence of the prolonged incubation period which leads to a prolonged PrPSc accumulation time.
FIG. 4.
Transmission of infected MSC-80 cells to mice. (A) Detection of PrPSc in brain extracts of MSCCh-inoculated tga20 mice. Four t ga20 mice were injected i.c. with Chandler-challenged MSC-80 cells homogenates after seven passages. For the control, five tga20 mice were inoculated i.c. with either the Chandler strain or noninfected MSC-80 cells. Scrapie was diagnosed according to standard clinical criteria (18). Mice inoculated with noninfected MSC-80 cells did not develop scrapie. Their brains were analyzed at the same time as the scrapie mice. At the terminal stage of the disease, immunoblot analysis of brain extracts from scrapie mice were performed. Tissue extracts were treated with PK. Immunoreactive PrPSc was detected with a mixture of three MAbs: SAF 60, SAF 69, and SAF 70 (10). The pattern identified in brain extract from MSCCh-inoculated mice (lane 3) was similar to the one observed with Chandler-infected mice (lane 1). Brain extract from noninfected MSC-inoculated mice did not show any PrPSc signal (lane 2). (B) The histological examination of brain sections colored with hematoxin-eosin showed an intense vacuolation of the neuropile in MSCCh-inoculated tga20 mice at the terminal stage of the disease. (C) An anti-glial fibrillary acidic protein immunolabeling exhibited a typical astrogliosis. Bar, 40 μm.
Histopathological studies were also realized to confirm transmissible spongiform encephalopathy and showed both an intense vacuolation of the neuropile (Fig. 4B) and an astrogliosis observed after an anti-glial fibrillary acidic protein labeling (Fig. 4C). These data demonstrated that MSC-80 Schwann cells can be infected in vitro with the mouse-adapted Chandler scrapie strain and can support the conversion of PrPc to PrPSc until after 18 weeks of culture.
DISCUSSION
There is increasing evidence indicating a central role of PrPc in the PNS in prion neuroinvasion (14). However, at the cellular level, the mode of transport of infectivity in peripheral nerves has not been determined yet. Our data demonstrate for the first time that PrPc is expressed in Schwann cell on mouse sciatic nerve sections and in a mouse Schwann cell line. We showed that this cell line can be infected in vitro and replicate the disease-specific isomer PrPSc, suggesting that prion propagation can occur along peripheral nerves via Schwann cells. The replication of prion in cell culture has previously been described only in neuronal cell lines (8, 25, 29, 30) but never in a glial cell line. Furthermore, Nishida et al. (25) have shown that only stably transfected N2a cells expressing a high level of mouse PrPc can efficiently replicate the mouse scrapie Chandler strain. In our experiment, untransfected Schwann cells can replicate the Chandler strain and support the conversion from the cellular to the protease resistant form. The susceptibility of Schwann cell to other scrapie strains remains however to be tested. These results also support earlier findings by others using transgenic mice with tissue specific hamster PrP expression to demonstrate the crucial role of PrP-positive peripheral nerves in the process of neuroinvasion following peripheral infection (28). These findings are similar to previous experiments with SCID mice infected intraperitoneally with a high dose of mouse scrapie strain ME7, where neuroinvasion also appeared to proceed directly via peripheral nerves without a susceptible lymphoreticular system or evidence of replication in the spleen (12, 20). However, the possibility that PrPSc transport in the PNS may not occur through axonal transport mechanisms was raised by Groschup et al. (15), Hainfeller et al. (16), and more recently by Glatzel et al., (14) who estimated the velocity of transport of infectivity in the PNS to be 0.7 mm per day. This value, as pointed out by Glatzel et al. (14) does not correspond to either fast nor slow axonal transport, indicating that PrPSc transport in the PNS may occur through another mechanism. Together with these findings, our data suggest a unifying concept for neuroinvasion via the peripheral nerves and bring a new insight in prion propagation along the peripheral nerves: the peripheral spread of prion through the PNS could occur in a domino effect fashion via PrPc-paved chain of Schwann cells, capable of supporting prion replication through conversion of PrPc by adjacent PrPSc. Finally, the expression of PrPc at the cell surface of Schwann cells suggests that PrPc does not play a structural nor a functional role in the myelin sheath, but may explain the peripheral neuropathies observed in some Creutzfeldt-Jacob Diseases patients (24) and in mice overexpressing PrPc (32). These data provide a basis for investigation into the cellular mechanisms of TSE transmission by the peripheral nerves and of therapeutic approaches to the TSEs.
Acknowledgments
We thank J.-J. Hauw (Hôpital Pitié Salpêtrière, France) for providing MSC-80 cells, C. Weissmann and A. Aguzzi for providing tga20 mice, and J. R. Barta (University of Guelph, Guelph, Ontario, Canada) and J. Gagnon (IBS, Grenoble, France) for critical reading of the manuscript.
J.F. has a fellowship with the Ministère de l'Education, de la Recherche et de la Technologie. This work was supported by the CNRS, grants from the “Programme de Recherche sur les ESST et les prions,” and CCE-FAIR grant JCT-6022.
REFERENCES
- 1.Beekes, M., P. A. McBride, and E. Baldauf. 1998. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. J. Gen. Virol. 79:601-607. [DOI] [PubMed] [Google Scholar]
- 2.Berryman, M. A., and R. D. Rodewald. 1990. An enhanced method for post-embedding immunocytochemical staining which preserves cell membranes. J. Histochem. Cytochem. 38:159-170. [DOI] [PubMed] [Google Scholar]
- 3.Blattler, T., S. Brandner, A. J. Raeber, M. A. Klein, T. Voigtlander, C. Weissmann, and A. Aguzzi. 1997. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature 389:69-73. [DOI] [PubMed] [Google Scholar]
- 4.Bons, N., N. Mestre-Frances, P. Belli, F. Cathala, D. C. Gajdusek, and P. Brown. 1999. Natural and experimental oral infection of nonhuman primates by bovine spongiform encephalopathy agents. Proc. Natl. Acad. Sci. USA 96:4046-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutry, J. M., J. J. Hauw, A. Gansmuller, N. Di-Bert, M. Pouchelet, and A. Baron-Van Evercooren. 1992. Establishment and characterization of a mouse Schwann cell line which produces myelin in vivo. J. Neurosci. Res. 32:15-26. [DOI] [PubMed] [Google Scholar]
- 6.Bruce, M. E., R. G. Will, J. W. Ironside, I. McConnell, D. Drummond, A. Suttie, L. McCardle, A. Chree, J. Hope, C. Birkett, S. Cousens, H. Fraser, and C. J. Bostock. 1997. Transmissions to mice indicate that ‘new variant' CJD is caused by the BSE agent. Nature 389:498-501. [DOI] [PubMed] [Google Scholar]
- 7.Bueler, H., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 8.Butler, D. A., M. R. Scott, J. M. Bockman, D. R. Borchelt, A. Taraboulos, K. K. Hsiao, D. T. Kingsbury, and S. B. Prusiner. 1988. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J. Virol. 62:1558-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandler, R. L. 1965. An experimental mixed infection of mice with scrapie and an oncogenic virus. J. Comp. Pathol. 75:323-326. [DOI] [PubMed] [Google Scholar]
- 10.Demart, S., J. G. Fournier, C. Creminon, Y. Frobert, F. Lamoury, D. Marce, C. Lasmezas, D. Dormont, J. Grassi, and J. P. Deslys. 1999. New insight into abnormal prion protein using monoclonal antibodies. Biochem. Biophys. Res. Commun. 265:652-657. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, J. R. 1996. Infectivity in extraneural tissues following intraocular scrapie infection. J. Gen. Virol. 77:2663-2668. [DOI] [PubMed] [Google Scholar]
- 13.Gajdusek, D. C., C. J. Gibbs, and M. Alpers. 1966. Experimental transmission of a kuru-like syndrome to chimpanzees. Nature 209:794-796. [DOI] [PubMed] [Google Scholar]
- 14.Glatzel, M., and A. Aguzzi. 2000. PrPC expression in the peripheral nervous system is a determinant of prion invasion. J. Gen. Virol. 81:2813-2821. [DOI] [PubMed] [Google Scholar]
- 15.Groschup, M. H., M. Beekes, P. A. McBride, M. Hardt, J. A. Hainfellner, and H. Budka. 1999. Deposition of disease-associated prion protein involves the peripheral nervous system in experimental scrapie. Acta Neuropathol. 98:453-457. [DOI] [PubMed] [Google Scholar]
- 16.Hainfellner, J. A., and H. Budka. 1999. Disease associated prion protein may deposit in the peripheral nervous system in human transmissible spongiform encephalopathies. Acta Neuropathol. 98:458-460. [DOI] [PubMed] [Google Scholar]
- 17.Kimberlin, R. H., and C. A. Walker. 1980. Pathogenesis of mouse scrapie: evidence for neural spread of infection to the CNS. J. Gen. Virol. 51:183-187. [DOI] [PubMed] [Google Scholar]
- 18.Kimberlin, R. H., and C. A. Walker. 1989. The role of the spleen in the neuroinvasion of scrapie in mice. Virus Res. 12:201-211. [DOI] [PubMed] [Google Scholar]
- 19.Krasemann, S., M. Groschup, G. Hunsmann, and W. Bodemer. 1996. Induction of antibodies against human prion proteins (PrP) by DNA-mediated immunization of PrP0/0 mice. J. Immunol. Methods 199:109-118. [DOI] [PubMed] [Google Scholar]
- 20.Lasmezas, C. I., J. Y. Cesbron, J. P. Deslys, R. Demaimay, K. T. Adjou, R. Rioux, C. Lemaire, C. Locht, and D. Dormont. 1996. Immune system-dependent and -independent replication of the scrapie agent. J. Virol. 70:1292-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasmezas, C. I., J. P. Deslys, O. Robain, A. Jaegly, V. Beringue, J. M. Peyrin, J. G. Fournier, J. J. Hauw, J. Rossier, and D. Dormont. 1997. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 275:402-405. [DOI] [PubMed] [Google Scholar]
- 22.Lemaire-Vieille, C., T. Schulze, V. Podevin-Dimster, J. Follet, Y. Bailly, F. Blanquet-Grossard, J. P. Decavel, E. Heinen, and J. Y. Cesbron. 2000. Epithelial and endothelial expression of the green fluorescent protein reporter gene under the control of bovine prion protein (PrP) gene regulatory sequences in transgenic mice. Proc. Natl. Acad. Sci. USA 97:5422-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montrasio, F., R. Frigg, M. Glatzel, M. A. Klein, F. Mackay, A. Aguzzi, and C. Weissmann. 2000. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 288:1257-1259. [DOI] [PubMed] [Google Scholar]
- 24.Neufeld, M. Y., J. Josiphov, and A. D. Korczyn. 1992. Demyelinating peripheral neuropathy in Creutzfeldt-Jakob disease. Muscle Nerve 15:1234-1239. [DOI] [PubMed] [Google Scholar]
- 25.Nishida, N., D. A. Harris, D. Vilette, H. Laude, Y. Frobert, J. Grassi, D. Casanova, O. Milhavet, and S. Lehmann. 2000. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J. Virol. 74:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne, R. J., and D. C. Krakauer. 1998. The spatial dynamics of prion disease. Proc. R. Soc. Lond. B 265:2341-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prusiner, S. B. 1991. Molecular biology of prion diseases. Science 252:1515-1522. [DOI] [PubMed] [Google Scholar]
- 28.Race, R., M. Oldstone, and B. Chesebro. 2000. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J. Virol. 74:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubenstein, R., R. I. Carp, and S. M. Callahan. 1984. In vitro replication of scrapie agent in a neuronal model: infection of PC12 cells. J. Gen. Virol. 65:2191-2198. [DOI] [PubMed] [Google Scholar]
- 30.Schatzl, H. M., L. Laszlo, D. M. Holtzman, J. Tatzelt, S. J. DeArmond, R. I. Weiner, W. C. Mobley, and S. B. Prusiner. 1997. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J. Virol. 71:8821-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilette, D., O. Andreoletti, F. Archer, M. F. Madelaine, J. L. Vilotte, S. Lehmann, and H. Laude. 2001. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc. Natl. Acad. Sci. USA 98:4055-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westaway, D., S. J. DeArmond, J. Cayetano-Canlas, D. Groth, D. Foster, S. L. Yang, M. Torchia, G. A. Carlson, and S. B. Prusiner. 1994. Degeneration of skeletal muscle, peripheral nerves, and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell 76:117-129. [DOI] [PubMed] [Google Scholar]