Abstract
Herpes simplex keratitis (HSK) is an inflammatory response to viral infection and self antigens in the cornea and is a major cause of blindness. Using two strains of mice which are susceptible (129/SVEV) and resistant (C57BL/6) to herpes simplex virus (HSV) strain KOS, (129/SVEV × C57BL/6)F2 mice were generated and examined for their disease susceptibility in terms of clinical symptoms, ocular disease, and antibody production following corneal scarification with HSV (KOS). A genome-wide screen was carried out using microsatellite markers to determine the genetic loci involved in this response. Loci on chromosomes 4, 5, 12, 13, and 14 were shown to be involved in general susceptibility to clinical disease, whereas loci on chromosomes 10 and 17 were shown to be unique to ocular disease.
Herpes simplex virus (HSV) is a neurotropic DNA virus. When HSV is introduced into the eye, it infects corneal epithelial cells, keratocytes, and endothelial cells and induces herpes simplex keratitis (HSK), an inflammation of the cornea which is a major cause of corneal blindness. In addition to the direct cytopathic consequences of HSV infection, there is a considerable autoimmune component to this disease. There have been many studies showing the role of T cells in the disease (12, 13, 18, 31, 32, 34, 40, 41). That an autoimmune response is induced after HSV infection via corneal scarification has been shown in both the mouse and rat (2, 9, 17, 50), though evidence for this mechanism has not been found in humans (21, 46).
It is also clear that there are strain differences in the mouse for susceptibility to herpes keratitis (13, 32, 34, 41). Genes from the murine major histocompatibility locus (H-2) as well as non-H-2 genes were found to be involved in immune responsiveness to this infection, which relates directly to susceptibility. Genetic studies using congenics have identified a keratitis-modifying locus on chromosome 12 of the mouse that is close to or identical with the immunoglobulin heavy chain (Igh) locus (13). This finding led to studies of the potential role of idiotypic Igh determinants in initiating and/or maintaining a state of self-tolerance (2). Such determinants have been shown to be cross-reactive with HSV determinants as well (50; K. Norose and E. Heber-Katz, unpublished data). However, the roles of other genes in resistance or susceptibility to HSK is unknown. Such genes might control the initial effectiveness of the immune response to the virus or the subsequent pathogenic autoreactivity unrelated to the Igh idiotype. These questions can be addressed by mapping genes that modify the severity and incidence of HSK in susceptible and resistant inbred mice and their progeny. This approach has been employed to understand the genetic basis underlying other autoimmune diseases (6, 16, 22, 28, 29, 49), but genes that control the autoimmune outcome of infectious agents are less well characterized (4, 8, 30, 37, 42).
In the present study, we carried out a genome-wide screen using the DNA-based mapping reagents or microsatellite markers (simple sequence length polymorphism [SSLP]) on chromosomes 1 through 19 of two parental mouse strains, 129/SVEV and C57BL/6, with the same major histocompatibility complex (MHC) haplotype (H-2b). Using markers that exhibited polymorphism, we analyzed the F2 progeny derived from matings of these two strains. Keratitis was measured by both clinical and histopathological parameters, and each was used independently as a quantitative trait phenotype for analysis with respect to the inheritance of microsatellite markers. As the first step in mapping the genes, we found multiple quantitative trait loci (QTL) that are involved in the clinical symptoms of keratitis susceptibility in addition to the previously identified locus on chromosome 12. We found multiple loci involved in ocular histopathology as well, which also included the QTL on chromosome 12. Like the QTL on chromosome 12, some of the loci were shared between these phenotypes, whereas some of the loci were unique to either clinical symptoms or histopathology. We also report the influence of sex on both the severity and incidence of HSK and the use of different genes in males and females for the particular phenotypes.
MATERIALS AND METHODS
Mice.
The parental 129/SVEV mice were obtained from the Jackson Laboratory (Bar Harbor, Maine), and the C57BL/6 parental mice were acquired from the Taconic Laboratory (Germantown, N.Y.). Mice from both parental strains were bred and maintained under standard conditions at The Wistar Institute (Philadelphia, Pa.). F1 and F2 populations were generated to conduct the genetic studies. The female parent used for generating F1 and intercross mice was 129/SVEV.
Infection.
HSV type 1 (HSV-1) strain KOS (kindly provided by Nigel Fraser, University of Pennsylvania, Philadelphia) at a concentration of 2 × 108 PFU/ml was used to infect the mice. The cornea of the right eye was scarified, and each mouse received 5 μl or 106 PFU. The left eye was untreated.
Histology.
For preparation of specimens for histology, mice were sacrificed on day 14 after infection and the eyes were enucleated, fixed in buffered 10% formalin, embedded in paraffin, cut in 5-μm-thick sections, and stained with hematoxylin and eosin.
Enzyme-linked immunosorbent assay (ELISA).
HSV-1 (KOS) (104 PFU/50 μl/well) was added to 96-well PRO-BIND assay plates (Falcon 3915; BD and Co., Lincoln Park, N.J.) overnight at room temperature. The wells were washed three times, coated with 100 μl of a solution of 1% bovine serum albumin in phosphate-buffered saline for 2 h, washed three times, and then incubated with 50 μl of serum. The serum was used at final dilutions of 1:200, 1:1,000, and 1:5,000. Sera from uninfected C57BL/6 mice were used as a negative control, and the monoclonal HSV glycoprotein D-specific antibody B35 was used as a positive control. After 2 h, the plates were washed three times, alkaline phosphatase-coupled goat anti-mouse immunoglobulin (catalog no. A3562; Sigma) was added at a concentration of 1:10,000 for 1 h, and the plates were washed four times. The substrate p-nitrophenylphosphate was then added, and the plates were developed. The plates were then read at 650 and 405 nm using an SLT Rainbow SPECTRA 96-well plate reader.
Phenotyping.
Scoring of susceptibility on day 6 for clinical disease and on day 15 for histological analysis after HSV infection was determined in several different ways.
(i) Clinical disease score.
The clinical disease score was indicated as follows: 0, normal; 1+, light sensitivity; 2+, mild blepharitis; 3+, severe blepharitis; and 4+, ruffled fur and shaking.
(ii) Histological score. (a) Corneal pathology (group I).
Corneal pathology was scored as follows: 0, clear cornea; 1+, slight cellular infiltration; 2+, moderate cellular infiltration; 3+, intense cellular infiltration; and 4+, severe necrotizing stromal keratitis.
(b) Anterior-chamber pathology (group II).
Anterior-chamber pathology was scored as follows: 0, clear; 1+, slight cellular infiltration and/or fibrin; 2+, moderate cellular infiltration and/or fibrin; 3+, intense cellular infiltration and/or fibrin; and 4+, anterior synechia.
(c) Stromal keratitis score (group III).
Stromal keratitis was scored as follows: 0, clear; 1+, corneal opacity and neovascularization in less than 25% of the cornea; 2+, less than 50% corneal opacity and neovascularization; 3+, less than 75% corneal opacity and neovascularization; and 4+, 75 to 100% corneal opacity and neovascularization.
Mice were assigned to one of three arbitrary severity classes based on their low, intermediate, or severe keratitis (HSK 0 to 1, 2, or 3 to 4). This trait was used to develop information on the linkage of loci that affected this qualitative severity and susceptibility trait. In addition, mice that showed at least a 1 in either of the histopathology scores were separately evaluated for the presence of HSK to determine the loci that are involved in progression from infiltration to frank disease.
Genetic analysis.
Genomic DNA was prepared from the liver of each animal in the (129/SVEV × C57BL/6) × (129/SVEV × C57BL/6)F2 population. DNA from the frozen liver was prepared using a DNA tissue extraction kit (Qiagen Inc., Valencia, Calif.). PCR primers, purchased from Research Genetics (Huntsville, Ala.), were employed to perform a genome-wide scan of the mouse. Amplification was conducted using Boehringer Mannheim reagents with the following concentrations: 1× PCR buffer, 0.375 mM deoxynucleoside triphosphates, 0.5 U of of Taq polymerase/μl, 0.165 μM (each) primer, and 160 ng of genomic DNA/20 μl. The cycling conditions included a 1-min denaturing at 95°C; 35 to 50 cycles of 1 min at 94°C, 1 min 30 s at 55°C, and 2 min 10 s at 72°C; and a 6-min final extension at 72°C. The PCR products were resolved using 3% Metaphor agarose (FMC, Rockland, Maine) and were visualized through ethidium bromide staining. This method was followed for the majority of polymorphic markers.
Statistics.
Genotype data were organized and analyzed through the use of Map Manager QT (27). For quantitative trait analysis, SSLP markers were evaluated individually based on linkage to the phenotypes, and intervals containing likely QTL were identified by interval mapping using the Zmap program in QTL Cartographer model 3 (http://statgen.ncsu.edu/qtlcart/cartographer.htm4). Critical values for the declaration of significance were determined by the permutation test routine in QTL Cartographer, based on a regression model developed by Churchill and Doerge (10, 11). The values were given in terms of a likelihood ratio statistic (LRS) and were based on the following values: α = 0.37 (suggestive) (27), α = 0.1 (strongly suggestive), α = 0.05 (significant), and α = 0.01 (highly significant) 7; http://mapmgr.roswellpark.org/mapmgr.html). LRS is equivalent to 4.6 LOD (likelihood of linkage).
RESULTS
Susceptibility to ocular HSV infection.
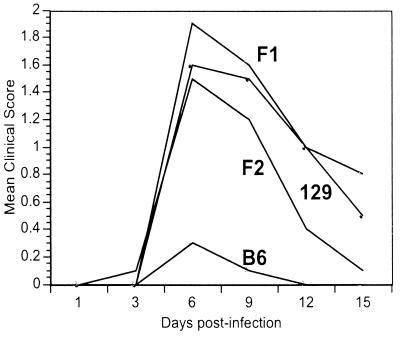
Corneal scarification was carried out using the KOS strain of HSV-1 at 106 PFU/mouse. In the initial susceptibility experiments, male and female C57BL/6 and 129/SVEV mice were infected and followed for disease. Peak disease occurred on day 6, and after this time point, all of the animals recovered (Fig. 1). It can be seen in Fig. 1 that C57BL/6 mice were relatively resistant to disease and that 129/SVEV mice were relatively susceptible to disease.
FIG. 1.
Kinetics of disease susceptibility. Ten mice each of the parental strains, 129/SVEV (129) and C57BL/6 (B6), and (129/SVEV × C57BL/6)F1 and F2 mice were infected with 106 PFU of HSV/mouse and followed for clinical symptoms of disease for the next 15 days.
We next crossed the parental strains, generating (129/SVEV × C57BL/6)F1 mice, and intercrossed those F1 mice to generate F2 mice. Both F1 and F2 mice (10 mice each) were infected with virus. The disease kinetics in all groups were similar (Fig. 1), and day 6 was chosen as the time point to analyze disease susceptibility in all further studies.
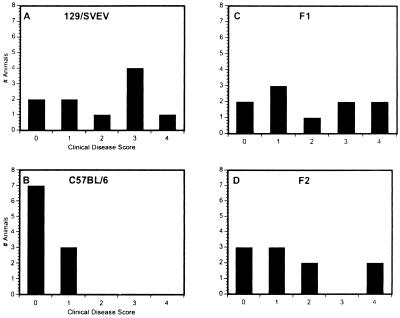
In contrast, the incidence and disease severity in all of the groups of parental and offspring mice were different from one another. On day 6, less-severe disease was seen in C57BL/6 mice (Fig. 2B), whereas the majority of 129/SVEV mice achieved a disease level of ≥3 (Fig. 2A). Both F1 (Fig. 2C) and F2 (Fig. 2D) populations showed an intermediate level of keratitis that was less severe than that shown by the 129/SVEV mice but more severe than that in C57BL/6 mice.
FIG. 2.
Severity and incidence of disease. Ten mice were examined on day 6 after HSV infection. The number of mice with an incidence at each clinical disease score is shown. The clinical scores are as follows: 0, normal; 1, light sensitivity; 2, mild blepharitis; 3, severe blepharitis; and 4, ruffled fur and shaking.
To ensure that the mice had been infected, lymph nodes and spleens were removed from the parental strains after day 15, and antigen-specific proliferative studies showed that all of the mice, both C57BL/6 and 129/SVEV, responded to glycoprotein D of HSV (data not shown), indicating that all of the mice had been infected. Therefore, the relative resistance of C57BL/6 mice seen in Fig. 1 reflects a relatively efficient virus clearance mechanism and/or genetic resistance to the immune consequences of HSV infection and not a failure to induce that infection per se.
Mapping of SSLP markers and significant threshold values.
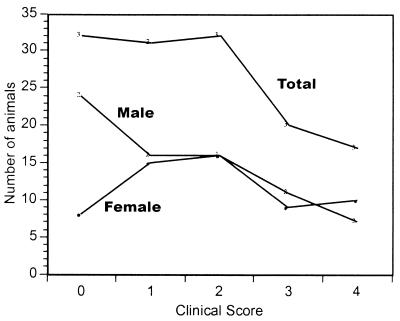
To carry out mapping studies, we generated 132 F2 mice. All of the mice were infected at 8 weeks of age and scored for disease 6 days after infection. A graph showing disease incidence and severity in the whole F2 population and also in the males (n = 74) and females (n = 58) can be seen in Fig. 3. The incidence of disease (score, >0) in the total population was 76%. More males (32%) were resistant to disease than females (14%), and their clinical signs were less severe. The mean of the disease level achieved in the total population was 1.7, with females achieving a mean score of 2.0 and males achieving a mean score of 1.5.
FIG. 3.
Disease incidence and severity in mapped F2 population. One hundred and thirty-two F2 mice were infected with HSV and examined on day 6 after infection for clinical symptoms as described in the legend to Fig. 2. The data presented are the number of mice at each level of clinical disease achieved. The three populations presented in this graph are the total population, the female group in the total population, and the male group in the total population.
All F2 progeny mice were also scored by ELISA for the presence of antiviral antibody. F2 mice that did not respond to the virus inoculation either by making specific antibody to HSV (KOS) or by displaying evidence of keratitis (n = 4) were considered possibly uninfected and removed from the analysis. The markers were distributed on average every 8.0 ± 4.9 centimorgans (cM) over the 19 autosomes.
The HSK susceptibility traits were mapped using the 128 mice from the (129/SVEV × C57BL/6)F2 intercross. For assessment of the probability of genetic linkage, critical values were calculated from this database using the permutation test (7). Proposed thresholds for highly significant (α = 0.01), significant (α = 0.05), strongly suggestive (α = 0.1), and suggestive (α = 0.37) linkage (27; http://mapmgr.roswellpark.org/mapmgr.html) were assigned to maximum LRS scores, which are indicated in the tables. The order of all markers in this linkage analysis was consistent with the order predicted by the available genomic maps (Whitehead Institute-MIT and Mouse Genome Database, The Jackson Laboratory, Bar Harbor, Maine [http://www.informatics.jax.org]).
Mapping of QTL linked to the HSV clinical disease susceptibility phenotype in the F2 population.
Tables 1 and 2 (All F2) show the microsatellite markers that were positive for linkage to the HSV susceptibility phenotype. In the F2 cross, QTL that contribute to this phenotype and are derived from 129/SVEV exist in five primary support intervals on chromosomes 4, 5, 12, 13, and 14. Of these five intervals, the QTL on chromosome 12 had the highest likelihood of linkage to the HSK-related clinical susceptibility trait, with a strongly suggestive likelihood of linkage (P = 0.0005). The other four QTL are also suggestive and are located on chromosome 4 near D4MIT332 (54 cM), on proximal chromosome 5 near D5Mit145 (0 cM), in the middle of chromosome 13 near D13Mit13 (35 cM), and in the middle of chromosome 14 near D14Mit197 (52 cM).
TABLE 1.
Locations of susceptibility QTL (clinical HSK)
| Marker (cM) | All F2
|
Male F2
|
Female F2
|
|||
|---|---|---|---|---|---|---|
| LRS | P value | LRS | P value | LRS | P value | |
| D4Mit332 (54) | 11.1a | 0.00400 | 8.0 | 0.01829 | 2.5 | 0.28419 |
| D4Mit278 (55) | 10.2 | 0.00600 | 7.1 | 0.02914 | 1.9 | 0.39919 |
| D5Mit145 (0) | 10.3 | 0.00580 | 0.8 | 0.67749 | 17.8c | 0.00013 |
| D5Mit48 (1) | 10.3 | 0.00580 | 0.8 | 0.67749 | 17.8c | 0.00013 |
| D5Mit1 (5) | 6.3 | 0.04379 | 0.7 | 0.71595 | 10.5 | 0.00525 |
| D5Mit294 (8) | 8.8 | 0.01221 | 2.1 | 0.35630 | 10.5 | 0.00525 |
| D12Mit20 (58) | 15.1a | 0.00053 | 9.9 | 0.00706 | 8.7 | 0.01270 |
| D12Mit134 (59) | 15.1a | 0.00053 | 9.9 | 0.00706 | 8.7 | 0.01270 |
| D13Mit137 (21) | 10.4 | 0.00544 | 8.3 | 0.01599 | 3.0 | 0.22396 |
| D13Mit139 (32) | 11.2a | 0.00374 | 8.8 | 0.01239 | 4.7 | 0.09469 |
| D13Mit13 (35) | 13.4a | 0.00125 | 8.2 | 0.01694 | 7.0 | 0.03006 |
| D13Mit254 (40) | 10.1 | 0.00647 | 8.3 | 0.01607 | 4.2 | 0.12360 |
| D13Mit103 (44) | 10.9a | 0.00433 | 10.1 | 0.00641 | 4.2 | 0.12360 |
| D14Mit228 (46) | 11.5a | 0.00323 | 2.0 | 0.36372 | 15.8b | 0.00037 |
| D14Mit265 (48) | 10.6 | 0.00502 | 1.2 | 0.55730 | 18.2c | 0.00011 |
| D14Mit269 (50) | 11.4a | 0.00340 | 1.9 | 0.38469 | 16.1b | 0.00032 |
| D14Mit197 (52) | 12.2a | 0.00226 | 2.9 | 0.23853 | 14.6a | 0.00069 |
| D14Mit185 (54) | 10.0 | 0.00658 | 0.8 | 0.68400 | 18.7c | 8.5e05 |
Suggestive.
Strongly suggestive.
Significant.
TABLE 2.
Critical values for susceptibility QTL (clinical HSK) in Table 1
| Critical value (α) | LRS
|
|||
|---|---|---|---|---|
| Total | Male | Female | ||
| 0.37 | Suggestive | 10.9 | 10.3 | 10.3 |
| 0.1 | Strongly suggestive | 15.4 | 15.2 | 15.9 |
| 0.05 | Significant | 17.2 | 17.4 | 17.6 |
| 0.01 | Highly significant | 21.2 | 19.2 | 20.8 |
Mapping of QTL associated with the HSK clinical susceptibility phenotype in the male and female populations.
Tables 1 and 2 also show the analysis of the clinical HSK-related susceptibility trait examined in the male and female populations separately. The suggestive loci on chromosomes 5 and 14 seen in the whole F2 population reach significance only in the female population; these loci are not significantly linked to clinical susceptibility in the male population. The QTL on chromosome 12 displays about equal significance in both males and females (P = 0.007 and 0.013, respectively), but the significance of this linkage reaches a suggestive LRS value only when the combined population is analyzed. In the case of the suggestive locus on chromosome 4, this is seen only in the male population. Finally, in the case of chromosome 13, there is a region at 32 to 35 cM which gives suggestive values in both males and females and perhaps a separate locus at 44 cM which appears to be male associated.
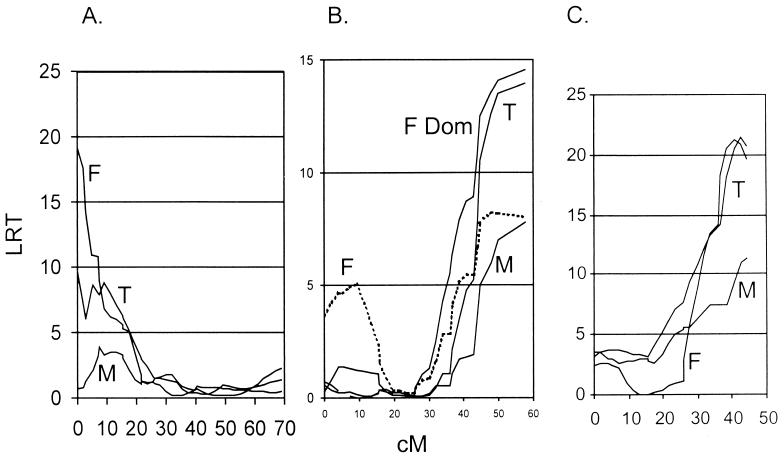
Interval-mapping analyses supported the pointwise linkages to all the traits and enabled the accurate placement of the QTL on their respective chromosomes. In addition, the mode of inheritance was analyzed for each of the QTL, and several of them demonstrated a relationship to the phenotype that was best explained by a dominant or recessive inheritance. For example, the susceptibility allele of the QTL on chromosome 12 is recessive, consistent with its identity as a self antigen encoded by the Igh locus, in a model of self-tolerance where heterozygous expression of the deleting allele is sufficient to prevent self-recognition (2, 13, 50). Nevertheless, some females homozygous for the C57BL/6 allele at this QTL were severely affected with clinical HSK-related symptoms. The QTL on chromosome 5 shows recessive protection against clinical symptoms in females with homozygous expression of the C57BL/6 alleles. The QTL on chromosome 14 showed additive inheritance, where heterozygotes on average were characterized by intermediate clinical scores. The most significant intervals containing QTL are shown in Fig. 4. As with the pointwise linkages, the interval analysis showed strong sexual dimorphism in the QTL that underlie clinical disease in these progeny.
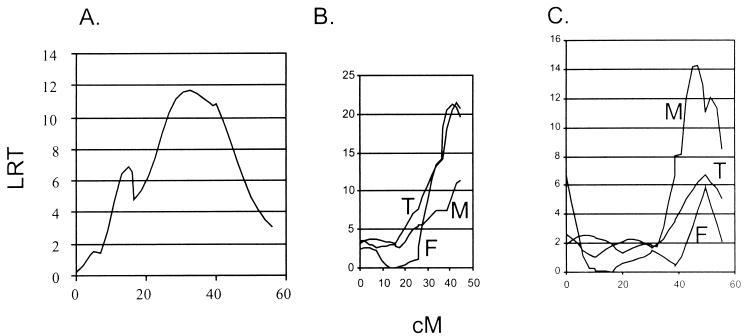
FIG. 4.
Intervals for HSK loci. Interval maps for the trait of HSK clinical disease show significant linkages on chromosome 12 (both sexes) (B), chromosome 5 (significant in females only) (A), and chromosome 14 (females only) (C). T, total; F, female; M, male. The QTL shown are calculated with free regression statistics except for chromosome 12 in females, where the dominant inheritance pattern (F Dom) is also depicted. In all three of the intervals shown, a down slope on both sides of the peaks could not be shown; thus, end effects need to be considered.
HSK histopathology.
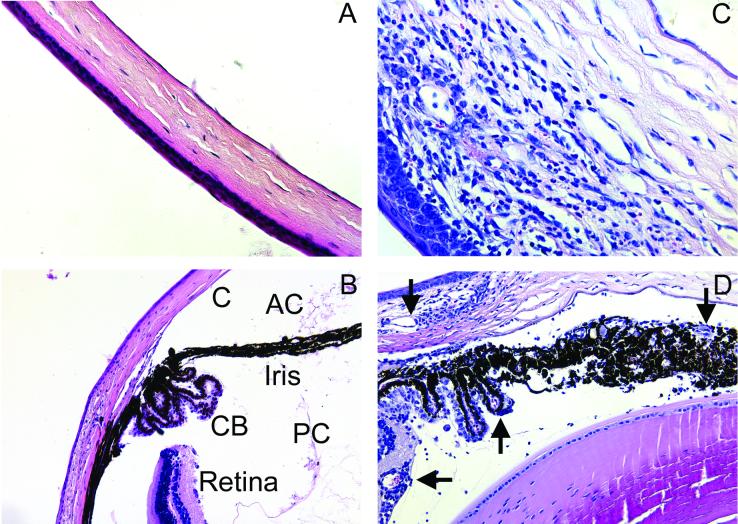
The analyses discussed above were all based on clinical disease caused by corneal scarification with HSV (KOS). However, we were interested in all loci involved not only in general disease symptoms but in keratitis and other ocular features of disease. This included iritis, uveitis, ulcerations, and neovascularization (Fig. 5).
FIG. 5.
Histological analysis of the eye after HSV (KOS) infection. Normal uninfected tissue from C57BL/6 mice (A and B) and tissue from infected (129/SVEV × C57BL/6)F2 mice (C and D) were fixed in buffered 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. Infected mice showed corneal (C) infiltrates and swelling, neovascularization (arrows), and cellular infiltrates in the ciliary body (CB), iris, and anterior (AC) and posterior (PC) chambers. Magnification, ×40 (A, C, and D) or ×20 (B).
When the analysis of the progeny was restricted to the corneal-pathology phenotype (Tables 3 and 4 [we used the histological scoring group I described in Materials and Methods]) or histopathology in general (Tables 5 and 6 [we used the histological scoring groups I to III]), two major QTL were seen on chromosomes 10 and 12. The C57BL/6-derived allele at the chromosome 12 QTL behaved as a dominant protective gene, with the 129/SVEV alleles associated with severe corneal pathology; protection due to the inheritance of C57BL/6 alleles at the chromosome 10 QTL behaved as a recessive trait. A locus on chromosome 17 (near 46 cM) was also modestly linked to total histopathology of the eye in males.
TABLE 3.
Locations of susceptibility QTL involved in corneal pathology
| Marker (cM) | All F2
|
Male
|
Female
|
|||
|---|---|---|---|---|---|---|
| LRS | P value | LRS | P value | LRS | P value | |
| D10Mit186 (40) | 10.8 | 0.0045 | 4.0 | 0.1341 | 9.2 | 0.0100 |
| D10Mit42 (44) | 11.0a | 0.0040 | 3.5 | 0.1746 | 9.8 | 0.0074 |
| D12Mit102 (50) | 9.2 | 0.0099 | 4.3 | 0.1165 | 8.3 | 0.0157 |
| D12Mit20 (58) | 15.2b | 0.0005 | 8.3 | 0.0157 | 10.7a | 0.0047 |
| D13MIT142 (37) | 5.5 | 0.0637 | 5.0 | 0.0811 | 7.0 | 0.0297 |
| D13Mit254 (40) | 9.7 | 0.0077 | 7.0 | 0.0305 | 7.0 | 0.0306 |
| D13Mit103 (44) | 9.8 | 0.0073 | 8.1 | 0.0173 | 7.0 | 0.0306 |
| D13MIT191 (45) | 6.1 | 0.0482 | 8.0 | 0.0179 | 3.3 | 0.1883 |
Suggestive.
Strongly suggestive.
TABLE 4.
Critical values for susceptibility QTL involved in corneal pathology in Table 3
| Critical value (α) | LRS
|
|||
|---|---|---|---|---|
| Total | Male | Female | ||
| 0.37 | Suggestive | 10.2 | 10.3 | 10.3 |
| 0.10 | Strongly suggestive | 15.2 | 15.4 | 15.4 |
| 0.05 | Significant | 16.7 | 16.9 | 17.6 |
| 0.01 | Highly significant | 20.3 | 22.0 | 20.8 |
TABLE 5.
Locations of susceptibility QTL involved with total histopathology
| Marker (cM) | All F2
|
Male F2
|
Female F2
|
|||
|---|---|---|---|---|---|---|
| LRS | P value | LRS | P value | LRS | P value | |
| D10Mit186 (40) | 8.6 | 0.0136 | 2.7 | 0.2620 | 9.3 | 0.0095 |
| D10Mit42 (44) | 9.0 | 0.0113 | 2.3 | 0.3212 | 10.2a | 0.0061 |
| D12Mit102 (50) | 15.4b | 0.0005 | 9.2 | 0.0100 | 10.2a | 0.0061 |
| D12Mit20 (58) | 21.9c | 1.8e5 | 14.0a | 0.0009 | 12.3a | 0.0022 |
| D17MIT158 (34) | 3.6 | 0.1650 | 8.2 | 0.0164 | 0.6 | 0.7515 |
| D17MIT93 (46) | 6.3 | 0.0429 | 9.8 | 0.0075 | 5.5 | 0.0626 |
| D17MIT155 (56) | 4.5 | 0.1049 | 6.7 | 0.0360 | 0.7 | 0.6954 |
Suggestive.
Strongly suggestive.
Highly significant.
TABLE 6.
Critical values for susceptibility QTL involved with total histopathology in Table 5
| Critical value (α) | LRS
|
|||
|---|---|---|---|---|
| Total | Male | Female | ||
| 0.37 | Suggestive | 9.9 | 10.4 | 10.2 |
| 0.10 | Strongly suggestive | 14.9 | 15.9 | 15.4 |
| 0.05 | Significant | 17.0 | 21.8 | 21.0 |
| 0.01 | Highly significant | 19.9 | 21.8 | 21.0 |
Interval analysis was performed to position the QTL for the total histopathology index (Fig. 6) and corneal inflammation (not shown). As with the inheritance of clinical disease, an interval on chromosome 12 that encompasses the Igh locus showed linkage to the histology scores in a dominant fashion. In addition, two other intervals showed a sexually dimorphic effect.
FIG. 6.
Intervals for ocular histopathology. Interval maps for the trait of total ocular histopathology show significant linkages on chromosome 10 (the total population) (A), chromosome 12 (both sexes) (B), and chromosome 17 (males only) (C). The QTL shown are calculated with free regression statistics. T, total; F, female; M, male.
Incidence and progression to HSK.
The F2 progeny were arbitrarily grouped into three discrete classes based on the presence and severity of keratitis (clinical disease scores 0, 1 to 2, and 3 to 4, respectively), and this discrete phenotype was tested for linkage to the markers used in the genome scan. Table 7 shows that markers on five chromosomes (chromosomes 4, 5, 12, 13, and 14) show linkage (LOD ≥ 3.0) to this incidence and severity trait. In addition, we determined whether genes in these five intervals could act as “progressor” loci, which are genetic loci capable of producing the keratitis trait from underlying inflammation. Of the 128 mice infected with HSV, 38 failed to develop any evidence of ocular inflammation. Of the remaining 90 mice with histological evidence of ocular disease, 14 had no keratitis signs and another 16 had very modest keratitis scores (<1). The 90 mice with histological evidence of disease were scored separately for the keratitis incidence and severity trait, and these results can be seen in Table 7. Markers on chromosomes 4, 5, and 12 were associated with the progression phenotype, whereas markers on chromosomes 13 and 14 did not contribute to progression. Sexual dimorphism of this trait was also noted.
TABLE 7.
Locations of loci that control severity and susceptibility to HSK
| Marker (cM) | All F2
|
Male F2
|
Female F2
|
|||
|---|---|---|---|---|---|---|
| LODa | Progression to HSK (P value)b | LOD | Progression to HSK (P value) | LOD | Progression to HSK (P value) | |
| D4Mit332 (54) | 4.4 | 3.2 | 1.2 | |||
| D4Mit278 (55) | 4.4 | 0.006 | 2.8 | 0.004 | 1.5 | NS |
| D4Mit12 (58) | 4.0 | 2.8 | 1.2 | |||
| D5Mit145 (0) | 3.7 | NS | 3.1 | |||
| D5Mit294 (8) | 3.4 | 0.04 | NS | 3.1 | 0.005 | |
| D12Mit102 (50) | 1.3 | NS | NS | |||
| D12Mit20 (58) | 3.0 | 0.03 | 1.8 | 0.12 | NS | 0.19 |
| D13Mit13 (35) | 3.9 | NSc | NS | NS | 2.9 | NS |
| D13Mit51 (59) | 4.2 | NS | NS | NS | 2.5 | NS |
| D14Mit193 (40) | 3.2 | NS | 2.7 | |||
| D14Mit204 (44) | 4.0 | 0.27 | NS | NS | 3.1 | 0.02 |
| D14Mit265 (48) | 4.0 | NS | 3.5 | |||
| D14Mit197 (52) | 3.0 | NS | 2.6 | |||
LOD scores for linkage of the qualitative traits of severity and susceptibility to markers on four chromosomes and their relative strengths of linkage in male and female F2 mice. Scores of ≥3.0 are in boldface.
Progression to HSK. Among those mice with histological evidence of HSV-induced ocular pathology, each of the markers in intervals shown to be linked to HSK susceptibility and severity was evaluated for linkage to progression to frank clinical signs of keratitis by analysis of variance, and the probability is indicated in the columns. All of the marker alleles linked to greater severity or progression are derived from the 129/SVEV (susceptible) strain, except those on chromosome 13.
NS, not significant.
Antibody titers in F2 progeny.
As a control for the success of infection in the F2 progeny, mice were bled on day 15, at the time of sacrifice, and sera were collected for analysis by ELISA for the presence and level of antibodies to HSV (KOS). In addition, these antibody titers were used to determine if a strong anti-HSV (KOS) antibody response was correlated with either HSK susceptibility or corneal-ocular histopathology results. A linear regression between HSK susceptibility scores and the titer of anti-HSV (KOS) antibody (expressed as the optical density of binding at either of two dilutions) was performed and suggested that the two measures of response to HSV were linearly related (high disease scores were correlated with high antibody levels and vice versa: r = 0.125; P = 0.0003). The correlation was less significant when antibody titers were regressed against HSV-induced ocular or corneal histopathology (P = 0.11 and 0.012, respectively). In all cases, the presence of high antibody titers did not correlate with protection against subsequent histopathology or disease but rather correlated with the presence and severity of these pathologies, indicating once again that HSK is the result of excessive viral immunity rather than deficient immune response to the virus.
DISCUSSION
HSV infections.
HSK is an infection of the cornea caused by HSV that leads to an inflammatory disease and consequent blindness. Susceptibility to this infectious disease has been shown to be related to both viral factors and factors involved in cellular infectibility; immune responses to the virus, both specific and innate; and immune responses to host antigens (18, 40).
In mice, HSK progression occurs after virus is cleared and requires infiltrates containing neutrophils (44) and T cells (9, 12, 17, 18, 21, 31, 40, 46). The T-cell response is predominantly a TH1 response (33), and it has been reported to be due to a cross-reaction between a corneal self antigen and a determinant on the HSV protein, UL6 (2, 50), as well as to a determinant on the immunoglobulin molecule, immunoglobulin G2. On the other hand, there have been studies showing that T-cell factors in the absence of antigen-specific T cells are sufficient, since a T-cell receptor (TcR) transgenic mouse without cross-reactive T cells can show symptoms of keratitis (15).
Confirmation of a previously identified HSK susceptibility locus.
Previous results examining multiple mouse strains for susceptibility to HSV-1 keratitis showed that genes close to the Igh-1 immunoglobulin allotype locus on chromosome 12 were associated with this trait; the H-2 haplotype, on the other hand, was not (13). Susceptibility was associated with Igh-1c, Igh-1dM, and Igh-1e alleles; resistance was associated with the Igh-1a, Igh-1b, and Igh-1j alleles. Igh-congenic mice on an H-2d background show HSK according to their allotype.
In the present study, we used two inbred strains of mice, C57BL/6 and 129/SVEV, which share an MHC haplotype (both are H-2b) but differ in their non-MHC genes. That the two strains differ greatly in their susceptibilities to HSV infection, with C57BL/6 being highly resistant, has been previously reported (31, 32, 41). In this study, using HSV-1 (KOS) at 106 PFU/mouse, 30% of the C57BL/6 mice showed disease symptoms compared to 80% of the 129/SVEV mice. That this was not due to a difference in infectibility was shown by the fact that in both groups of parental mice given virus, all of the mice responded to HSV antigen in culture. This indicated that our method of inoculation resulted in 100% virus exposure. In the F2 population that was generated and then mapped, the incidence of disease symptoms was 76%. Of the 32 F2 mice that did not show disease symptoms, almost half (15 of 32) showed evidence of viral infection histologically, increasing the incidence of measured infectivity to 87%. Finally, all but 4 of the 132 F2 mice showed titers of anti-HSV antibody that exceeded 1/1,000 dilution (not shown), again indicating the success of infection in all animals.
Carrying out a genome-wide screen at a 10-cM range, we found that in the whole F2 population, the locus at chromosome 12, close or identical to the Igh locus and previously identified, showed the most significant level of linkage with the susceptibility trait as determined by clinical disease symptoms. These included light sensitivity, blepharitis, and ruffled fur. However, we also found additional novel loci on chromosomes 4, 5, 13, and 14 that regulate this trait. Furthermore, in female mice, the level of significance of the QTL on chromosomes 5 and 14 exceeded that of the chromosome 12 QTL for HSK-related clinical disease incidence and severity.
This analysis examined the total response to virus after corneal scarification and included generalized symptoms as well as specific ocular effects seen only by histology. Thus, the virus infects keratocytes of the eye and induces an inflammatory response in multiple locations and also infects the epithelium surrounding the eye. Susceptibility probably involves factors besides cellular susceptibility to infection, including the immune response, both innate and antigen specific, to virus and to autoantigens.
Therefore, we also used the genome-wide screen to examine the ocular histopathological traits, determining involvement of the cornea as well as other components of the eye. In this case, we identified chromosomes 12 and 13 as loci with nearly suggestive to significant levels of association which were shared with the clinical disease symptoms described above. We also found new loci at chromosomes 10 and 17 which were unique to the analysis involving ocular histopathology.
Loci in males and females: potential modifiers.
We also examined the effect of sex on the response to HSV, which was made evident by the fact that the females were more susceptible to HSV disease, although there was no obvious evidence that they had a different disease phenotype.
Examination of the QTL screen in males and females separately revealed two different patterns of responsiveness. In one case, the LRS for the total population was greater than that for either the male or female group, although the QTL was linked to the phenotype at some level in each group. This could be due to the number of mice analyzed. This seems to be true for chromosome 12 and one interval on chromosome 13 at approximately 32 to 35 cM, which were only modestly significant in males and females when they were considered separately.
In the second case, the male or female value was higher than that of the total because the noncontributing male or female population caused the value in the total population to be lower. The QTL on chromosomes 5 and 14 were contributed by either females or males but not both. The QTL on chromosome 10 may be female specific, as it was quite modest in males for predicting histopathology scores.
An interesting interpretation of these data is that the locus on chromosome 12 (and possibly chromosome 13) is the key gene(s) in governing the level and character of the immune response to the virus and that the other QTL cause the disease to progress from inflammatory infiltrates to frank keratitis, with its associated clinical signs. This is consistent with the identity of the chromosome 12 QTL as a self antigen, because tolerance to the self antigen could predict the absence of an immune response to a cross-reactive viral antigen (2, 50). The fact that these two loci are also the loci shared between the different phenotypes also supports the idea that these are the key genes involved in susceptibility and that they act at an early stage. In males, the actions of the chromosome 12 and 13 QTL are apparently insufficient to create the environment necessary for frank keratitis, and a locus on chromosome 4 is necessary for full susceptibility and progression to disease (Tables 1 and 7). In females, the progressor loci are completely different and located on chromosomes 5 and 14. These QTL apparently govern the severity of the clinical signs of keratitis, and we would speculate that they act at a later stage. It should be noted that the clinical score is given on day 6 and the histological score is given on day 15, which may lead to some of the different loci seen.
Candidate genes.
The analysis presented in this study does not allow us to identify the exact genes involved in herpes keratitis, but there are many genes of interest related to already-identified factors involved in the severity of HSV infection, as seen in Table 8 and the genetic intervals presented in Fig. 4 and 6, which could provide potential targets for future study.
TABLE 8.
Candidate genes
| Chromo- some | QTa | cM | Gene | Name of product |
|---|---|---|---|---|
| 4 | C | 52 | Bmfr1 | B-cell maturation factor responsiveness 1 |
| 54.1 | Ckb-rs1 | Creatine kinase; brain; related sequence 1 | ||
| 57.2 | Grik3 | Glutamate receptor; ionotropic; kainate 3 | ||
| 5 | C | 19 | Nos3 | Nitric oxide synthase 3 |
| 17 | Il6 | Interleukin-6 | ||
| 20 | Fgfr3 | Fibroblast growth factor receptor 3 | ||
| 22 | Hs3st1 | Heparan sulfate 3-O-sulfotransferase 1 | ||
| 14 | C | 45 | Tnfsf11 | TNF superfamily member 11 |
| 53.5 | Irg1 | Immunoresponsive gene 1 | ||
| 59 | Fgf14 | Fibroblast growth factor 14 (FHF4) | ||
| 12 | C, E | 58-59 | Igh | Immunoglobulin heavy chain complex |
| 13 | C, E | 33 | Fgfr4 | Fibroblast growth factor receptor 4 |
| 35 | Il9 | Interleukin-9 | ||
| 10 | E | 40.9 | Mif | Macrophage migration inhibitory factor |
| 47 | Timp3 | Tissue inhibitor of MMP3 | ||
| 48 | Igf1 | Insulin-like growth factor 1 | ||
| 52 | Cradd | Caspases Rip adaptor with death domain | ||
| 67 | Ifng | Interferon gamma | ||
| 17 | E | 45 | Lamr9 | Laminin receptor 9 |
| 46.5 | Lhcgr | Choriogonadotropin receptor |
QT, qualitative trait; C, clinical disease; E, histopathology in the eye.
Perhaps the most intriguing candidate is on chromosome 5: Hs3st1, for heparan sulfate 3-O-sulfotransferase 1. A receptor for HSV entry, 3-O-sulfated heparan sulfate, has recently been described (35, 38). It has been shown that the heparan sulfate 3-O-sulfotransferase 3 but not the isoform encoded by Hst3st1, which has antithrombin activity (26), is necessary for the activation of this receptor.
Also on chromosome 5 is a gene, Nos3, for an endothelial and neuronal nitric oxide synthase. NO has been shown to be important in HSV infectivity after corneal infection (23, 43). Also at this locus is the gene Fgfr3. It has been previously reported that a receptor for basic FGF serves as a receptor for HSV-1 (19), and Fgfr3 and Fgfr4, a candidate gene on chromosome 13, could act as such receptors. Not surprisingly, there have also been many studies describing the importance of FGF in keratitis (14, 36). Another candidate gene, Fgf14 or Fhf-4, on chromosome 14, shown to be important in the nervous system (39, 47), could play a role in herpesvirus infectivity.
There is a large literature on cytokine and chemokine (24) involvement in HSV infection, and it includes molecules such as gamma interferon (5, 25). Granulocyte (44) and macrophage infiltrates in keratitis (3, 45) could be affected by interleukin-6 (Il6 on chromosome 5) and interleukin-9 (Il9 on chromosome 13), as well as the gene for the macrophage inhibitory factor, a candidate gene found on chromosome 10.
Finally, both tumor necrosis factor (TNF) in keratitis pathogenesis (20) and a member of the TNF receptor family acting as another HSV receptor (48) have been described. In the mapping studies here, the candidate genes include a gene for a novel member of the TNF family called TRANCE, or Tnfsf11 (chromosome 14), as well as a gene for a molecule involved in TNF receptor death domain adapter protein, or Cradd (1) (chromosome 10).
Acknowledgments
We thank Beth Ann McBrearty, Lise Clark, and Khamilia Bedelbaeva for all their very generous help. We also thank Mark Greene and Laszlo Otvos for useful discussions and for reading the manuscript.
This work was supported by the G. Harold and Leila Y. Mathers Charitable Foundation and by NIH grant AI42395.
REFERENCES
- 1.Ahmad, M., S. M. Srinivasula, L. Wang, R. V. Talanian, G. Litwack, T. Fernandes-Alnemri, and E. S. Alnemri. 1997. CRADD, a novel human apoptotic adaptor molecule for caspase-2, and FasL/tumor necrosis factor receptor-interacting protein RIP. Cancer Res. 157:615-619. [PubMed] [Google Scholar]
- 2.Avery, A. C., Z. S. Zhao, A. Rodriguez, E. K. Bikoff, M. Soheilian, C. S. Foster, and H. Cantor. 1995. Resistance to herpes stromal keratitis conferred by an IgG2a-derived peptide. Nature 376:431-434. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, D., S. Mrzyk, N. Van Rooijen, K. P. Steuhl, and A. Heiligenhaus. 2000. Macrophage-depletion influences the course of murine HSV-1 keratitis. Curr. Eye Res. 20:45-53. [PubMed] [Google Scholar]
- 4.Bihl, F., M. Brahic, and J. F. Bureau. 1999. Two loci, Tmevp2 and Tmevp3, located on the telomeric region of chromosome 10, control the persistence of Theiler's virus in the central nervous system of mice. Genetics 52:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouley, D. M., S. Kanangat, W. Wire, and B. T. Rouse. 1995. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J. Immunol. 155:3964-3971. [PubMed] [Google Scholar]
- 6.Butterfield, R., J. D. Sudweeks, E. P. Blankenhorn, R. Korngold, J. C. Marini, J. A. Tod, R. J. Roper, and C. Teuscher. 1998. New genetic loci that control susceptibility and clinical symptoms of EAE in inbred strains of mice. J. Immunol. 161:1860-1867. [PubMed] [Google Scholar]
- 7.Butterfield, R. J., E. P. Blankenhorn, R. J. Roper, J. F. Zachary, R. W. Doerge, and C. Teuscher. 2000. Identification of genetic loci controlling the characteristics and severity of brain and spinal cord lesions in experimental allergic encephalomyelitis. Am. J. Pathol. 157:637-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter, V. C., S. R. Jennings, P. L. Rice, and S. S. Tevethia. 1984. Mapping of a herpes simplex virus type 2-encoded function that affects the susceptibility of herpes simplex virus-infected target cells to lysis by herpes simplex virus-specific cytotoxic T lymphocytes. J. Virol. 49:766-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, L., M. Fareed, S. Miller, C. Merryman, and E. Heber-Katz. 1996. Corneal infection with herpes simplex virus type-1 leads to autoimmunity. J. Neurosci. Res. 45:770-775. [DOI] [PubMed] [Google Scholar]
- 10.Churchill, G. A., and R. W. Doerge. 1994. Empirical thresholds for quantitative trait mapping. Genetics 138:963-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doerge, R. W., and G. A. Churchill. 1996. Permutation tests for multiple loci affecting a quantitative character. Genetics 142:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doymaz, M., and B. T. Rouse. 1991. MHC II-restricted CD4+ cytotoxic T lymphocytes specific for HSV-1: implications for the development of herpetic stromal keratitis in mice. Clin. Immunol. Immunopathol. 61:398-409. [DOI] [PubMed] [Google Scholar]
- 13.Foster, C. S., Y. Tsai, J. G. Monroe, R. Campbell, M. Cestari, R. Wetzig, D. Knipe, and M. I. Greene. 1986. Genetic studies on murine susceptibility to herpes simplex keratitis. Clin. Immunol. Immunopathol. 40:313-325. [DOI] [PubMed] [Google Scholar]
- 14.Gamus, D., A. Romano, M. Rubinstein, and N. Savion. 1996. Moderation of herpetic stromal keratitis by basic fibroblast growth factor. Exp. Eye Res. 63:1-8. [DOI] [PubMed] [Google Scholar]
- 15.Gangappa, S., S. P. Deshpande, and B. T. Rouse. 1999. Bystander activation of CD4 T cells can represent an exclusive means of immunopathology in a virus infection. Eur. J. Immunol. 29:3674-3682. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths, M. M., J. Wang, B. Joe, S. Dracheva, Y. Kawahito, J. S. Shepard, V. R. Reese, S. McCall-Vining, A. Hashiramoto, G. W. Cannon, E. F. Remmers, and R. L. Wilder. 2000. Identification of four new quantitative trait loci regulating arthritis severity and one new quantitative trait locus regulating autoantibody production in rats with collagen-induced arthritis. Arthritis Rheum. 43:1278-1289. [DOI] [PubMed] [Google Scholar]
- 17.Heber-Katz, E. 1998. The interplay of T cell responses to viral and autoimmune epitopes. Immunol. Res. 17:83-87. [DOI] [PubMed] [Google Scholar]
- 18.Hendricks, R. L. 1997. An immunologist's view of herpes simplex keratitis. Cornea 16:503-506. [PubMed] [Google Scholar]
- 19.Kaner, R. J., A. Baird, A. Mansukhani, C. Basilico, B. D. Summers, R. Z. Florkiewicz, and D. P. Hajjar. 1990. Fibroblast growth factor receptor is a portal of cellular entry for herpes simplex virus type 1. Science 248:1410-1413. [DOI] [PubMed] [Google Scholar]
- 20.Keadle, T. L., N. Usui, K. A. Lavcock, J. K. Miller, J. S. Pepose, and P. M. Stuart. 2000. IL-1 and TNF-alpha are important factors in the pathogenesis of murine recurrent herpetic stromal keratitis. Investig. Ophthalmol. Vis. Sci. 41:98-102. [PubMed] [Google Scholar]
- 21.Koelle, D. M., S. N. Reymond, H. Chen, W. W. Kwok, C. McClurkan, T. Gyaltsong, E. W. Petersdorf, W. Rotkis, A. R. Talley, and D. A. Harrison. 2000. Tegument-specific, virus-reactive CD4 T cells localize to the cornea in herpes simplex virus interstitial keratitis in humans. J. Virol. 74:10930-10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kono, D. H., and A. N. Theofilopoulos. 2000. Genetics of systemic autoimmunity in mouse models of lupus. Int. Rev. Immunol. 19:367-387. [DOI] [PubMed] [Google Scholar]
- 23.Koprowski, H., Y. M. Zheng, E. Heber-Katz, N. Fraser, L. Rorke, Z. F. Fu, C. Hanlon, and B. Dietzschold. 1993. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc. Natl. Acad. Sci. USA 90:3024-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumaraguru, U., J. Davis, and B. T. Rouse. 1999. Chemokines and ocular pathology caused by corneal infection with herpes simplex virus. J. Neurovirol. 5:42-47. [DOI] [PubMed] [Google Scholar]
- 25.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, J., N. W. Shworak, P. Sinay, J. J. Schwartz, L. Zhang, L. M. S. Fritze, and R. D. Rosenberg. 1999. Expression of heparan sulfate D-glucosaminyl-3-O-sulfotransferase isoforms reveals novel substrate specificities. J. Biol. Chem. 274:5185-5192. [DOI] [PubMed] [Google Scholar]
- 27.Manly, K. F. 1993. A Macintosh program for storage and analysis of experimental genetic mapping data. Mamm. Genome 4:303-313. [DOI] [PubMed] [Google Scholar]
- 28.Martin, A. M., M. N. Maxson, J. Leif, J. P. Mordes, D. L. Greiner, and E. P. Blankenhorn. 1999. Diabetes-prone and diabetes-resistant BB rats share a common major diabetes susceptibility locus, iddm4: additional evidence for a “universal autoimmunity locus” on rat chromosome 4. Diabetes 48:2138-2144. [DOI] [PubMed] [Google Scholar]
- 29.Meeker, N. D., W. F. Hickey, R. Korngold, W. K. Hansen, J. D. Sudweeks, B. B. Wardell, J. S. Griffith, and C. Teuscher. 1995. Multiple loci govern the bone marrow-derived immunoregulatory mechanism controlling dominant resistance to autoimmune orchitis. Proc. Natl. Acad. Sci. USA 92:5684-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melvold, R. W., D. M. Jokinen, S. D. Miller, M. C. Dal Canto, and H. L. Lipton. 1990. Identification of a locus on mouse chromosome 3 involved in differential susceptibility to Theiler's murine encephalomyelitis virus-induced demyelating disease. J. Virol. 64:686-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metcalf, J. F., D. Hamilton, and R. Reichert. 1979. Herpetic keratitis in athymic nude mice. Infect. Immun. 26:1164-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metcalf, J. F., and B. A. Michaelis. 1984. Herpetic keratitis in inbred mice. Investig. Ophthalmol. Vis. Sci. 25:1222-1225. [PubMed] [Google Scholar]
- 33.Niemialtowski, M. G., and B. T. Rouse. 1992. Predominance of Th1 cells in ocular tissue during herpetic stromal keratitis. J. Immunol. 149:3035-3039. [PubMed] [Google Scholar]
- 34.Pepose, J. S., and J. A. Whittum-Hudson. 1987. An immunogenetic analysis of resistance to herpes simplex virus retinitis in inbred strains of mice. Investig. Ophthalmol. Vis. Sci. 28:1549-1552. [PubMed] [Google Scholar]
- 35.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 36.Rieck, P., J. Denis, D. Peters, C. Hartmann, Y. Pouliquen, and Y. Courtois. 1997. Fibroblast growth factor 2, heparin and suramin reduce epithelial ulcer development in experimental HSV-1 keratitis. Graefes Arch. Clin. Exp. Ophthalmol. 235:733-740. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez, M., J. Leibowitz, and C. S. David. 1986. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J. Exp. Med. 163:620-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 39.Smallwood, P. M., I. Munoz-Sanjuan, P. Tong, J. P. Macke, S. H. Hendry, D. J. Gilbert, N. G. Copeland, N. A. Jenkins, and J. Nathans. 1996. Fibroblast growth factor (FGF) homologous factors: new members of the FGF family implicated in nervous system development. Proc. Natl. Acad. Sci. USA 93:9850-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Streilein, J. W., M. Dana, and B. R. Ksander. 1998. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol. Today 18:443-449. [DOI] [PubMed] [Google Scholar]
- 41.Stulting, R. D., J. C. Kindle, and A. J. Nahmias. 1985. Patterns of herpes simplex keratitis in inbred mice. Investig. Ophthalmol. Vis. Sci. 26:1360-1367. [PubMed] [Google Scholar]
- 42.Teuscher, C., D. M. Rhein, K. D. Livingstone, R. A. Paynter, R. W. Doerge, S. M. Nicholson, and R. W. Melvold. 1997. Evidence that Tmevd2 and eae3 may represent either a common locus or members of a gene complex controlling susceptibility to immunologically-mediated demyelination in mice. J. Immunol. 159:4930-4934. [PubMed] [Google Scholar]
- 43.Thakur, A., S. Athmanathan, and M. Willcox. 2000. The differential regulation of nitric oxide by herpes simplex virus-1 and -2 in a corneal epithelial cell line. Clin. Exp. Ophthalmol. 28:188-190. [DOI] [PubMed] [Google Scholar]
- 44.Thomas, J., S. Gangappa, S. Kanangat, and B. T. Rouse. 1997. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J. Immunol. 158:1383-1391. [PubMed] [Google Scholar]
- 45.Tumpey, T. M., H. Cheng, D. N. Cook, O. Smithies, J. E. Oakes, and R. N. Lausch. 1998. Absence of macrophage inflammatory protein-1 alpha prevents the development of blinding herpes stromal keratitis. J. Virol. 72:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verjans, G. M., L. Remeijer, C. M. Mooy, and A. D. M. E. Osterhaus. 2000. Herpes simplex virus-specific T cells infiltrate the cornea of patients with herpetic stromal keratitis: no evidence of autoreactive T cells. Investig. Ophthalmol. Vis. Sci. 41:2607-2612. [PubMed] [Google Scholar]
- 47.Wang, Q., D. G. McEwen, and D. M. Ornitz. 2000. Subcellular and developmental expression of alternatively spliced forms of fibroblast growth factor 14. Mech. Dev. 90:283-287. [DOI] [PubMed] [Google Scholar]
- 48.Whitbeck, J. C., S. A. Connolly, S. H. Willis, W. Hou, C. Krummenacher, M. Ponce De Leon, H. Lou, I. Baribaud, R. J. Eisenberg, and G. H. Cohen. 2001. Localization of the gD-binding region of the human herpes simplex virus receptor, hveA. J. Virol. 75:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wicker, L. S., J. A. Todd, and L. B. Peterson. 1995. Genetic control of autoimmune diabetes in the NOD mouse. Annu. Rev. Immunol. 13:179-200. [DOI] [PubMed] [Google Scholar]
- 50.Zhao, Z. S., F. Granucci, L. Yeh, P. A. Schaffer, and H. Cantor. 1998. Molecular mimicry by herpes simplex virus type 1: autoimmune disease after viral infection. Science 279:1344-1347. [DOI] [PubMed] [Google Scholar]