Abstract
Coxsackievirus B3 (CVB3) is the most common human pathogen for viral myocarditis. We have previously shown that the signaling protein p21ras GTPase-activating protein (RasGAP) is cleaved and that mitogen-activated protein kinases (MAPKs) ERK1/2 are activated in the late phase of CVB3 infection. However, the role of intracellular signaling pathways in CVB3-mediated myocarditis and the relative advantages of such pathways to host or virus remain largely unclear. In this study we extended our prior studies by examining the interaction between CVB3 replication and intracellular signaling pathways in HeLa cells. We observed that CVB3 infection induced a biphasic activation of ERK1/2, early transient activation versus late sustained activation, which were regulated by different mechanisms. Infection by UV-irradiated, inactivated virus capable of receptor binding and endocytosis triggered early ERK1/2 activation, but was insufficient to trigger late ERK1/2 activation. By using a general caspase inhibitor (zVAD.fmk) we further demonstrated that late ERK1/2 activation was not a result of CVB3-mediated caspase cleavage. Treatment of cells with U0126, a selective inhibitor of MAPK kinase (MEK), significantly inhibited CVB3 progeny release and decreased virus protein production. Furthermore, inhibition of ERK1/2 activation circumvented CVB3-induced apoptosis and viral protease-mediated RasGAP cleavage. Taken together, these data suggest that ERK1/2 activation is important for CVB3 replication and contributes to virus-mediated changes in host cells. Our findings demonstrate coxsackievirus takeover of a particular host signaling mechanism and uncover a prospective approach to stymie virus spread and preserve myocardial integrity.
Coxsackievirus B3 (CVB3), a member of the Picornaviridae family, is the most common human pathogen that has been associated with the pathogenesis of myocarditis and idiopathic dilated cardiomyopathy (DCM) (5, 42). Although viral myocarditis was originally considered predominantly an immune system-mediated disease of the heart (39), recently early direct virus-mediated injury occurring prior to infiltrating immune responses has been shown to have important implications in the progression of CVB3 myocarditis. In cultured cells, CVB3 infection is capable of inducing a direct cytopathic effect (CPE) and cell apoptosis (10, 61). Immunocompromised mice demonstrate an early and extended coagulative necrosis and contraction band necrosis following CVB3 infection (11, 43). Prominent cytopathic alterations colocalized to cells with viral replication by in situ hybridization of both positive- and negative-strand viral RNA reinforce the importance of direct virus-induced damage (29). Previous studies by our laboratory (56, 64) and others (33, 44) have suggested that early host gene responses to viral infection play a key role in determining the severity of myocarditis and disease progression to DCM. However the early determining factors, in particular, the interplay of virus-host signaling pathways, remain to be determined.
CVB3 has a short life cycle, which typically culminates in rapid cell death and release of progeny virus. Subsequent to virus attachment to a target cell receptor, viral RNA is released into the cell and acts as a template for the translation of the virus polyprotein and replication of the virus genome. Viral receptors include the coxsackievirus and adenovirus receptor (6, 22, 38, 59) and the decay-accelerating factor (DAF) coreceptor (37, 50). Viral proteins are initially synthesized as a large polyprotein, which is subsequently cleaved into individual structural and nonstructural proteins by virus-encoded proteases 2A, 3C, and 3CD. In addition to degrading the viral polyprotein, viral proteases can cleave multiple host proteins (4, 15). CVB3 protein 3D, an RNA-dependent RNA polymerase, is essential for transcription of the negative-strand viral RNA intermediate, which then serves as a template for synthesis of multiple progeny genomes.
Many viruses are known to manipulate host signaling machinery to regulate virus replication and host gene responses. Such pathways include the mitogen-activated protein kinases (MAPKs), which respond to diverse extracellular stimuli and which transduce signals from the cell membrane to the nucleus (7, 30). MAPKs constitute a superfamily of highly related serine/threonine kinases. At least seven members of the MAPK family have been identified in mammals: extracellular signal-regulated kinases 1 and 2 (ERK1/2) (7, 30), c-Jun NH2-terminal kinase (JNK)/stress-activated protein kinase (7, 30), p38 MAPK (7, 30), big MAPK 1 (BMK1) (32), ERK6 (31), and ERK7 (1). Each MAPK pathway generally consists of three kinase modules composed of a MAPK, a MAPK kinase (MAPKK), and a MAPKK kinase. These kinase modules are differentially activated by a variety of cellular stimuli and contribute to distinct cellular function. The ERK1/2 module includes Raf, MEK1/2, and ERK1/2, which regulate a wide range of cellular functions including cell proliferation, transformation, differentiation, and, notably, cell survival and death (14, 35). Small GTP-binding protein Ras has been shown to activate the Raf/MEK/ERK cascade by binding Raf and anchoring it at the cell membrane, where it is phosphorylated and activated by other kinases (34). Recently, the ERK pathway has been implicated in the regulation of viral gene expression and replication for human cytomegalovirus (26), simian virus 40 (53), human immunodeficiency virus type 1 (HIV-1) (23, 66), and influenza virus (46). However, to date, the host signaling mechanisms involved in CVB3-mediated myocarditis are still largely unclear.
In a previous study, we observed that CVB3 infection resulted in the cleavage of RasGAP and activation of ERK1/2 late in viral infection (21). Here, we extend such findings and report that CVB3 infection leads to a biphasic activation of ERK1/2, each activation triggered by very different mechanisms. We further show that inhibition of this pathway results in decreased viral protein synthesis and viral progeny release and augmented cell survival.
MATERIALS AND METHODS
Cell culture, virus, and materials.
HeLa cells (American Type Culture Collection) were grown and maintained in Dulbecco's modified Eagle's media (DMEM) supplemented with 10% heat-inactivated fetal calf serum. CVB3 (Kandolf strain) was propagated in HeLa cells and stored at −80°C. Virus titer was routinely determined prior to infection by a plaque assay of HeLa cell monolayers as described below. UV-irradiated virus was prepared as described previously (5).
All other supplies were purchased from Sigma Chemical Co. unless otherwise specified. Polyclonal phosphorylated ERK1/2 and ERK1/2 antibodies were purchased from New England Biolabs. The polyclonal CVB3 VP1 antibody was obtained from Accurate Chemicals. The polyclonal anti-caspase 3 antibody was obtained from Santa Cruz Biotechnology, and the monoclonal RasGAP antibody was obtained from Transduction Laboratories. U0126, the MEK inhibitor, was purchased from Promega. General caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD.fmk) was obtained from Bachem.
Virus infection.
HeLa cells were grown in complete medium, and upon reaching 70 to 80% confluence cells were serum starved by incubation in serum-free DMEM for 24 h. For viral infection, growth-arrested HeLa cells were infected at a multiplicity of infection (MOI) of 10 with CVB3 or sham treated with phosphate-buffered saline (PBS) for 1 h. Cells were washed with PBS and cultured in serum-free DMEM. For inhibitor experiments, HeLa cells were incubated with MEK inhibitor U0126 or caspase inhibitor zVAD.fmk for 30 min. Cells were then infected for 1 h, washed with PBS, and placed in serum-free media containing fresh inhibitor unless otherwise specified.
Western blot analysis.
Cell lysates were prepared as described previously (63). Equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. Membranes were blocked for 1 h with nonfat dry milk solution (5% in Tris-buffered saline) containing 0.1% Tween 20. Blots were then incubated for 1 h with the primary antibody followed by incubation for 1 h with the secondary antibody (horseradish peroxidase conjugated). Immunoreactive bands were visualized by chemiluminescence (ECL; Amersham).
Cell viability assay.
A modified 3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTS) assay (Promega), which measures mitochondrial function, was used to determine cell viability (13). HeLa cells were grown in 96-well plates and serum starved for 24 h. Following CVB3 infection, culture medium was replaced with serum-free DMEM. Twenty-four hours postinfection, cells were incubated for 4 h in MTS solution, and absorbance was measured with an enzyme-linked immunosorbent assay plate reader (490 nm). MTS assays were performed in triplicate.
Plaque assay.
CVB3 titer in cell supernatant was determined on monolayers of HeLa cells by an agar overlay plaque assay in triplicate as previously described (3). Briefly, samples were serially diluted 10-fold and overlaid on 90 to 95% confluent monolayers of HeLa cells in six-well plates and incubated for 1 h. Medium was removed, and 2 ml of complete DMEM containing 0.75% agar was overlaid in each well. Cells were incubated at 37°C for 72 h, fixed with Carnoy's fixative (75% ethanol-25% acetic acid) for 30 min, and stained with 1% crystal violet. Plaques were counted, and viral concentration was calculated as PFU per milliliter.
Morphological analysis.
HeLa cells are examined following CVB3 infection for cellular morphological changes by phase-contrast microscopy.
RESULTS
CVB3 leads to biphasic activation of ERK1/2.
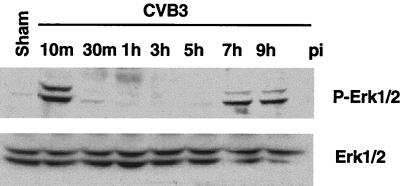
To determine the roles of intracellular signaling pathways in CVB3 replication, we first examined the kinetics of MAPK activation. We have previously shown that CVB3 stimulated ERK1/2 activity beginning 6 h postinfection and that ERK1/2 were still activated after 9 h (21). In the present series of experiments, extended time courses were studied. Growth arrested HeLa cells were infected with CVB3 at an MOI of 10 for 1 h, and cells were harvested at 10 and 30 min and at 1, 3, 5, 7, and 9 h postinfection. ERK1/2 activity was determined with the phosphorylated-ERK1/2 antibody. As shown in Fig. 1, exposure of HeLa cells to CVB3 stimulated ERK1/2 activity, with a first peak at 10 min and a return to baseline at 30 min; a second peak of ERK1/2 activation was apparent at 7 h, and activation remained elevated to 9 h postinfection. Therefore, CVB3 infection triggers early transient and late sustained biphasic activation of ERK1/2.
FIG. 1.
Time course for CVB3 stimulation and ERK1/2 phosphorylation. Growth-arrested HeLa cells were incubated with CVB3 at an MOI of 10 for 1 h, and then the cells were washed with PBS twice and replenished with serum-free medium. Cell lysates were collected for the indicated times following CVB3 infection (pi) and subjected to Western blotting. ERK1/2 activities were analyzed based on phosphorylated ERK1/2 (P-Erk1/2). To verify equal loading, Western blotting was performed with an antibody to ERK1/2. Data are from one of three different experiments. Sham, sham treatment with PBS for 1 h.
To determine whether ERK1/2 activity was influenced by other proteins present in the media during virus preparation, we used HeLa cell extract processed in an identical freeze-thaw protocol as that used during virus propagation but in the absence of virus to treat growth-arrested HeLa cells. We did not observe significant increases of ERK1/2 phosphorylation compared to that of the PBS-treated control (data not shown). Therefore, we believe that the activation of ERK1/2 is due to the direct interaction between virus and host cells.
Recently, several reports have described the induction of the activity of other MAPK submembers, including JNK and p38, following cytomegalovirus (25), simian immunodeficiency virus (48), herpes simplex virus (41), and HIV infection (51). To determine if CVB3 infection also increased other MAPK activities, we assayed the activities of p38 MAPK, JNK, and BMK1 by using phosphorylated-p38 and phosphorylated-JNK antibodies or a mobility shift on a Western blot for BMK1 (28). However, there was no evidence of significant increases for other MAPKs throughout the time course of CVB3 infection (data not shown), which suggests that activation of ERK was a rather specific event.
UV-irradiated CVB3 stimulates early-phase phosphorylation of ERK1/2.
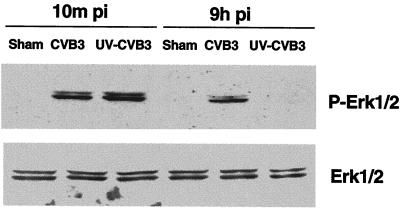
To further elucidate the mechanism of CVB3-mediated ERK1/2 activation, we used UV-irradiated virus. Such inactivated virus fails to express viral proteins due to thymidine dimers, which prevent transcription of viral genes, but retains the capability for receptor binding and endocytosis into host cells (5). Since specific ligand-receptor interactions can result in rapid and transient activation of ERK1/2 (35), the presence of ERK activation immediately following infection suggests that this is a direct receptor-mediated event. As Fig. 2 shows, the first (early) ERK1/2 phosphorylation event remains, whereas the second (late) event completely disappears, following UV-irradiated virus infection, suggesting that CVB3-receptor interaction is responsible for early ERK1/2 activation while viral protein production appears necessary for late-phase ERK1/2 activation following CVB3 infection.
FIG. 2.
UV-irradiated CVB3 stimulates early-phase phosphorylation of ERK1/2. HeLa cells were infected with either wild-type virus or UV-irradiated virus, and 10 min and 9 h after infection (pi) cell lysates were harvested and Western blotting was performed to determine ERK activation. To verify equal loading, Western blotting was performed with an anti-ERK1/2 antibody. Data are from one of two different experiments. Sham is as defined for Fig. 1.
Inhibition of ERK1/2 activation results in significant reduction in viral progeny production and viral protein synthesis.
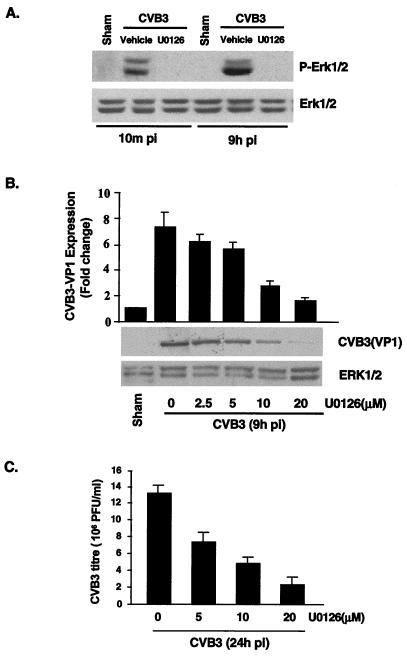
To determine the potential role of ERK activation in viral replication, we used a selective inhibitor of the ERK pathway, U0126, which inhibits MEK, immediately upstream of ERK. Treatment of cells with U0126 (20 μM) resulted in a complete inhibition of both CVB3-induced early- and late-phase ERK phosphorylation (Fig. 3A).
FIG. 3.
MEK inhibitor reduces viral progeny release and viral protein synthesis. (A) Inhibition of ERK activation by MEK inhibitor U0126 was determined by Western blotting with an anti-phosphorylated ERK antibody. HeLa cells were preincubated with U0126 (20 μM) for 30 min and then were infected with CVB3 (MOI = 10). One hour later, cells were washed twice with PBS and replenished with serum-free medium containing fresh U0126. To verify equal loading, Western blotting was performed with an anti-ERK1/2 antibody. The data are representative of two different experiments. Sham, pi, and P-Erk1/2 are as defined for Fig. 1. (B) HeLa cells were treated with U0126 exactly as for panel A. Cellular lysates were collected from CVB3-infected HeLa cells 9 h postinfection, and Western blot analysis using a CVB3 polyclonal antibody that recognizes viral structure protein VP1 was performed. Results (means ± standard errors [SE]; n = 3) were quantitated by densitometric analysis using National Institutes of Health Image, version 1.61, and normalized to control levels (sham-infected cells without U0126) arbitrarily set to 1.0. (C) HeLa cells were treated with different concentrations of U0126 exactly as for panel A. Medium was collected from CVB3-infected HeLa cells 24 h after infection, and virus titers were determined by plaque assays on HeLa cell monolayers. Values are means ± SE from three independent experiments, in each of which titrations were carried out in triplicate.
First, we investigated the effect of the MEK inhibitor on the expression of viral proteins. HeLa cells were incubated with different concentrations of U0126 for 30 min prior to infection. U0126 was also present during infection and in subsequent incubation periods. Nine hours postinfection cell lysates were collected and Western blotting was performed using a CVB3 polyclonal antibody that recognizes viral structural protein VP1. Densitometric analysis of Western blotting showed that the intracellular expression of the VP1 protein was reduced by U0126 in a dose-dependent manner (Fig. 3B). Next, we wanted to examine whether U0126 affected viral titers. Twenty-four hours postinfection supernatants were collected and viral titers were determined by plaque assay of monolayer HeLa cells. As Fig. 3C shows, the presence of U0126 reduced viral progeny release in a dose-dependent manner. Compared with controls, 5, 10, and 20 μM U0126 reduced viral progeny by 46, 61, and 85%, respectively. It should be noted that, at all concentrations of U0126 used in this study, there was no evidence of cell death, as detected by the MTS assay (data not shown).
Exposure to U0126 following the first peak of ERK1/2 activation (3 h postinfection) attenuated viral progeny production, but to a lesser extent than administration prior to infection (data not shown), suggesting that both phases of ERK activation contribute to viral replication. Taken together, these results indicate that ERK plays an important role in CVB3 replication.
Inhibition of ERK1/2 activation prevents CVB3-mediated CPE and caspase activation.
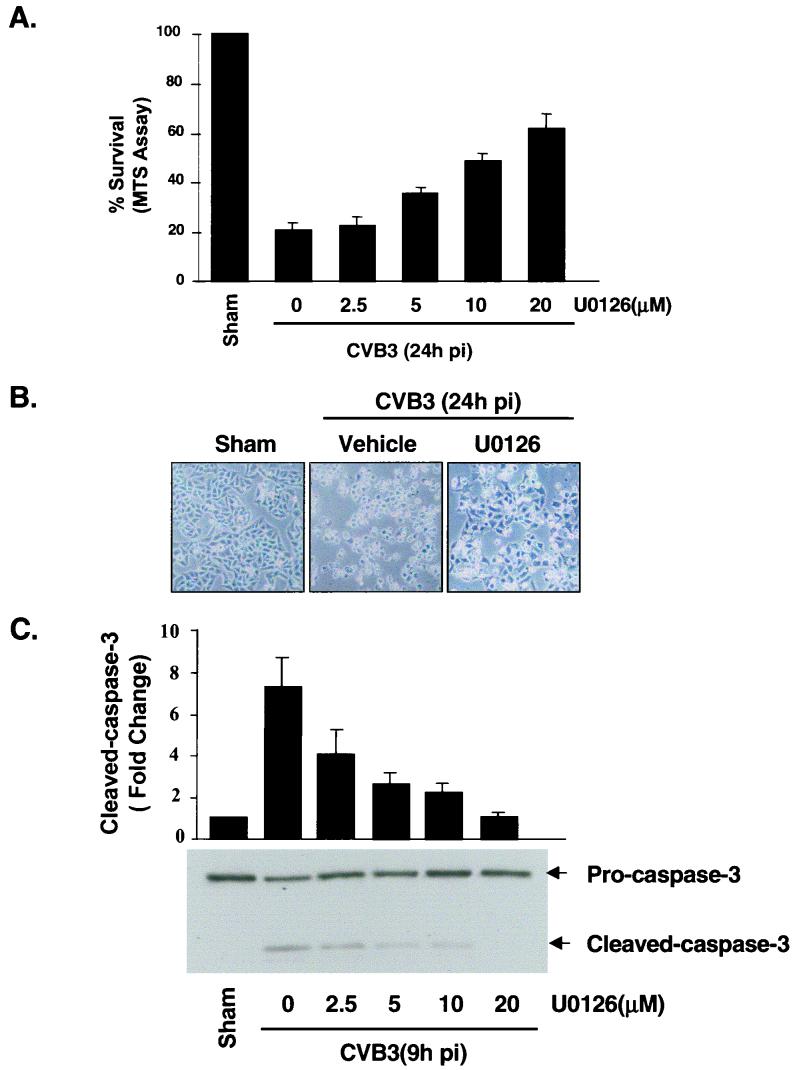
Apoptosis may play a critical role in viral myocarditis (12, 19, 20). We have previously demonstrated two separate but related phenomena, CPE and apoptosis, following CVB3 infection of HeLa cells (10). We therefore decided to see whether ERK1/2 activation is required for CVB3-induced CPE and apoptosis. Cell viability was determined by the MTS assay, which measures mitochondrial function. As shown in Fig. 4A, inhibition of ERK1/2 by U0126 resulted in a dose-dependent reduction of CVB3-mediated cell death 24 h postinfection, with 20 μM U0126 producing an approximate 60% increase of viable cells. Morphology of cells treated with U0126 is shown in Fig. 4B. CVB3-infected cells displayed typical features of apoptosis by 24 h postinfection. Consistent with the cell viability change, pretreatment with U0126 markedly suppressed the morphological changes induced by CVB3 infection. We also examined the effect of U0126 on CVB3-mediated caspase activation. As shown in Fig. 4C, U0126 significantly inhibited CVB3-induced caspase 3 cleavage in a dose-dependent manner. Therefore, we believe that ERK1/2 activation is required for CVB3-induced apoptosis, although it is unclear whether the apoptosis and CPE are directly mediated by the ERK signaling pathway or whether they are indirectly associated with ERK-regulated viral replication or viral infectivity.
FIG. 4.
MEK inhibitor blocks CVB3-induced CPE and apoptosis. (A) HeLa cells were treated with U0126 as described for Fig. 3A. Cell viability was determined at 24 h postinfection (pi) by the MTS assay, which measures mitochondrial function, at 24 h after infection. Values are means ± standard errors (SE; n = 6). The level of MTS in sham-infected cells in the absence of U0126 was defined as 100% survival. Similar results were obtained in three independent experiments. (B) Representative phase-contrast microscopy of HeLa cells treated with medium containing or lacking U0126 24 h postinfection. (C) HeLa cells were pretreated with vehicle or various concentrations of U0126 for 30 min, followed by infection with CVB3 for 1 h, 9 h after infection. Western blotting was performed to examine the cleavage of caspase 3. Results were quantitated by densitometric analysis using National Institutes of Health Image, version 1.61, and normalized to the control level (sham-infected cells without U0126), which was arbitrarily set to 1.0. Values are means ± SE (n = 3).
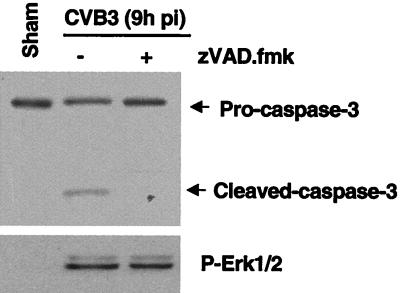
Caspases are the main executioners of apoptosis through a number of cleavage events. It has been reported that many host proteins are cleaved during the course of apoptosis (57). We thus determined whether ERK1/2 activity was a result of caspase activation by using general caspase inhibitor zVAD.fmk. As shown in Fig. 5, caspase 3 activation could be demonstrated at 9 h postinfection by the presence of the 17-kDa cleavage product. ERK1/2 activation was also increased at 9 h postinfection. Preincubation of cells with 100 μM zVAD.fmk mostly blocked CVB3-induced caspase cleavage but had no effect on ERK activation. This result shows that the observed second peak of ERK1/2 phosphorylation occurred before initiation of caspase activity and suggests that the caspase pathway and subsequent apoptotic processes do not influence MAPK.
FIG. 5.
ERK1/2 activation was not due to caspase activation. HeLa cells were preincubated with zVAD.fmk (100 μM) for 30 min and then infected with CVB3 for 1 h. Cell lysates were collected 9 h postinfection (pi). ERK1/2 activation and caspase 3 cleavage were determined by Western blotting using a phosphorylated ERK1/2 (P-Erk1/2) antibody and a caspase 3 antibody. The data are representative of two different experiments.
MEK inhibitor blocks CVB3-mediated RasGAP cleavage.
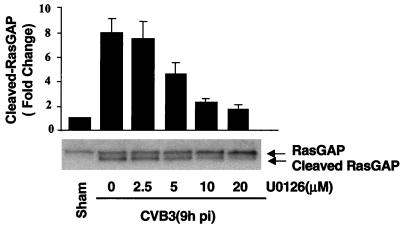
To gain further insight into the mechanisms and regulation of intracellular signaling pathways in CVB3-mediated host cell expression, we next considered the contribution of the MEK inhibitor to CVB3-induced RasGAP cleavage. HeLa cells were treated with different concentrations of U0126. Nine hours postinfection cell lysates were collected and subjected to Western blotting for RasGAP, a protein which negatively regulates the activation of Ras by hydrolysis of GTP to GDP. The results, depicted in Fig. 6, show that MEK inhibition significantly blocked CVB3-mediated RasGAP cleavage. This result suggests that ERK activation may be achieved through a positive-feedback mechanism. As such, ERK activation enhances viral replication, resulting in a cleavage of host signaling protein RasGAP, further promoting Ras activity and subsequent activation of the ERK cascade (Fig. 7).
FIG. 6.
MEK inhibitor inhibits CVB3-mediated RasGAP cleavage. HeLa cells were pretreated for 30 min with various concentrations of U0126 and then were infected by CVB3 for 1 h. At 9 h after addition of CVB3, HeLa cells were harvested and Western blot analysis was performed using an antibody that recognizes RasGAP. Results were quantitated by densitometric analysis using National Institutes of Health Image, version 1.61, and normalized to the control level (sham-infected cells without U0126), which was arbitrarily set to 1.0. Values are means ± standard errors (n = 3).
FIG. 7.
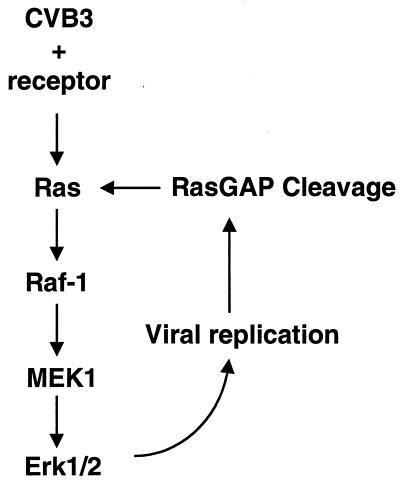
A proposed model of the mechanism of ERK activation during CVB3 infection. CVB3 binds with its receptor and initiates early transient ERK phosphorylation. Virus replication mediates RasGAP cleavage, which triggers late-phase ERK phosphorylation. Subsequently, there is a positive feedback to augment ERK activation and viral replication.
DISCUSSION
In the present study, we have shown that virus infection induces biphasic activation of MAPK ERK1/2 in target cells. Immediately following CVB3 or UV-irradiated CVB3 infection, ERK is transiently phosphorylated. Toward the final stages of virus replication, sustained activation of ERK was observed, but this event was absent in infection by UV-irradiated virus. We further show that specific inhibition of ERK leads to attenuation of the virus life cycle, as determined by lower virus protein and progeny production, decreased virus cleavage of host proteins, and attenuation of host cell death. This study has provided new insights into our understanding of the interplay of virus and host signaling induced by CVB3 infection and demonstrates that ERK1/2 signaling is required for CVB3 pathogenesis.
The significance of host activation of MAPK in other models of viral infection has been reported. In the HIV-1 model, MAPK activation is beneficial to viral replication and inhibition of these phosphorylation events seems to inhibit viral replication (23). Similarly, the following viruses also interact with ERK: adenovirus type 7 (2), Borna disease virus (45), influenza A virus (46), and hepatitis C virus (17). Such widespread ERK involvement suggests either involvement in a global virus strategy to enhance its own replicative machinery or a universal host protective response to infection.
We have found that CVB3 infection induces a biphasic activation of ERK1/2 at 10 min and 7 h postinfection. The mechanisms for ERK1/2 activation following viral infection may be induced by direct virus-receptor binding, such as for HIV activation of ERK (47), or by exposure to a viral protein such as the hepatitis C virus core protein (16) or HIV Tat protein (49). The first peak occurs immediately following infection, which strongly suggests that signaling is initiated directly from a receptor-coreceptor complex. We further showed that UV-irradiated and inactivated CVB3 does indeed activate early but not late ERK signaling. The DAF coreceptor is anchored to the extracellular surface by glycosylphosphatidylinositol (GPI), which is localized to cholesterol-rich invaginations of the plasma membrane, or caveolae, which are proposed sites for increased outside-to-inside signal transduction and transcytosis (40). This virus receptor has been found to associate with tyrosine kinases p56lck and p59fyn, among others, and with downstream ERK1/2 as an essential part of T-cell development (52). Recent evidence has shown that p56lck is required for CVB3 infection of T-cell lines and that ERK may be a downstream target of such signaling pathways (33). Since DAF expression is not always necessary for viral entry, this evidence raises the question of whether CVB3 binding to coxsackievirus and adenovirus receptor can trigger MAPK activation or whether the intracellular presence, with or without replication, of viral RNA is required (38). We therefore speculate that such a DAF-GPI signaling complex is responsible for early ERK activation in CVB3-infected HeLa cells, but further studies are necessary to confirm the nature of intersection between receptor complex, tyrosine kinases, and ERK signaling.
A plausible explanation for these early events in the interaction between CVB3 and host cells is that ERK signaling is indeed a host protective mechanism to which CVB3 has adapted. Activation of ERK1/2 is a well-documented cell-protective and cell-beneficial response in the heart to a wide variety of damaging agents such as pressure or volume overload and ischemia-reperfusion injury (9, 67). It is conceivable that cardiac myocytes may also invoke the ERK response to CVB3 as a defense mechanism. The virus, in turn, may have adapted to such preexisting signaling pathways to benefit its own replication. A relatively short CVB3 life cycle and lack of RNA polymerase proofreading function may allow for rapid adjustments to changing characteristics of the host cell system, which would beget an overall high mutation rate.
We also show that ERK is stimulated 7 h postinfection, which is consistent with peak virus replication and caspase 3 activation (10). We show that UV-irradiated virus, incapable of replication, does not trigger late ERK activation, which suggests that the observed high level of late-phase ERK activation is dependent on viral gene expression. Several viral gene products have been shown to intersect the ERK1/2 signaling pathway, including the hepatitis C virus core protein (16) and the HIV Tat protein (49). Although no comparable data for a picornavirus protein have yet been identified, previous work in our laboratory has shown that RasGAP is cleaved and ERK1/2 is activated during virus infection (21). Such findings raise the possibility that a viral protease is responsible for ERK1/2 activation via cleavage of an upstream effector molecule. These findings indicate that CVB3 may have developed multiple mechanisms to ensure activation of the MAPK ERK1/2 and that timely activation may have important consequences during the course of an infection.
Activation of the ERK pathway results in phosphorylation of numerous ERK target proteins, which mediate multiple cellular functions. In the HIV-1 model, ERK1/2 activation augments viral infectivity and replication, which may occur by direct phosphorylation of viral protein Vif (65). It is not known at present how CVB3 viral replication is regulated by the ERK signaling pathway. Perhaps this process involves direct phosphorylation of intracellular components which are required for viral replication. Alternatively, ERK1/2 activity may be necessary to activate CVB3 viral proteins, for example, RNA-dependent RNA polymerase 3D, which is essential for the initiation of viral RNA replication.
Recently MEK inhibitors have been reported to prevent the activation of both ERK1/2 and BMK1. MEK inhibitors, including U0126 and PD98059, are specific for ERK1/2 at low doses, but high doses block mitogen-induced activation of both ERK1/2 and BMK1 (27). The dose of U0126 used here is sufficiently specific for CVB3-medicated ERK1/2 activation; BMK1 was not activated throughout the course of CVB3 infection.
CVB3 infection, as well as expression of viral capsid proteins and proteases, induces direct CPE and cell apoptosis (10, 18, 19). Inhibition of such caspase activation blocks the loss of viability and prevents progeny virus release. Since caspase inhibition does not affect ERK activation, but ERK inhibition protects against CVB3-induced CPE and apoptosis, we conclude that ERK activation occurs upstream of caspase activation. The mechanism by which inhibition of ERK prevents CVB3-induced apoptosis is likely an indirect result of a decrease in virus replication and/or infectivity, analogous to the ERK contribution to influenza virus infection (46). In addition to the effects of virus replication on host cell apoptotic pathways, we offer the possibility of a more direct role for ERK in the CVB3-induced cell death signaling pathway in HeLa cells. Although many studies have supported the general view that activation of the ERK pathway delivers a survival signal that counteracts proapoptotic effects associated with p38 and JNK activation (62), there are some studies that relate ERK and the apoptosis cascade. For example, it has been reported that ERK activation is required for cisplatin-induced apoptosis of HeLa cells and functions upstream of caspase activation to initiate the apoptotic signal (60). Overproduction of proto-oncogenes downstream of ERK, such as c-myc, may trigger apoptosis at the level of the mitochondria (54). Joe et al. (24) have also shown that dominant inhibitory Ras delays Sindbis virus-induced apoptosis in neuronal cells. The mechanism of indirect induction of apoptosis by CVB3 replication and the possible contribution by direct host signaling events remain an important area for further investigation.
Ras is a 21-kDa, GTP-binding protein that plays a critical role in signal transduction pathways mediating many important cellular functions. In the GTP-bound state, Ras interacts with and transmits signals to downstream effector molecules, such as those in the ERK signaling pathway. Hydrolysis of GTP to GDP switches Ras to the inactive state. RasGAP negatively regulates the GTP-bound state by stimulating the intrinsic Ras GTPase by hydrolysis of GTP to GDP (8, 58). We have previously shown that RasGAP is cleaved after CVB3 infection, potentially triggering the Ras pathway and subsequent phosphorylation of ERK1/2 (21). In this study we report that a MEK inhibitor blocks viral replication and CVB3-induced RasGAP cleavage. This result suggests a possible mechanism by which viral protein cleavage of RasGAP and late-phase ERK activation prompt a positive-feedback loop to further increase viral replication, with subsequent cleavage of RasGAP.
Increasing our understanding of virus-induced death signaling in viral myocarditis has become particularly important in light of direct virus-mediated mechanistic connections between myocarditis and DCM. These findings are qualified by the presence of the CVB3 genome in patients with DCM (36) and more-recent findings that suggest that CVB3 may persist in such tissues disguised in a stable double-stranded form (55). The contribution of virus-myocyte interactions, as opposed to immune infiltration, has profound consequences for the degree of myocardial injury. Therefore, greater understanding of key signaling pathways that are beneficial to the virus may lead to novel therapies to stymie the progression from myocarditis to an end-stage disease which impacts a significant and growing population.
Acknowledgments
This work was funded by grants from the Heart and Stroke Foundation of British Columbia and Yukon (B.M.M.) and the Medical Research Council of Canada (B.M.M.) and by doctoral traineeships from the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada (joint; B.Y.), the Heart and Stroke Foundation of British Columbia and Yukon (M.E.), and the Michael Smith Foundation for Health Research (B.Y. and M.E.).
We thank Reinhard Kandolf (University of Tübingen, Tübingen, Germany) for providing the CVB3.
REFERENCES
- 1.Abe, M. K., W. L. Kuo, M. B. Hershenson, and M. R. Rosner. 1999. Extracellular signal-regulated kinase 7 (ERK7), a novel ERK with a C-terminal domain that regulates its activity, its cellular localization, and cell growth. Mol. Cell. Biol. 19:1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcorn, M. J., J. L. Booth, K. M. Coggeshall, and J. P. Metcalf. 2001. Adenovirus type 7 induces interleukin-8 production via activation of extracellular regulated kinase 1/2. J. Virol. 75:6450-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, D. R., J. E. Wilson, C. M. Carthy, D. Yang, R. Kandolf, and B. M. McManus. 1996. Direct interactions of coxsackievirus B3 with immune cells in the splenic compartment of mice susceptible or resistant to myocarditis. J. Virol. 70:4632-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badorff, C., G. H. Lee, B. J. Lamphear, M. E. Martone, K. P. Campbell, R. E. Rhoads, and K. U. Knowlton. 1999. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat. Med. 5:320-326. [DOI] [PubMed] [Google Scholar]
- 5.Beck, M. A., N. M. Chapman, B. M. McManus, J. C. Mullican, and S. Tracy. 1990. Secondary enterovirus infection in the murine model of myocarditis. Pathologic and immunologic aspects. Am. J. Pathol. 136:669-681. [PMC free article] [PubMed] [Google Scholar]
- 6.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 7.Blenis, J. 1993. Signal transduction via the MAP kinases: proceed at your own RSK. Proc. Natl. Acad. Sci. USA 90:5889-5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bollag, G., and F. McCormick. 1991. Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature 351:576-579. [DOI] [PubMed] [Google Scholar]
- 9.Bueno, O. F., L. J. De Windt, K. M. Tymitz, S. A. Witt, T. R. Kimball, R. Klevitsky, T. E. Hewett, S. P. Jones, D. J. Lefer, C. F. Peng, R. N. Kitsis, and J. D. Molkentin. 2000. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 19:6341-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carthy, C. M., D. J. Granville, K. A. Watson, D. R. Anderson, J. E. Wilson, D. Yang, D. W. Hunt, and B. M. McManus. 1998. Caspase activation and specific cleavage of substrates after coxsackievirus B3-induced cytopathic effect in HeLa cells. J. Virol. 72:7669-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow, L. H., K. W. Beisel, and B. M. McManus. 1992. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab. Investig. 66:24-31. [PubMed] [Google Scholar]
- 12.Colston, J. T., B. Chandrasekar, and G. L. Freeman. 1998. Expression of apoptosis-related proteins in experimental coxsackievirus myocarditis. Cardiovasc. Res. 38:158-168. [DOI] [PubMed] [Google Scholar]
- 13.Cory, A. H., T. C. Owen, J. A. Barltrop, and J. G. Cory. 1991. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 3:207-212. [DOI] [PubMed] [Google Scholar]
- 14.Davis, R. J. 1993. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 268:14553-14556. [PubMed] [Google Scholar]
- 15.Ehrenfeld, E. 1982. Poliovirus-induced inhibition of host-cell protein synthesis. Cell 28:435-436. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda, K., K. Tsuchihara, M. Hijikata, S. Nishiguchi, T. Kuroki, and K. Shimotohno. 2001. Hepatitis C virus core protein enhances the activation of the transcription factor, Elk1, in response to mitogenic stimuli. Hepatology 33:159-165. [DOI] [PubMed] [Google Scholar]
- 17.Giambartolomei, S., F. Covone, M. Levrero, and C. Balsano. 2001. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the hepatitis C virus (HCV) core protein. Oncogene 20:2606-2610. [DOI] [PubMed] [Google Scholar]
- 18.Goldstaub, D., A. Gradi, Z. Bercovitch, Z. Grosmann, Y. Nophar, S. Luria, N. Sonenberg, and C. Kahana. 2000. Poliovirus 2A protease induces apoptotic cell death. Mol. Cell. Biol. 20:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henke, A., H. Launhardt, K. Klement, A. Stelzner, R. Zell, and T. Munder. 2000. Apoptosis in coxsackievirus B3-caused diseases: interaction between the capsid protein VP2 and the proapoptotic protein siva. J. Virol. 74:4284-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzum, M., V. Ruppert, B. Kuytz, H. Jomaa, I. Nakamura, and B. Maisch. 1994. Coxsackievirus B3 infection leads to cell death of cardiac myocytes. J. Mol. Cell. Cardiol. 26:907-913. [DOI] [PubMed] [Google Scholar]
- 21.Huber, M., K. A. Watson, H. C. Selinka, C. M. Carthy, K. Klingel, B. M. McManus, and R. Kandolf. 1999. Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J. Virol. 73:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito, M., M. Kodama, M. Masuko, M. Yamaura, K. Fuse, Y. Uesugi, S. Hirono, Y. Okura, K. Kato, Y. Hotta, T. Honda, R. Kuwano, and Y. Aizawa. 2000. Expression of coxsackievirus and adenovirus receptor in hearts of rats with experimental autoimmune myocarditis. Circ. Res. 86:275-280. [DOI] [PubMed] [Google Scholar]
- 23.Jacque, J. M., A. Mann, H. Enslen, N. Sharova, B. Brichacek, R. J. Davis, and M. Stevenson. 1998. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 17:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joe, A. K., G. Ferrari, H. H. Jiang, X. H. Liang, and B. Levine. 1996. Dominant inhibitory Ras delays Sindbis virus-induced apoptosis in neuronal cells. J. Virol. 70:7744-7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, R. A., S. M. Huong, and E. S. Huang. 2000. Activation of the mitogen-activated protein kinase p38 by human cytomegalovirus infection through two distinct pathways: a novel mechanism for activation of p38. J. Virol. 74:1158-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, R. A., X. L. Ma, A. D. Yurochko, and E. S. Huang. 2001. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J. Gen. Virol. 82:493-497. [DOI] [PubMed] [Google Scholar]
- 27.Kamakura, S., T. Moriguchi, and E. Nishida. 1999. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J. Biol. Chem. 274:26563-26571. [DOI] [PubMed] [Google Scholar]
- 28.Kato, Y., R. I. Tapping, S. Huang, M. H. Watson, R. J. Ulevitch, and J. D. Lee. 1998. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 395:713-716. [DOI] [PubMed] [Google Scholar]
- 29.Klingel, K., P. Rieger, G. Mall, H. C. Selinka, M. Huber, and R. Kandolf. 1998. Visualization of enteroviral replication in myocardial tissue by ultrastructural in situ hybridization: identification of target cells and cytopathic effects. Lab. Investig. 78:1227-1237. [PubMed] [Google Scholar]
- 30.Lange-Carter, C. A., C. M. Pleiman, A. M. Gardner, K. J. Blumer, and G. L. Johnson. 1993. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science 260:315-319. [DOI] [PubMed] [Google Scholar]
- 31.Lechner, C., M. A. Zahalka, J. F. Giot, N. P. Moller, and A. Ullrich. 1996. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc. Natl. Acad. Sci. USA 93:4355-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, J. D., R. J. Ulevitch, and J. Han. 1995. Primary structure of BMK1: a new mammalian map kinase. Biochem. Biophys. Res. Commun. 213:715-724. [DOI] [PubMed] [Google Scholar]
- 33.Liu, P., K. Aitken, Y. Y. Kong, M. A. Opavsky, T. Martino, F. Dawood, W. H. Wen, I. Kozieradzki, K. Bachmaier, D. Straus, T. W. Mak, and J. M. Penninger. 2000. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nat. Med. 6:429-434. [DOI] [PubMed] [Google Scholar]
- 34.Marais, R., Y. Light, H. F. Paterson, and C. J. Marshall. 1995. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 14:3136-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 36.Martino, T. A., P. Liu, and M. J. Sole. 1994. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ. Res. 74:182-188. [DOI] [PubMed] [Google Scholar]
- 37.Martino, T. A., M. Petric, M. Brown, K. Aitken, C. J. Gauntt, C. D. Richardson, L. H. Chow, and P. P. Liu. 1998. Cardiovirulent coxsackieviruses and the decay-accelerating factor (CD55) receptor. Virology 244:302-314. [DOI] [PubMed] [Google Scholar]
- 38.Martino, T. A., M. Petric, H. Weingartl, J. M. Bergelson, M. A. Opavsky, C. D. Richardson, J. F. Modlin, R. W. Finberg, K. C. Kain, N. Willis, C. J. Gauntt, and P. P. Liu. 2000. The coxsackie-adenovirus receptor (CAR) is used by reference strains and clinical isolates representing all six serotypes of coxsackievirus group B and by swine vesicular disease virus. Virology 271:99-108. [DOI] [PubMed] [Google Scholar]
- 39.Mason, J. W., J. B. O'Connell, A. Herskowitz, N. R. Rose, B. M. McManus, M. E. Billingham, T. E. Moon, et al. 1995. A clinical trial of immunosuppressive therapy for myocarditis. N. Engl. J. Med. 333:269-275. [DOI] [PubMed] [Google Scholar]
- 40.Mayor, S., K. G. Rothberg, and F. R. Maxfield. 1994. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science 264:1948-1951. [DOI] [PubMed] [Google Scholar]
- 41.McLean, T. I., and S. L. Bachenheimer. 1999. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J. Virol. 73:8415-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McManus, B. M., L. H. Chow, S. J. Radio, S. M. Tracy, M. A. Beck, N. M. Chapman, K. Klingel, and R. Kandolf. 1991. Progress and challenges in the pathological diagnosis of myocarditis. Eur. Heart J. 12:18-21. [DOI] [PubMed] [Google Scholar]
- 43.McManus, B. M., L. H. Chow, J. E. Wilson, D. R. Anderson, J. M. Gulizia, C. J. Gauntt, K. E. Klingel, K. W. Beisel, and R. Kandolf. 1993. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin. Immunol. Immunopathol. 68:159-169. [DOI] [PubMed] [Google Scholar]
- 44.Peng, T., T. Sadusky, Y. Li, G. R. Coulton, H. Zhang, and L. C. Archard. 2001. Altered expression of Bag-1 in coxsackievirus B3 infected mouse heart. Cardiovasc. Res. 50:46-55. [DOI] [PubMed] [Google Scholar]
- 45.Planz, O., S. Pleschka, and S. Ludwig. 2001. MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells. J. Virol. 75:4871-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 47.Popik, W., J. E. Hesselgesser, and P. M. Pitha. 1998. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J. Virol. 72:6406-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popik, W., and P. M. Pitha. 1998. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology 252:210-217. [DOI] [PubMed] [Google Scholar]
- 49.Rusnati, M., C. Urbinati, B. Musulin, D. Ribatti, A. Albini, D. Noonan, C. Marchisone, J. Waltenberger, and M. Presta. 2001. Activation of endothelial cell mitogen activated protein kinase ERK(1/2) by extracellular HIV-1 Tat protein. Endothelium 8:65-74. [DOI] [PubMed] [Google Scholar]
- 50.Shafren, D. R., R. C. Bates, M. V. Agrez, R. L. Herd, G. F. Burns, and R. D. Barry. 1995. Coxsackieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J. Virol. 69:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shapiro, L., K. A. Heidenreich, M. K. Meintzer, and C. A. Dinarello. 1998. Role of p38 mitogen-activated protein kinase in HIV type 1 production in vitro. Proc. Natl. Acad. Sci. USA 95:7422-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shenoy-Scaria, A. M., J. Kwong, T. Fujita, M. W. Olszowy, A. S. Shaw, and D. M. Lublin. 1992. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn 1. J. Immunol. 149:3535-3541. [PubMed] [Google Scholar]
- 53.Sontag, E., S. Fedorov, C. Kamibayashi, D. Robbins, M. Cobb, and M. Mumby. 1993. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell 75:887-897. [DOI] [PubMed] [Google Scholar]
- 54.Soucie, E. L., M. G. Annis, J. Sedivy, J. Filmus, B. Leber, D. W. Andrews, and L. Z. Penn. 2001. Myc potentiates apoptosis by stimulating Bax activity at the mitochondria. Mol. Cell. Biol. 21:4725-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tam, P. E., and R. P. Messner. 1999. Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J. Virol. 73:10113-10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor, L. A., C. M. Carthy, D. Yang, K. Saad, D. Wong, G. Schreiner, L. W. Stanton, and B. M. McManus. 2000. Host gene regulation during coxsackievirus B3 infection in mice: assessment by microarrays. Circ. Res. 87:328-334. [DOI] [PubMed] [Google Scholar]
- 57.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 58.Trahey, M., G. Wong, R. Halenbeck, B. Rubinfeld, G. A. Martin, M. Ladner, C. M. Long, W. J. Crosier, K. Watt, K. Koths. 1988. Molecular cloning of two types of GAP complementary DNA from human placenta. Science 242:1697-1700. [DOI] [PubMed] [Google Scholar]
- 59.Wang, X., and J. M. Bergelson. 1999. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J. Virol. 73:2559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, X., J. L. Martindale, and N. J. Holbrook. 2000. Requirement for ERK activation in cisplatin-induced apoptosis. J. Biol. Chem. 275:39435-39443. [DOI] [PubMed] [Google Scholar]
- 61.Wessely, R., A. Henke, R. Zell, R. Kandolf, and K. U. Knowlton. 1998. Low-level expression of a mutant coxsackieviral cDNA induces a myocytopathic effect in culture: an approach to the study of enteroviral persistence in cardiac myocytes. Circulation 98:450-457. [DOI] [PubMed] [Google Scholar]
- 62.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 63.Yan, C., H. Luo, J. D. Lee, J. Abe, and B. C. Berk. 2001. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J. Biol. Chem. 276:10870-10878. [DOI] [PubMed] [Google Scholar]
- 64.Yang, D., J. Yu, Z. Luo, C. M. Carthy, J. E. Wilson, Z. Liu, and B. M. McManus. 1999. Viral myocarditis: identification of five differentially expressed genes in coxsackievirus B3-infected mouse heart. Circ. Res. 84:704-712. [DOI] [PubMed] [Google Scholar]
- 65.Yang, X., and D. Gabuzda. 1998. Mitogen-activated protein kinase phosphorylates and regulates the HIV-1 Vif protein. J. Biol. Chem. 273:29879-29887. [DOI] [PubMed] [Google Scholar]
- 66.Yang, X., and D. Gabuzda. 1999. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J. Virol. 73:3460-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue, T. L., C. Wang, J. L. Gu, X. L. Ma, S. Kumar, J. C. Lee, G. Z. Feuerstein, H. Thomas, B. Maleeff, and E. H. Ohlstein. 2000. Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ. Res. 86:692-699. [DOI] [PubMed] [Google Scholar]