Abstract
Candida albicans ECM33 encodes a glycosylphosphatidylinositol-linked cell wall protein that is important for cell wall integrity. It is also critical for normal virulence in the mouse model of hematogenously disseminated candidiasis. To identify potential mechanisms through which Ecm33p contributes to virulence, we investigated the interactions of C. albicans ecm33Δ mutants with endothelial cells and the FaDu oral epithelial cell line in vitro. The growth rate of blastospores of strains containing either one or no intact copies of ECM33 was 50% slower than that of strains containing two intact copies of ECM33. However, all strains germinated at the same rate, forming similar-length hyphae on endothelial cells and oral epithelial cells. Strains containing either one or no intact copies of ECM33 had modestly reduced adherence to both types of host cells, and a markedly reduced capacity to invade and damage these cells. Saccharomyces cerevisiae expressing C. albicans ECM33 did not adhere to or invade epithelial cells, suggesting that Ecm33p by itself does not act as an adhesin or invasin. Examination of ecm33Δ mutants by transmission electron microscopy revealed that the cell wall of these strains had an abnormally electron-dense outer mannoprotein layer, which may represent a compensatory response to reduced cell wall integrity. The hyphae of these mutants also had aberrant surface localization of the adhesin Als1p. Collectively, these results suggest that Ecm33p is required for normal cell wall architecture as well as normal function and expression of cell surface proteins in C. albicans.
Candida albicans causes both hematogenously disseminated and oropharyngeal disease. During hematogenously disseminated candidiasis, the blood-borne organisms must adhere to and penetrate the endothelial cell lining of the blood vessels to invade the deep tissues of the target organs. These target organs include the kidney, liver, spleen, heart, brain, and eye (6). During oropharyngeal candidiasis, C. albicans not only adheres to the epithelial cells of the oral mucosa, but also invades these cells. Invasion into the epithelial cell lining of the oral mucosa is characteristic of both human and experimental animal models of oropharyngeal candidiasis (3, 7, 15, 18, 23).
The mechanism by which C. albicans invades endothelial cells and oral epithelial cells has been investigated in vitro. These in vitro studies demonstrate that one mechanism by which C. albicans invades both endothelial cells and oral epithelial cells is by inducing its own endocytosis (8, 20, 21). Once C. albicans is endocytosed by either endothelial cells or oral epithelial cells, it damages these cells. In vitro, host cell damage requires the endocytosis of live organisms (8, 20). Importantly, we have found that mutants of C. albicans that are endocytosed poorly and cause little damage to endothelial cells and oral epithelial cells have significantly reduced virulence in mouse models of hematogenously disseminated or oropharyngeal candidiasis (20, 21, 25). The association between the inability of these strains to invade and damage host cells in vitro and their attenuated virulence suggests that fungal invasion and damage of endothelial cells and oral epithelial cells contribute to the pathogenesis of hematogenously disseminated and oropharyngeal candidiasis, respectively.
The cell wall represents the initial point of interaction between the host and pathogen. The C. albicans ECM33 gene product is predicted to be a glycosylphosphatidylinositol (GPI)-linked cell wall protein (5). Recently, it was found that Ecm33p is required for normal cell wall integrity and the yeast-to-hypha transition in vitro (16). Blastospores of a C. albicans ecm33Δ/ecm33Δ mutant were larger and flocculated more extensively than blastospores of the wild-type strain. The mutant also exhibited enhanced susceptibility to compounds, such as calcofluor white and Congo red, that interfere with cell wall integrity. In addition, the ecm33Δ/ecm33Δ mutant had delayed hypha formation in liquid and solid media. Consistent with these in vitro defects, this mutant had severely attenuated virulence in the mouse model of hematogenously disseminated candidiasis. Finally, these investigations showed that both copies of ECM33 were required for a normal phenotype. The phenotype of strains that contained only one copy of ECM33 was more similar to that of the homozygous ecm33Δ/ecm33Δ mutant than to that of strains that contained two copies of ECM33.
We hypothesized that an additional reason Ecm33p is required for normal virulence is that this protein is necessary for specific interactions of C. albicans with endothelial and oral epithelial cells. Therefore, we investigated the role of Ecm33p in the ability of C. albicans to adhere to, invade, and damage endothelial cells and an oral epithelial cell line in vitro.
MATERIALS AND METHODS
Organisms, media, and plasmids.
The C. albicans and Saccharomyces cerevisiae strains used in this study are listed in Table 1. All C. albicans strains were maintained on YPD (1% yeast extract, 2% peptone, 2% glucose) agar plates at 37°C. This medium was supplemented with 80 μg of uridine per ml for growing Ura− strains. Ura+ transformants were selected on synthetic complete medium without uridine (2% glucose, 0.67% yeast nitrogen base without amino acids, 0.065% synthetic complete supplement mixture without uridine) (Qbiogene, Carlsbad, CA). To select Ura− strains, the organisms were grown on synthetic complete medium containing uridine and 0.1% 5-fluoroorotic acid (Zymo Research, Orange, CA). For use in the experiments, all organisms were grown in liquid YPD medium on a rotary shaker overnight at 30°C, harvested by centrifugation, and washed twice in Dulbecco's phosphate-buffered saline (PBS). The organisms were resuspended to the appropriate concentration in RPMI 1640 medium (Irvine Scientific, Santa Ana, CA).
TABLE 1.
Strains of C. albicans and S. cerevisiae used in this study
| Species | Strain | Relevant genotype | Doubling time (h) | Reference |
|---|---|---|---|---|
| C. albicans | CAI4 | ECM33/ECM33 ura3Δ::imm434/ura3Δ::imm434 | 9 | |
| CAI4-URA | ECM33/ECM33 ura3Δ::imm434/ura3Δ::imm434::URA3 | 1.3 | 20 | |
| RML1a | ECM33/ecm33Δ::hisG ura3Δ::imm434/ura3Δ::imm434 | 16 | ||
| RML1U | ECM33/ecm33Δ::hisG ura3Δ::imm434/ura3Δ::imm434::URA3 | 2.0 | This study | |
| RML2a | ecm33Δ::hisG/ecm33Δ::hisG ura3Δ::imm434/ura3Δ::imm434 | 16 | ||
| RML2U | ecm33Δ::hisG/ecm33Δ::hisG ura3Δ::imm434/ura3Δ::imm434::URA3 | 2.0 | This study | |
| RML3a | ecm33Δ::hisG/ecm33Δ::hisG::ECM33-cat ura3Δ::imm434/ura3Δ::imm434 | 16 | ||
| RML3U | ecm33Δ::hisG/ecm33Δ::hisG::ECM33-cat ura3Δ::imm434/ura3Δ::imm434::URA3 | 2.0 | This study | |
| RML4 | ecm33Δ::hisG::ECM33-cat/ecm33Δ::hisG::ECM33-cat-URA3-cat ura3Δ::imm434/ura3Δ::imm434 | 16 | ||
| RML4a | ecm33Δ::hisG::ECM33-cat/ecm33Δ::hisG::ECM33-cat ura3Δ::imm434/ura3Δ::imm434 | This study | ||
| RML4U | ecm33Δ::hisG::ECM33-cat/ecm33Δ::hisG::ECM33-cat ura3Δ::imm434/ura3Δ::imm434::URA3 | 1.3 | This study | |
| CAYF4 | als1Δ::hisG/als1Δ::hisG ura3Δ::imm434/ura3Δ::imm434::URA3 | NDa | Unpublished | |
| S. cerevisiae | BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | ND | EUROSCARF |
| Y03215 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ecm33::kanMX4 | ND | EUROSCARF |
ND, not determined.
Strain construction.
The C. albicans ECM33 mutants used in the previous study all had URA3 integrated at the ECM33 locus (16). The chromosomal locus at which URA3 is integrated can influence the proteome of C. albicans, its adherence to host cells in vitro, and its virulence (2, 4, 28, 29). Therefore, to avoid the potential confounding effects of the chromosomal location of URA3 on the phenotypes of the ECM33 mutants, we modified them so that URA3 was integrated at its native locus in all strains. The ura3Δ/ura3Δ strains RML1a (ECM33/ecm33Δ), RML2a (ecm33Δ/ecm33Δ), and RML3a (ecm33Δ/ecm33Δ::ECM33) were transformed with a 3.9-kb NheI/PstI fragment encompassing the C. albicans URA3 and adjacent IRO1 genes, as described previously (20), to produce RML1U, RML2U, and RML3U, respectively. Integration of the URA3-IRO1 fragment into the correct chromosomal locus was confirmed by PCR using the primers 5′-TGCTGGTTGGAATGCTTATTTG-3′ and 5′-TGCAAATTCTGCTACTGGAGTT-3′.
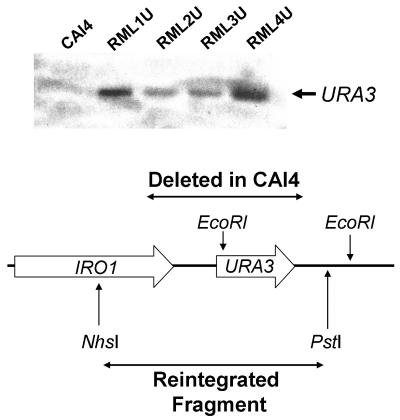
A Ura− clone of strain RML4 (ecm33Δ::ECM33/ecm33Δ::ECM33) was selected by plating it on 5-fluoroorotic acid. This Ura− clone was named RML4a. The absence of URA3 in RML4a was confirmed by PCR using the primers 5′-TAGGACGTGACAAGATACAGGATCGCA-3′ and 5′-GTTTTCCGCCATCGCAATCAGGC-3′. Next, RML4a was transformed with the URA3-IRO1 fragment as outlined above to make RML4U. The integration of URA3 into the correct locus was confirmed by Southern blotting (Fig. 1).
FIG. 1.
Southern blot analysis of restoration of the URA3 locus in the various C. albicans strains. Genomic DNA from the indicated strains was digested with EcoRI. and the blot was probed with an EcoRI fragment encompassing URA3 and its 3′-flanking region.
Growth rate determination.
The doubling times of the C. albicans strains were determined by growing them in liquid YPD medium on a rotary shaker at 30°C. At 1-hour intervals, an aliquot was removed and sonicated briefly, and then the optical density at 600 nm was measured.
Expression of C. albicans ECM33 in S. cerevisiae.
To analyze the effects of expression of C. albicans ECM33 (CaECM33) in S. cerevisiae, the plasmid pYEPCaECM33, which contained the CaECM33 protein coding sequence, 330 bp of promoter sequence, and 495 bp of 3′ untranslated region, was used (16). As a control, plasmid pYEPScerevisiaeECM33, containing the protein coding sequence of S. cerevisiae ECM33 (ScECM33), 1,102 bp of promoter, and 565 bp of 3′ untranslated region, was used (16). An additional control was the empty plasmid pYEP352. The plasmids containing CaECM33 and ScECM33 were transformed into strains of S. cerevisiae with either an intact copy of ScECM33 (strain BY4741) or a disrupted copy of this gene (strain Y03215) (Table 1).
Endothelial cells and oral epithelial cells.
Endothelial cells were isolated from the veins of human umbilical cords by the method of Jaffe et al. (14). They were grown in M-199 medium (Gibco-BRL, Gaithersburg, MD) containing 10% fetal bovine serum and 10% defined bovine calf serum (both from Gemini BioProducts, Inc., Calabasas, CA), and supplemented with 2 mM l-glutamine with penicillin and streptomycin (Irvine Scientific) as described previously (8).
The FaDu oropharyngeal epithelial cell line was purchased from the American Type Culture Collection (Manassas, VA). It was maintained in Eagle's minimal essential medium with Earle's balanced salt solution (Irvine Scientific) adjusted to contain 1.5 g/liter sodium bicarbonate, 2 mM l-glutamine, 0.1 mM nonessential amino acids, and 1.0 mM sodium pyruvate. This medium was supplemented with 10% fetal bovine serum, penicillin, and streptomycin.
Both cell types were grown at 37°C in a humidified environment containing 5% CO2 and used at 95% confluence in all experiments.
Endocytosis assay.
The number of organisms that were endocytosed by the endothelial cells and FaDu oral epithelial cells was determined using a differential fluorescence assay exactly as described previously (20, 22). Briefly, endothelial cells or FaDu cells were grown on fibronectin-coated glass coverslips and infected with 105 cells of either C. albicans or S. cerevisiae in RPMI 1640 medium. After incubating for 90 min, the cells were rinsed once with Hanks' balanced salt solution (Irvine Scientific) and then fixed in 3% paraformaldehyde. The nonendocytosed organisms were stained with an anti-C. albicans rabbit serum (Biodesign International, Kennebunkport, ME) conjugated with Alexa 568 (Molecular Probes, Eugene, OR). This antiserum recognizes both C. albicans and S. cerevisiae (27). Afterwards, the FaDu cells were rinsed extensively with PBS and then permeabilized with 0.2% (vol/vol) Triton X-100 (Sigma-Aldrich, St. Louis, MO) in PBS. Next, the cell-associated organisms (the endocytosed plus nonendocytosed organisms) were stained with the anti-C. albicans rabbit serum conjugated with Alexa 488 (Molecular Probes). The coverslips were observed with an epifluorescence microscope. The number of organisms endocytosed by the host cells was determined by subtracting the number of cell-associated organisms (labeled with Alexa 568, which fluoresces red) from the total number of organisms (labeled with Alexa 488, which fluoresces green). At least 100 organisms were counted on each coverslip, and all experiments were performed in triplicate.
Damage assay.
The extent of damage caused by the various C. albicans strains to the endothelial cells and the FaDu cell line was measured using a 51Cr release assay as described previously (1, 20, 21). The host cells were grown in a 96-well tissue culture plate with detachable wells and incubated overnight with Na251CrO4 (MP Biomedicals, Inc., Irvine, CA) per well. The following day, the unincorporated tracer was removed by rinsing. When endothelial cells were used, they were infected with 4 × 104 organisms in RPMI 1640. Because FaDu cells are less susceptible to damage by C. albicans, they were infected with 105 organisms in the same medium. To measure the spontaneous release of 51Cr, uninfected host cells were exposed to medium alone. After a 3-h incubation, the upper 50% of medium was removed from each well and then the wells were manually detached from one another. The amount of 51Cr in the aspirates and the well was determined by gamma counting. After correcting for the amount of 51Cr incorporated in each well, the specific release of 51Cr was calculated by the formula (experimental release − spontaneous release)/(total incorporation − spontaneous release). Experimental release was the amount of 51Cr released into the medium by cells infected with C. albicans. Spontaneous release was the amount of 51Cr released into the medium by uninfected host cells. Total incorporation was the sum of the amount of 51Cr released into the medium and remaining in the host cells.
Transmission electron microscopy.
Samples for transmission electron microscopy were prepared according to the protocol described by Miret et al. (17). Briefly, 2 × 107 blastospores of each strain were collected after 24 h of growth in liquid YPD medium and fixed at 4°C for 24 h in 500 μl fixative solution (sodium cacodylate buffer, pH 7.2, containing 1.2% glutaraldehyde and 2% paraformaldehyde). The samples were then washed with saline and postfixed for 90 min with 1% potassium permanganate. The fixed cells were dehydrated through a graded series of ethanol and embedded in Embed 812 resin (Electron Microscopy Sciences, Hatfield, PA). Thin sections were stained with uranyl acetate and lead citrate and then imaged with a Zeiss EM902 electron microscope. Multiple cells of each strain were imaged.
Detection of Als1p on the surface of C. albicans.
The localization of the adhesin, Als1p on the surface of the various strains of C. albicans was determined by direct immunofluorescence using a modification of our previously described method (10). Briefly, 2 × 105 blastospores of the different C. albicans strains were incubated for 90 min with endothelial cells on glass coverslips as in the endocytosis assay. Next, the cells were rinsed once with Hanks' balanced salt solution, fixed with 3% paraformaldehyde, and blocked with 1% goat serum containing 1% Triton X-100. The cells were incubated with an Alexa 488-conjugated murine monoclonal antibody directed against the N terminus of Als1p (10). After extensive rinsing with PBS, the cells were stained with the Alexa 568-conjugated rabbit anti-C. albicans antiserum and viewed by confocal microscopy. To enable comparison of the intensity of staining among the different strains, the image acquisition parameters were set using the wild-type strain and these parameters were used for imaging all of the other strains. The final confocal images were produced by combining optical sections taken through the z axis.
To determine if some fraction of Als1p was not detected by the anti-Als1p antibody because it was buried in the cell wall, 3 × 106 blastospores of the different strains were added to endothelial cells in a six-well tissue culture plate and allowed to germinate for 90 min. Next, the medium was aspirated and replaced with distilled water to lyse the endothelial cells. The germ tubes were scraped from the wells into 1.5-ml centrifuge tubes, fixed in 3% paraformaldehyde for 15 min, and washed twice with PBS. They were resuspended in KS buffer (0.1 M potassium phosphate buffer, 1 M sorbitol, pH 7.0,) containing 0.5 units of zymolyase (Zymo Research Corporation, Orange, Calif.) per ml. The germ tubes were allowed to settle onto poly-l-lysine coated coverslips where they were incubated for 5 to 30 min. At each time point, the coverslips were washed three times with KS buffer, blocked with 1% bovine serum albumin in PBS, and then stained with the anti-Als1p monoclonal antibody, followed by the rabbit anti-C. albicans antiserum as above.
Statistical analysis.
Differences among the various strains of C. albicans in their interactions with endothelial cells and the FaDu oral epithelial cell line were compared by analysis of variance. P values of ≤0.05 were considered significant.
RESULTS
Strains lacking one or both copies of ECM33 had slower growth rates in vitro than strains containing two copies of ECM33.
Strains of C. albicans that grow slowly in vitro frequently have attenuated virulence when tested in the mouse model of hematogenously disseminated candidiasis (24). Growth rate is also likely to be an important factor for virulence during oropharyngeal candidiasis. Therefore, we determined the growth rates of blastospores of the various ECM33 strains in vitro. The doubling time of all C. albicans strains containing either one or no copies of ECM33 was approximately 50% longer than that of either the wild-type strain or the ecm33Δ::ECM33/ecm33Δ::ECM33 double-complemented strain (Table 1). Therefore, both copies of ECM33 are critical for the normal growth of C. albicans blastospores in vitro and probably in vivo.
Hyphal formation on endothelial cells and the FaDu oral epithelial cell line is independent of ECM33.
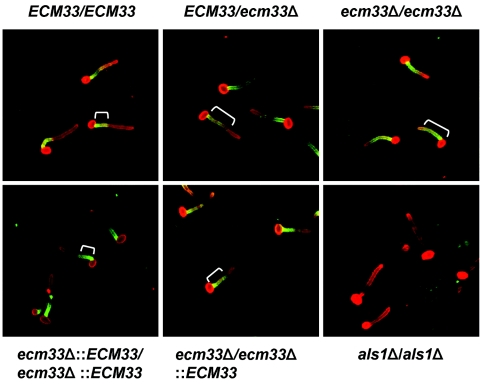
The slow growth rates of the ecm33Δ strains precluded a meaningful analysis of the contribution of Ecm33p to virulence in relevant animal models of candidiasis. Therefore, for a surrogate for virulence studies, we examined the interactions of the ECM33 mutants with endothelial cells and an oral epithelial cell line in vitro. Although the organisms were added to these host cells as blastospores, they began germinating within 45 min. Previously, it was reported that ecm33Δ mutants had filamentation defects on some liquid and solid media (16). Interestingly, we observed that all of the mutant strains produced normal length hyphae on the endothelial cells and oral epithelial cells after 90 min of incubation (Fig. 2). Therefore, the slow doubling time of the blastospores did not translate into delayed hyphal formation or elongation under the conditions used in the assays.
FIG. 2.
ECM33 is not required for hyphal formation on endothelial cells, but it does influence the localization of Als1p on the hyphal surface. Endothelial cells were infected with the indicated strains of C. albicans. After 90 min, the cells were fixed and stained with an anti-Als1p monoclonal antibody (green) and an anti-C. albicans antiserum (red). The cells were viewed by confocal microcopy and the images were produced by merging the red and green channels. The white brackets indicate the extent of Als1p expression on the surface of the hyphae.
ECM33 is required for maximal C. albicans adherence to and invasion of endothelial cells and the FaDu oral epithelial cell line.
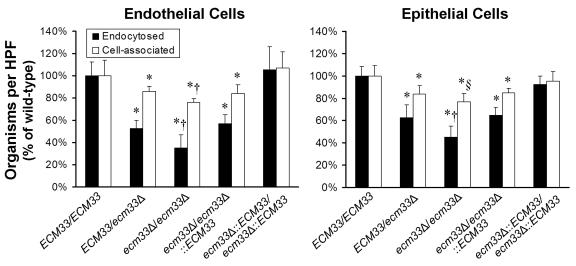
The capacity of each ECM33 mutant strain to adhere to endothelial cells and the FaDu oral epithelial cell line after 90 min of infection was investigated. Fewer organisms of the heterozygous ecm33Δ/ECM33 mutant were cell-associated (defined as the sum of the adherent and endocytosed organisms) with either type of host cell compared to the wild-type strain (Fig. 3). The number of cell-associated organisms was even lower when the host cells were infected with the homozygous ecm33Δ/ecm33Δ strain, suggesting that there was a gene dosage effect. When the homozygous ecm33Δ/ecm33Δ strain was complemented with a single copy of wild-type ECM33, the number of organisms that were cell associated with either type of host cell was similar to that of the heterozygous ecm33Δ/ECM33 mutant. Complementing the homozygous ecm33Δ/ecm33Δ strain with two copies of ECM33 resulted in a phenotype that was indistinguishable from that of the wild-type strain. These results indicate that both copies of ECM33 are required for maximal adherence to both endothelial cells and oral epithelial cells.
FIG. 3.
Two copies of ECM33 are required for maximal C. albicans adherence to and endocytosis by endothelial cells and FaDu oral epithelial cells. The indicated strains of C. albicans were incubated with either endothelial cells or the FaDu oral epithelial cell line for 90 min, after which the numbers of endocytosed and cell-associated organisms were determined by a differential fluorescence assay. Results are the mean ± standard deviation of three experiments, each performed in triplicate. *, P < 0.01 compared to either the wild-type ECM33/ECM33 or the ecm33Δ::ECM33/ecm33Δ::ECM33 strain. †, P < 0.02 compared to all other strains. §, P = 0.06 compared to the ECM33/ecm33Δ strain and P < 0.02 compared to all other strains. HPF, high-power field.
Strains containing either one or no copies of ECM33 were also endocytosed poorly by both endothelial cells and FaDu oral epithelial cells (Fig. 3). The homozygous ecm33Δ/ecm33Δ strain had a greater endocytosis defect than did the strains containing a single copy of wild-type ECM33. Paralleling the adherence results, the endocytosis defect of the ecm33Δ/ecm33Δ:: ECM33 singly complemented strain was similar to that of the ECM33/ecm33Δ heterozygous strain, whereas the ecm33Δ::ECM33/ecm33Δ::ECM33 double-complemented strain had no endocytosis defect.
For strains containing either one or no intact copies of ECM33, the reduction in endocytosis was greater than the reduction in adherence (Fig. 3). For example, 24% fewer cells of the homozygous ecm33Δ/ecm33Δ mutant were cell associated with endothelial cells, compared to the wild-type strain. In contrast, 65% fewer cells of the ecm33Δ/ecm33Δ mutant were endocytosed by endothelial cells compared to the wild-type strain. Therefore, the endocytosis defect of the ecm33Δ strains was not merely the result of decreased adherence.
ECM33 is essential for C. albicans to cause maximal damage to oral epithelial cells in vitro.
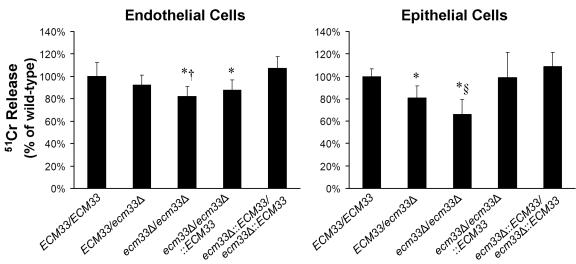
We compared the extent of damage caused by the different C. albicans strains to endothelial cells and FaDu oral epithelial cells. Strains containing a single intact copy of ECM33 caused slightly less damage to both types of host cells compared to the wild-type strain. The homozygous ecm33Δ/ecm33Δ mutant was the only strain that consistently caused significantly less damage to both endothelial and epithelial cells than did the wild-type strain (P = 0.003 and P < 0.0001, respectively) (Fig. 4).
FIG. 4.
Role of ECM33 in C. albicans-induced damage to endothelial cells and FaDu oral epithelial cells. The indicated host cells were loaded with 51Cr and then incubated with the indicated strains of C. albicans for 3 h. The extent of host cell damage was measured by the release of 51Cr into the medium. Results are the mean ± standard deviation of at least independent experiments, each performed in triplicate. *, P < 0.04 compared to both the wild-type ECM33/ECM33 and the ecm33Δ::ECM33/ecm33Δ::ECM33 strain. †, P = 0.03 compared to the ecm33Δ/ECM33 strain and P = 0.19 compared to the ecm33Δ/ecm33Δ::ECM33 strain. §, P < 0.002 compared to both the ECM33/ecm33Δ and the ecm33Δ/ecm33Δ::ECM33 strains.
ecm33Δ mutants have aberrant cell wall architecture.
There are at least two nonexclusive explanations for the defects in the interactions of the ecm33Δ mutants with endothelial and oral epithelial cells. Biochemical studies have indicated that Ecm33p is present in the cell wall of C. albicans, and it may be expressed on the cell surface (5). Therefore, it is possible that Ecm33p functions as an adhesin or invasin. However, it is also known that Ecm33p is required for normal cell wall integrity in C. albicans (16). Similarly, in Saccharomyces cerevisiae, Ecm33p is necessary for normal cell wall architecture (19). Thus, it is also possible that Ecm33 is required for the normal functioning of other C. albicans cell wall proteins that are adhesins or invasins.
To investigate whether Ecm33p itself mediates adherence to or invasion of host cells, we expressed either CaECM33 or ScECM33 in both a wild-type and an Scecm33Δ mutant strain of S. cerevisiae. We then measured the adherence to and endocytosis of these strains by the FaDu oral epithelial cell line. For a negative control, we also tested the same strains of S. cerevisiae transformed with empty plasmid. We found that all strains had similarly low levels of adherence and endocytosis (data not shown). CaECM33 was functional in S. cerevisiae because it reversed the cell wall integrity defects of the Scecm33Δ strain (16). These results suggest that Ecm33p by itself is not sufficient to mediate adherence or endocytosis, at least in S. cerevisiae.
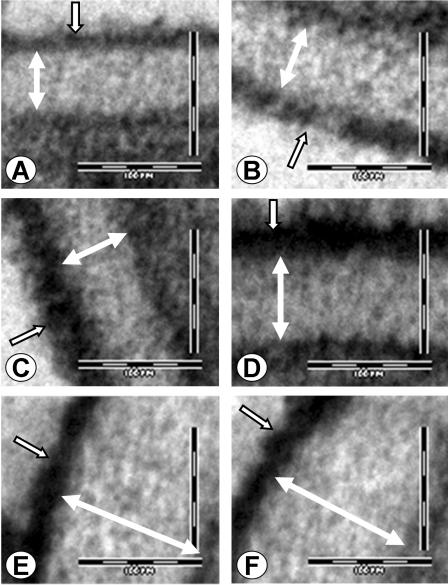
Next, we used transmission electron microscopy to investigate the structure of the cell walls of the ecm33Δ mutants. Multiple cells of each strain were imaged and several different sections were viewed. The wild-type and ecm33Δ::ECM33/ecm33Δ::ECM33 double-complemented strains both had normal cell wall architecture, consisting of an electron dense outer layer of mannoproteins, and an electron lucent inner layer composed mainly of 1,3 β-glucan and chitin (Fig. 5A and B). In contrast, the cell walls of all strains containing either one or no functional copies of ECM33 were consistently abnormal. In the strains with only one copy of ECM33, the outer mannoprotein layer of the cell wall was abnormally wide and electron dense (Fig. 5C and D). In the homozygous ecm33Δ/ecm33Δ mutant, not only was the outer layer of the cell wall abnormally electron dense, but the electron lucent inner layer was also much wider and appeared to be less electron dense than that of the other strains (Fig. 5E and F). These results indicate that ECM33 is required for the normal cell wall architecture of C. albicans.
FIG. 5.
ECM33 is necessary for the normal cell wall architecture of C. albicans. Representative transmission electron micrographs of the cell walls of blastospores of the wild-type (A), ecm33Δ::ECM33/ecm33Δ::ECM33 (B), ecm33Δ/ECM33 (C), ecm33Δ/ecm33Δ::ECM33 (D), and ecm33Δ/ecm33Δ (E and F) strains. White, double-headed arrows indicate the thickness of the internal layer of 1,3 β-glucans and chitin. Open, single-headed arrows indicate the outer mannoprotein layer. Scale bars indicate 100 nm.
ECM33 is necessary for normal Als1p localization on hyphae.
The aberrant cell wall architecture of the ecm33Δ mutants suggested that there may be alteration in the surface expression or localization of other proteins in these strains. Therefore, we analyzed the expression of Als1p on the surface of the various strains by using direct immunofluorescence with a monoclonal antibody directed against the N terminus of Als1p. Als1p is a GPI-linked protein that is expressed on the surface of C. albicans hyphae, where it functions as an adhesin (10, 11, 27). In the wild-type and the ecm33Δ::ECM33/ecm33Δ::ECM33 double-complemented strains, Als1p was observed to be present in a relatively narrow band at the base of the hyphae, adjacent to the blastospore-hyphal junction (Fig. 2). In the strains containing a single functional copy of ECM33, this band of Als1p extended slightly further along the length of the hyphae. Importantly, in the homozygous ecm33Δ/ecm33Δ mutant, Als1p was expressed diffusely over the majority of the hyphal surface, except at the very tip. Therefore, ECM33 is necessary for the normal localization of Als1p.
Although Als1p is known to be expressed on the cell surface of C. albicans (10), it is possible that some fraction of the total pool of Als1p is buried within the cell wall and is therefore not accessible to the monoclonal antibody that was used to detect this protein. If this possibility were true, then the aberrant localization of Als1p in the ecm33Δ mutants could have been due to the exposure of some Als1p that is normally inaccessible to the anti-Als1p antibody. To test this possibility, we incubated germ tubes of the wild-type, homozygous ecm33Δ/ecm33Δ mutant, and ecm33Δ::ECM33/ecm33Δ::ECM33 double-complemented strain for various times in zymolyase to remove the cell wall. After a 5-min exposure to this enzyme, there was a slight reduction in the intensity of Als1p staining in all strains. However the distribution of Als1p along the hyphae of the different strains remained similar to that of control hyphae that were not exposed to the enzyme (data not shown). Longer exposure to the enzyme resulted in the progressive loss of Als1p staining on all hyphae, but it did not change the distribution of the remaining Als1p. Therefore, the aberrant localization of Als1p in the ecm33Δ strains was not due to the cell surface expression of protein that was normally buried within the cell wall of the wild-type strain.
DISCUSSION
Ecm33p is important for several factors that contribute to the virulence of C. albicans. One of these factors is growth rate. The growth rate of C. albicans strains is known to have a significant effect on their virulence in the mouse model of hematogenously disseminated infection (24). Although the relationship between C. albicans growth rate and virulence during oropharyngeal infection has not been reported, it is highly probable that strains that grow slowly have attenuated virulence during this infection, as well.
It is unlikely that the slow growth rate of blastospores of the ecm33Δ mutants played a significant role in their interactions with endothelial cells and oral epithelial cells in vitro. These organisms germinated normally on both types of host cells and the resultant hyphae were of normal length. Previously, it was reported that ecm33Δ mutants had filamentation defects in both liquid and solid media (16). However, these prior experiments were performed under different conditions from the ones used in the current studies. Also, the previous ecm33Δ mutants had URA3 integrated at a different chromosomal locus. Thus, our current results are not directly comparable to the previous data.
We observed that hyphae of the ecm33Δ mutants adhered to and were endocytosed poorly by both endothelial cells and oral epithelial cells. The adherence defect of these mutants was not as great as their endocytosis defect, indicating that their reduced endocytosis was not solely the result of decreased adherence.
Ecm33p is located in the C. albicans cell wall, and it is known to be important for cell wall integrity (5, 16). We found that the ecm33Δ strains had markedly aberrant cell wall architecture with an abnormally electron dense outer mannoprotein layer. We speculate that the enhanced mannoprotein synthesis in these mutants may represent a compensatory response to a weakened cell wall. This abnormal mannoprotein layer likely contributed to the reduced adherence and endocytosis of the ecm33Δ strains by interfering with the normal expression and/or function of C. albicans adhesin and invasin proteins. Consistent with this theory, we found that the Als1p adhesin was abnormally distributed on the surface of the ecm33Δ strains, probably as a result of either abnormal trafficking of Als1p or prolonged ALS1 gene expression. Furthermore, we found no evidence that Ecm33p by itself was able to mediate adherence or invasion of oral epithelial cells.
The ecm33Δ mutants also had diminished capacity to damage oral epithelial cells. Because endocytosis is required for C. albicans to damage endothelial cells and FaDu oral epithelial cells (8, 20), the decreased host cell damage caused by the ecm33Δ mutants is likely due in part to the reduced endocytosis of these strains. It is also possible that the ecm33Δ mutants have reduced secretion of lytic enzymes, such as secreted aspartyl proteases and phospholipases (12, 13, 26), which contributed to the host cell damage defect of these mutants.
A notable finding was that the phenotype of strains that still possessed one functional copy of ECM33 was much closer to the phenotype of the strain that had no functional ECM33 than to phenotype of the strains containing two copies of ECM33. Previous Northern blot analysis of ECM33 transcript levels in the various strains confirmed that strains containing one intact copy of ECM33 expressed approximately 50% less ECM33 mRNA than did strains containing two intact copies of ECM33 (16). Although the exact amount of Ecm33p expressed by these stains is currently unknown, it is likely that at least some of this protein is expressed by strains containing a single functional copy of ECM33. Therefore, there appears to be a critical threshold of cellular Ecm33p content. When the amount of Ecm33p drops below this threshold, there is a significant alteration in cell wall architecture and growth rate. These changes result in a reduction in virulence-related traits, including the capacity of C. albicans to adhere to, invade, and damage host cells. Why Ecm33p is so vital for C. albicans pathogenicity is currently under investigation.
Acknowledgments
We thank Norma Solis and Quynh Trang Phan for help with tissue culture studies, Donald Sheppard for invaluable discussion, and the perinatal nurses at the Harbor-UCLA Medical Center Pediatric Clinical Research Center for collection of umbilical cords.
This work was supported in part by grants R01DE013974, DE017088, R01AI054928, R01AI19990, and MO1RR00425 from the U.S. National Institutes of Health and by BIO 2003-00030 from the Comision Interministerial de Ciencia y Tecnologýa (CYCIT, Spain), and CPGE 1010/2000 for Strategic Groups from Comunidad Autonoma de Madrid. Raquel Martinez-Lopez is the recipient of a fellowship from the Ministerio de Educacion y Ciencia de España.
REFERENCES
- 1.Bensen, E. S., S. G. Filler, and J. Berman. 2002. A forkhead transcription factor is important for true hyphal as well as yeast morphogenesis in Candida albicans. Eukaryot. Cell 1:787-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand, A., D. M. MacCallum, A. J. Brown, N. A. Gow, and F. C. Odds. 2004. Ectopic Expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot. Cell 3:900-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cawson, R. A., and K. C. Rajasingham. 1972. Ultrastructural features of the invasive phase of Candida albicans. Br. J. Dermatol. 87:435-443. [DOI] [PubMed] [Google Scholar]
- 4.Cheng, S., M. H. Nguyen, Z. Zhang, H. Jia, M. Handfield, and C. J. Clancy. 2003. Evaluation of the roles of four Candida albicans genes in virulence by using gene disruption strains that express URA3 from the native locus. Infect. Immun. 71:6101-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Groot, P. W., A. D. de Boer, J. Cunningham, H. L. Dekker, L. de Jong, K. J. Hellingwerf, C. de Koster, and F. M. Klis. 2004. Proteomic analysis of Candida albicans cell walls reveals covalently bound carbohydrate-active enzymes and adhesins. Eukaryot. Cell 3:955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards, J. E., Jr. 2000. Candida species, p. 2656-2674. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 5th ed. Churchill Livingston, Philadelphia, Pa.
- 7.Eversole, L. R., P. A. Reichart, G. Ficarra, A. Schmidt-Westhausen, P. Romagnoli, and N. Pimpinelli. 1997. Oral keratinocyte immune responses in HIV-associated candidiasis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodont. 84:372-380. [DOI] [PubMed] [Google Scholar]
- 8.Filler, S. G., J. N. Swerdloff, C. Hobbs, and P. M. Luckett. 1995. Penetration and damage of endothelial cells by Candida albicans. Infect. Immun. 63:976-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu, Y., A. S. Ibrahim, D. C. Sheppard, Y. C. Chen, S. W. French, J. E. Cutler, S. G. Filler, and J. E. Edwards. 2002. Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol. Microbiol. 44:61-72. [DOI] [PubMed] [Google Scholar]
- 11.Hoyer, L. L., S. Scherer, A. R. Shatzman, and G. P. Livi. 1995. Candida albicans ALS1: domains related to a Saccharomyces cerevisiae sexual agglutinin separated by a repeating motif. Mol. Microbiol. 15:39-54. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim, A. S., S. G. Filler, D. Sanglard, J. E. Edwards, Jr., and B. Hube. 1998. Secreted aspartyl proteinases and interactions of Candida albicans with human endothelial cells. Infect. Immun. 66:3003-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim, A. S., F. Mirbod, S. G. Filler, Y. Banno, G. T. Cole, Y. Kitajima, J. E. Edwards, Jr., Y. Nozawa, and M. A. Ghannoum. 1995. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect. Immun. 63:1993-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamai, Y., M. Kubota, T. Hosokawa, T. Fukuoka, and S. G. Filler. 2001. New model of oropharyngeal candidiasis in mice. Antimicrob. Agents Chemother. 45:3195-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Lopez, R., L. Monteoliva, R. Diez-Orejas, C. Nombela, and C. Gil. 2004. The GPI-anchored protein CaEcm33p is required for cell wall integrity, morphogenesis and virulence in Candida albicans. Microbiology 150:3341-3354. [DOI] [PubMed] [Google Scholar]
- 17.Miret, J. J., A. J. Solari, P. A. Barderi, and S. H. Goldemberg. 1992. Polyamines and cell wall organization in Saccharomyces cerevisiae. Yeast 8:1033-1041. [DOI] [PubMed] [Google Scholar]
- 18.Montes, L. F., and W. H. Wilborn. 1968. Ultrastructural features of host-parasite relationship in oral candidiasis. J. Bacteriol. 96:1349-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardo, M., L. Monteoliva, P. Vazquez, R. Martinez, G. Molero, C. Nombela, and C. Gil. 2004. PST1 and ECM33 encode two yeast cell surface GPI proteins important for cell wall integrity. Microbiology 150:4157-4170. [DOI] [PubMed] [Google Scholar]
- 20.Park, H., C. L. Myers, D. C. Sheppard, Q. T. Phan, A. A. Sanchez, J. E. Edwards, Jr., and S. G. Filler. 2005. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell. Microbiol. 7:499-510. [DOI] [PubMed] [Google Scholar]
- 21.Phan, Q. T., P. H. Belanger, and S. G. Filler. 2000. Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect. Immun. 68:3485-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan, Q. T., R. A. Fratti, N. V. Prasadarao, J. E. Edwards, Jr., and S. G. Filler. 2005. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J. Biol. Chem. 280:10455-10461. [DOI] [PubMed] [Google Scholar]
- 23.Reichart, P. A., H. P. Philipsen, A. Schmidt-Westhausen, and L. P. Samaranayake. 1995. Pseudomembranous oral candidiasis in HIV infection: ultrastructural findings. J. Oral Pathol. Med. 24:276-281. [DOI] [PubMed] [Google Scholar]
- 24.Rieg, G., Y. Fu, A. S. Ibrahim, X. Zhou, S. G. Filler, and J. E. Edwards, Jr. 1999. Unanticipated heterogeneity in growth rate and virulence among Candida albicans AAF1 null mutants. Infect. Immun. 67:3193-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez, A. A., D. A. Johnston, C. Myers, J. E. Edwards, Jr., A. P. Mitchell, and S. G. Filler. 2004. Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro. Infect. Immun. 72:598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaller, M., H. C. Korting, W. Schafer, J. Bastert, W. Chen, and B. Hube. 1999. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol. Microbiol. 34:169-180. [DOI] [PubMed] [Google Scholar]
- 27.Sheppard, D. C., M. R. Yeaman, W. H. Welch, Q. T. Phan, Y. Fu, A. S. Ibrahim, S. G. Filler, M. Zhang, A. J. Waring, and J. E. Edwards, Jr. 2004. Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 279:30840-30849. [DOI] [PubMed] [Google Scholar]
- 28.Staab, J. F., and P. Sundstrom. 2003. URA3 as a selectable marker for disruption and virulence assessment of Candida albicans genes. Trends Microbiol. 11:69-73. [DOI] [PubMed] [Google Scholar]
- 29.Sundstrom, P., J. E. Cutler, and J. F. Staab. 2002. Reevaluation of the role of HWP1 in systemic candidiasis by use of Candida albicans strains with selectable marker URA3 targeted to the ENO1 locus. Infect. Immun. 70:3281-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]