Abstract
Separation of an extract of corpora cardiaca from the protea beetle, Trichostetha fascicularis, by single-step RP (reverse-phase)-HPLC and monitoring of tryptophan fluorescence resulted in two distinctive peaks, the material of which mobilized proline and carbohydrates in a bioassay performed using the beetle. Material from one of these peaks was; however, inactive in the classical bioassays of locusts and cockroaches that are used for detecting peptides belonging to the AKH (adipokinetic hormone) family. After enzymatically deblocking the N-terminal pyroglutamic acid (pGlu) residue in the peptide material and sequencing by Edman degradation, a partial sequence was obtained: (pGlu)-Ile-Asn-Met-Thr-Xaa-Gly-Trp. The complete sequence was deduced from ESI-MSn (electrospray ionization multi-stage-MS); position six was identified as a phosphothreonine residue and the C-terminus is amidated. The peptide, code-named Trifa-CC, was chemically synthesized and used in confirmatory experiments to show that the primary structure had been correctly assigned. To our knowledge, this is the first report of a phosphorylated invertebrate neuropeptide. Synthetic Trifa-CC co-elutes with the natural peptide, found in the gland of the protea beetle, after RP-HPLC. Moreover, the natural peptide can be dephosphorylated by alkaline phosphatase and the product of that reaction has the same retention time as a synthetic nonphosphorylated octapeptide which has the same sequence as Trifa-CC. Finally, synthetic Trifa-CC has hypertrehalosaemic and hyperprolinaemic biological activity in the protea beetle, but even high concentrations of synthetic Trifa-CC are inactive in locusts and cockroaches. Hence, the correct peptide structure has been assigned. Trifa-CC of the protea beetle is an unusual member of the AKH family that is unique in its post-translational modification. Since it increases the concentration of carbohydrates and proline in the haemolymph when injected into the protea beetle, and since these substrates are also used during flight, we hypothesize that Trifa-CC controls the mobilization of these metabolites in the protea beetle.
Keywords: adipokinetic hormone (AKH), arthropod, neuropeptide, phosphopeptide, phosphothreonine, protea beetle
Abbreviations: AKH, adipokinetic hormone; CID, collision-induced dissociation; DCM, dichloromethane; ESI-MSn, electrospray ionization multi-stage-MS (e.g. MS3 denotes three stages); Fmoc, fluoren-9-ylmethoxycarbonyl; HrTH, hypertrehalosaemic hormone; MALDI–TOF, matrix-assisted laser-desorption ionization–time-of-flight; MBHA, 4-methylbenzylhydrylamine; NMP, N-methylpyrrolidinone; pGlu, py4roglutamic acid; PTH, phenylthiohydantoin; RP, reverse phase; RPCH, red pigment-concentrating hormone; TFA, trifluoroacetic acid
INTRODUCTION
By analogy to the well known hypothalamo-hypophyseal neuroendocrine system of vertebrates, arthropods have very similar neuroendocrine systems, e.g. the X-organ sinus-gland complex in crustacean eyestalks and the retrocerebral corpora cardiaca in insects. In these neurosecretory organs both arthropod groups produce inter alia small peptide hormones which belong to the same peptide family, the AKH (adipokinetic hormone)/RPCH (red pigment-concentrating hormone) family. This family is named after the first fully characterized members and their most prominent functions (reviewed in [1–3]). These are the aggregation of pigment in the epidermal cells of crustaceans by RPCHs, whereas the AKHs in insects regulate the levels of circulating metabolites (lipids, carbohydrates and proline) by activating phosphorylases or lipases in the fat body cell (reviewed in [4,5]). To date, only one octapeptide family member, denoted Panbo-RPCH, has been identified in a relatively large number of crustaceans examined (reviewed in [3,6]), whereas more than 36 close analogues (isoforms), including Panbo-RPCH [7], have been elucidated from all major orders of insects (reviewed in [2,4,8]), including the newly discovered order Mantophasmatodea [9]. The members of this peptide family are structurally characterized by a chain length of eight to 10 amino acids, aromatic amino acids at positions four (phenylalanine or tyrosine) and eight (tryptophan), and by post-translational modifications that include a pyroglutamic acid (pGlu) residue at the N-terminus that is genetically coded for as glutamine, and a carboxyamide at the C-terminus; the amide group is contributed from a glycine-extended AKH via the action of a peptidylglycine-α-amidating mono-oxygenase [10]. In addition to these general modifications that characterize each member of the AKH/RPCH family, corpora cardiaca of the stick insect synthesizes a glycosylated AKH, denoted Carma-HrTH (hypertrehalosaemic hormone)-I [11]. This modification is unique because it is not the common O-glycosylation of serine or threonine, or N-glycosylation of asparagine residues, instead the hexose is thought to be linked by C-glycosylation to the C-2 atom of the indole ring of tryptophan (as shown to occur in human ribonuclease [12]). Another, as yet unidentified, modification for an AKH member isolated from the corpora cardiaca of cicadas has also been postulated independently by three research groups [13–15].
In the present study, we have isolated and identified a novel member of the AKH/RPCH family from the corpora cardiaca of the protea beetle Trichostetha fascicularis. This novel AKH is an octapeptide with a unique modification at position six, i.e. a phosphorylated threonine residue. Moreover, this peptide does not fit the classical structural scheme of AKH peptides (there is no aromatic amino acid at position four) and hence, it is devoid of biological activity in locusts and cockroaches (insects that are typically used for demonstrating the functional relevance of an AKH). However, the novel peptide is active in the protea beetle itself and apparently controls carbohydrate, and mainly, proline concentrations; both of these substrates act as important fuels during flight in this species.
EXPERIMENTAL
Insects
Adult specimens from both sexes of T. fascicularis were collected mostly from flowering heads of the oleander-leaved protea, Protea neriifolia, at Kirstenbosch National Botanical Gardens, Cape Town, South Africa, in the austral summers of 2002 to 2004 during October to March. The beetles were used for isolation of peptides and for bioassays (as described below). The insects were kept in captivity in the same conditions as described previously for keeping the fruit beetle, Pachnoda sinuata [16], with water and fruit ad libitum together with flowering twigs of various gum trees and proteas of which the protea beetles ate the stamen. For additional heterologous bioassays, adult males were used: 14–25-day-old migratory locusts (Locusta migratoria), American cockroaches (Periplaneta americana) and P. sinuata of unspecified age; rearing and maintenance conditions of locust, cockroach and fruit beetle colonies are described elsewhere [16–18].
Isolation of corpora cardiaca peptides
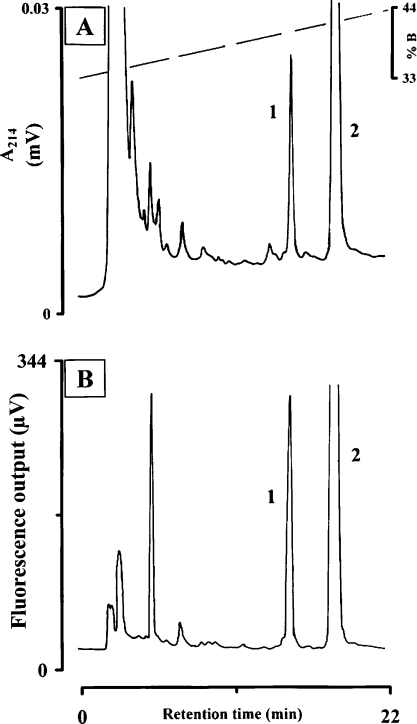
Corpora cardiaca were dissected in to 80% methanol immediately after arrival of protea beetles in the laboratory and were frozen at −25 °C. Methanolic extracts were prepared in batches of 10–45 glands as described previously [19]. Gland extracts were applied to a Nucleosil 100 C-18 column for RP (reverse phase)-HPLC using equipment as described previously ([20]; see Figure 1). Fractions displaying distinctive UV (214 nm) and/or fluorescence (excitation 276 nm, emission 350 nm) peaks were collected manually and were used either for biological assays or for determination of the primary structure (as described below).
Figure 1. Isolation of neuropeptides from corpora cardiaca of T. fascicularis.
Analysis was performed on a Nucleosil 100 C-18 column eluted with a linear gradient of 0.11% (v/v) TFA (solvent A) and 0.1% (v/v) TFA in 60% acetonitrile (solvent B). The gradient ran from 33 to 53% B within 40 min at a flow rate of 1 ml·min−1. A methanolic extract of 10 pairs of corpora cardiaca from T. fascicularis was injected and (A) UV absorbance at 214 nm and (B) fluorescence at 276 nm (excitation) and 350 nm (emission) were recorded.
Sequence determination via MS and Edman degradation
HPLC/MS analysis was performed using an LCQ mass spectrometer equipped with an ESI (electrospray ionization) ion source (Thermo Electron, San Jose, CA, U.S.A.) operated at 4.2 kV. The dried material of the purified active peak eluting at 14.2 min (see Figure 1) after RP-HPLC and representing 20 pair-equivalents of corpora cardiaca from T. fascicularis was dissolved in 100 μl of aqueous 0.04% TFA (trifluoroacetic acid); 5 μl of this solution was repeatedly injected into a 150 mm×1 mm ID RP-C8 Symmetry column (Waters, Milford, U.S.A.). An elution gradient from 28 to 50% B (solvent A, 0.04% TFA in water; solvent B, 0.04% TFA in acetonitrile) was used at a flow rate of 50 μl·min−1 within 8 min.
The primary sequence of the peptide eluting from the RP-HPLC column at 14.2 min (see Figure 1) was deduced using two different methods. First an aliquot of the peptide from 45 pair-equivalents of corpora cardiaca was enzymatically deblocked using L-pyroglutamate aminopeptidase as described previously [21]. The deblocked peptide was subsequently separated from the undigested peptide by RP-HPLC (as described above; gradient 33 to 53% in B for 40 min). Under the conditions employed, approx. 90% of the peptide material was deblocked. The deblocked material was spotted on to a polybrene-coated glass-fibre disc and subjected to automated Edman degradation (Model 477A; Applied Biosystem, Foster City, CA, U.S.A.). The sequencer was connected to an online PTH (phenylthiohydantoin) amino acid analyser (Model 120 A; ABI). Additional sequence information was deduced from a series of ESI-MSn (multi-stage MS; e.g. MS3 denotes three stages) experiments carried out on the detected (M+H)+ ion and its product ions; the ESI-MSn experiments were performed using an ion-trap mass spectrometer as described previously [22].
Synthetic peptides
The novel peptide, which we refer to as Trifa-CC, was synthesized on an Applied Bioystems 433 synthesizer using Fmoc (9-fluorenylmethoxycarbonyl) chemistry. Fmoc-L-amino acids and Rink amide MBHA (4-methylbenzylhydrylamine) resins were purchased from Novabiochem (EMD Biosciences, Inc., La Jolla, CA, U.S.A.). Standard side-chain protecting groups were incorporated during the synthesis except for Thr6, which was left unprotected to allow for post-synthesis incorporation of a phosphate group. After the completion of a 0.1 mM synthesis, a portion of resin was set aside for phosphorylation. Briefly, tetrazole (578 μl of 0.45 M tetrazole in acetronitrile; Fluka/Sigma–Aldrich) was dried under N2, then dissolved in 300 μl of NMP (N-methylpyrrolidinone; FisherBiotech) and added to the dried peptide-resin (75 mg). To initiate phosphorylation of the unprotected threonine, 112 μl of di-t-butyl diethylphosphoramidite (Sigma–Aldrich) was added, and the mixture was incubated overnight in a rotary mixer at room temperature. Subsequently, the sample was placed in a 0.8 mm×4 mm disposable column (Poly-Prep Chromatography Columns, Bio-Rad, Hercules, CA, U.S.A.) and washed with 15 ml of NMP. To oxidize the phosphite ester to its final phosphate form, the resin was resuspended in 0.5 ml of NMP, then transferred to a small screw-top glass container to which 100 μl of t-butyl hydroperoxide (Sigma–Aldrich) was added. After mixing for 5 min, the resin was allowed to settle and the supernatant was removed. t-Butyl hydroperoxide was added a second time (100 μl of t-butyl hydroperoxide+200 μl of NMP) and mixed for 1 h. The resin was transferred on to a disposable column and washed in succession with 15 ml of NMP, 15 ml of methanol, and 10 ml of DCM (dichloromethane), then dried under N2.
In addition, the nonphosphorylated Trifa-CC peptide and another peptide, L. migratoria hypertrehalosaemic peptide (Locmi-HrTH) with the sequence pEVTFSRDWSPamide [23], were also synthesized using conventional Fmoc chemistry. Each resin-peptide was cleaved and unprotected for 4 h in reagent K, a cocktail containing 5% phenol (Sigma–Aldrich), 5% HPLC-grade water, 5% thioanisole (Fluka) and 2.5% dithioethane (Fluka) in TFA (FisherBiotech) [24]. After removing the resin from the reaction mixture by filtration, the peptide was precipitated in cold t-butylmethyl ether (Fisherbiotech), followed by repeated ether washes and air-drying. Peptides were subsequently purified on a conventional C-18 RP-HPLC column using a linear gradient of acetonitrile from 10 to 80% for 70 min and a flow rate of 0.5 ml·min−1; both mobile phases contained 0.05% TFA. The desired peak was identified using a Bruker MALDI-TOF (matrix-assisted laser-desorption ionization–time-of-flight) mass spectrometer. The peptide denoted Melme-CC (pELNYSPDW amide [25]) was a gift from Novabiochem.
Dephosphorylation of native peptide
Native phosphorylated peptide, Trifa-CC, purified from 15 pair-equivalents of corpora cardiaca of T. fascicularis, was used as the substrate for alkaline phosphatase in order to show unequivocally that the peptide was phosphorylated. In brief, the native peptide was incubated in 50 μl of incubation mixture which contained Tris buffer (100 mM, pH 9.5) and approx. 1 m-unit of alkaline phosphatase. Immediately after combining the peptide and the incubation mixture, an aliquot of 25 μl was removed and added to 30 μl of methanol; the aliquot was centrifuged at 11000 g for 3 min at room temperature and the supernatant was processed by RP-HPLC (as described above). The remaining 25 μl of the peptide-plus-alkaline phosphatase mixture was further incubated at room temperature for 90 min and then prepared for RP-HPLC. A pilot experiment in which 60 pmol of synthetic Trifa-CC was incubated with alkaline phosphatase had shown, by removal of an aliquot for RP-HPLC every 15 min, that dephosphorylation was complete after approx. 60–90 min (results not shown).
Biological assays
Lipid and carbohydrate mobilization in L. migratoria and P. americana respectively, was measured as outlined elsewhere [26]. Changes in the concentrations of the two amino acids proline and alanine in the haemolymph of P. sinuata were determined as outlined previously [16]. Conspecific assays were performed as follows: the night before experimentation was started, protea beetles were kept individually at room temperature (25±2 °C) in small plastic containers (50 ml) which contained a piece of tissue paper soaked with tap water. Before injection of the appropriate test solution and 90 min thereafter, a 1 μl sample of haemolymph was taken from the neck membrane of the beetle with a microcapillary device to determine the concentration of total carbohydrates, lipids or the free amino acids proline and alanine. Total lipids were measured as described in [27], as vanillin-positive material; total carbohydrates were measured as described in [28] as anthrone-positive material; the concentrations of proline and alanine were also measured as described previously [16].
Flight experiments
Experiments using adult T. fascicularis were conducted in the laboratory in a temperature controlled room at 25 °C under a set of extra lights (800 W) which raised the temperature to 30 °C. Beetles were attached to a flight apparatus that was designed to allow tethered flight with generation of lift and is described in detail elsewhere [29]. Haemolymph was withdrawn, as described above, from individual beetles before flight (resting), after 1 min of flight (1 min-flown), and 60 min of rest following a flight of 1 min duration (1 min-flown-plus-60 min-resting). In another series of experiments, flight muscles were removed from resting, 1 min-flown and 1 min-flown-plus-60 min-resting beetles; tissues were immediately frozen in liquid nitrogen. Preparation of perchloric acid extracts and the determination of the concentrations of glycogen, proline and alanine were carried out as outlined previously [16].
RESULTS AND DISCUSSION
Peptide purification and biological activities
The UV and fluorescence profiles of a methanolic extract from 10 pairs of corpora cardiaca from T. fascicularis on an analytical RP-HPLC column are shown in Figure 1. Fluorescence (excitation at 276 nm and emission at 350 nm) targets for the presence of tryptophan, which is conserved in peptides of the AKH/RPCH family, were identified. There are two very distinct UV and fluorescent peaks in the chromatogram, namely at 14.2 min (peak 1) and at 17.1 min (peak 2; Figure 1). Peak 2 is the major peak (approx. 4-fold the area of peak 1) of a peptide with the same retention time as AKH material identified previously from other beetles [30]. Indeed, we established that the protea beetle peptide producing peak 2 is identical in structure and function to the octapeptide known as Melme-CC (results not shown). In the present study, we focus on the peptide that produces the smaller peak 1 that was eluted first (Figure 1), which we now name Trifa-CC. When such material was injected at a dose of one pair-equivalent of corpora cardiaca it significantly increased the proline and carbohydrate concentrations in the haemolymph of the protea beetle; it decreased the alanine level but did not affect the circulating levels of lipid (Table 1, upper panel). In heterologous assays, the material in the Trifa-CC peak did not affect the lipid or carbohydrate levels in the haemolymph of the recipient insects (locusts and cockroaches respectively) (Table 1, lower panel). However, when the fruit beetle P. sinuata was injected with one gland pair-equivalent of the Trifa-CC peak material, a significant hyperprolinaemic effect was elicited (Table 1, lower panel).
Table 1. Hypertrehalosaemic, adipokinetic and hyperprolinaemic activity of HPLC-purified peak 1 material from corpora cardiaca of protea beetles, T. fascicularis, in homologous and heterologous bioassays.
Insects were injected with 10 μl of the indicated substance, and haemolymph concentrations of total carbohydrates, total lipids, proline and alanine were determined. Means ± S.D. of changes in concentration between pre- and post-injection values are expressed as mg (carbohydrates, lipids) and μmol (proline, alanine) per ml of haemolymph. The significance of this difference is indicated by *P<0.05 (paired Student's t test); whereas the significance of the difference compared with the control injection is indicated by †P<0.05 (A11A with post-hoc Dunnett test). ‡pCC, pair-equivalent of corpora cardiaca.
| Concentration change | |||||||
|---|---|---|---|---|---|---|---|
| T. fascicularis | |||||||
| Treatment | n | Carbohydrates (mg·ml−1) | Lipids (mg·ml−1) | Proline (μmol·ml−1) | Alanine (μmol·ml−1) | ||
| Control (distilled water) | 6 | −3.4±3.5 | −1.1±0.9* | 1.9±10.3 | −0.1±0.8 | ||
| HPLC peak 1 | 5 | 4.5±2.8*† | −0.4±0.8 | 37.4±22.7*† | −2.4±2.0*† | ||
| Concentration change | |||||||
| P. americana | L. migratoria | P. sinuata | |||||
| Treatment | n | Carbohydrates (mg·ml−1) | n | Lipids (mg·ml−1) | n | Proline (μmol·ml−1) | Alanine (μmol·ml−1) |
| Control (distilled water) | 5 | 1.6±2.3 | 4 | −2.6±4.8 | 6 | −3.0±6.2 | −2.6±5.2 |
| 0.1 pCC‡ P. americana | 6 | 18.5±7.5*† | |||||
| 0.1 pCC‡ L. migratoria | 6 | 51.3±8.8*† | |||||
| 50 pmol of Melme-CC | 6 | 30.2±14.9*† | −8.4±3.7*† | ||||
| HPLC peak 1 | 8 | 0.7±4.7 | 9 | 0.8±3.8 | 10 | 12.6±9.1*† | −9.0±4.2*† |
To summarize, the corpora cardiaca of T. fascicularis contains two peptides which activate carbohydrate and proline metabolism in this species and in a related fruit beetle, P. sinuata. The most prominent of the two peptides in T. fascicularis is identical in primary structure to Melme-CC, a member of the AKH family that was first isolated from the cockchafer Melolontha melolontha [25]. The biological actions of the minor peptide (Trifa-CC) when assayed in the protea beetle and in the fruit beetle, clearly identify it as having AKH activity; yet surprisingly, Trifa-CC material was inactive in locusts and cockroaches, which are classical test animals used for detecting uncharacterized AKHs from other insect species [1]. These results could be interpreted in two ways: either Trifa-CC is not a member of the AKH peptide family or it is modified in key areas of the molecule so that it is not able to bind to the locust or cockroach AKH receptor and therefore could not elicit biological activity in these insects, despite an ability to interact with a receptor in T. fascicularis and P. sinuata to stimulate carbohydrate and proline metabolism. It was, therefore, paramount to elucidate the primary structure of Trifa-CC.
Peptide characterization and confirmation of structure
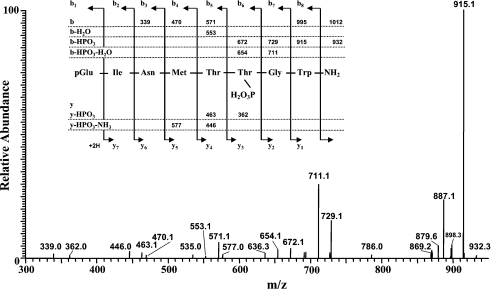
Purification of sufficient Trifa-CC material from glands of the protea beetle, followed by enzymatic deblocking of the N-terminal pyroglutamic acid residue and subsequent isolation of the des-pGlu peptide via RP-HPLC, resulted in homogeneous material for sequencing by automated Edman degradation. The sequence analysis yielded the following sequence: (pmol are given in brackets), (pGlu)-Ile (96.9)-Asn (72.1)-Met (74.3)-Thr (20.5)-Xaa (0.0)-Gly (37.2)-Trp (12.0). At cycle five during the sequencing process (thus, position six of the peptide when the Nterminal pyroglutamic residue is taken into account), no recognizable PTH-amino acid was detected. It was decided to employ MS to accurately determine the molecular mass of the peptide and to elucidate its sequence. When Trifa-CC material was injected into the LCQ instrument, a peak with an m/z value of 1012.4 for (M+H)+ was detected after 2.3 min (data shown in Supplementary Figures 1S A and 1S B at http://www.BiochemJ.org/bj/393/bj3930705add.htm). In addition, an m/z ion having (MH−80)+=932.3 was observed (data shown in Supplementary Figure 1S B at http://www.BiochemJ.org/bj/393/bj3930705add.htm). This difference of 80 Da was the first indication that the parent ion may be phosphorylated and had lost the phosphate (PO3) group. When the (MH)+ ion at m/z 1012 was subjected to CID (collision-induced dissociation) in the ion-trap, the resulting MS2 spectrum revealed only a few diagnostic product ions for the assignment of a sequence, namely (MH−NH3)+=m/z 995.2, (MH−HPO3)+=m/z 932.4, (b8−HPO3)+=m/z 915.3 and b5=m/z 571.1 of the b series (data shown in Supplementary Figure 2S at http://www.BiochemJ.org/bj/393/bj3930705add.htm). The intensive product ion (MH−HPO3)+ at m/z 932.4 was selected for analogous CID processes as described above; the resulting CID MS3 spectrum (Figure 2) revealed sufficient b, b−HPO3, b−H2O and b−HPO3−H2O product ions including a few y−HPO3 and y−HPO3−NH3 ions to confirm the sequence assigned by Edman degradation and to complete the existing gap at position six, namely a phosphothreonine residue, pT, (see scheme as inset in Figure 2). Consequently, the novel peptide pEINMTpTGWamide was chemically synthesized and a number of experiments were performed to verify the correct assignment. A nonphosphorylated form of Trifa-CC (pEINMTTGWamide) was also chemically synthesized for inclusion in comparative experiments.
Figure 2. The CID ESI-MS3 spectrum of (MH−HPO3)+ at m/z=932.4.
The inset shows the sequence of the assigned peptide, denoted Trifa-CC, together with the theoretical calculated masses for diagnostic ions, which were observed in the MS3 mass spectrum.
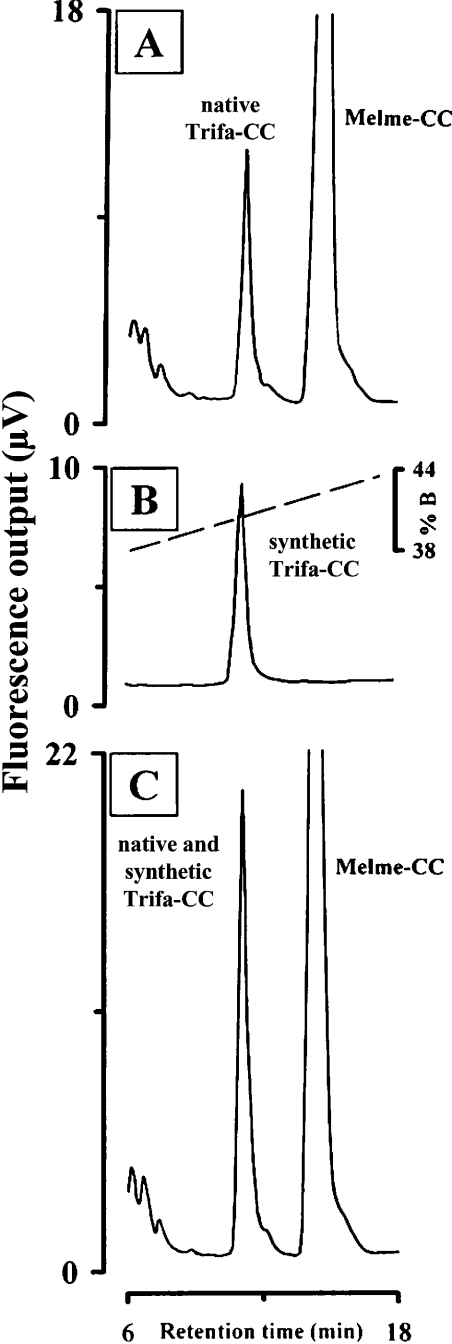
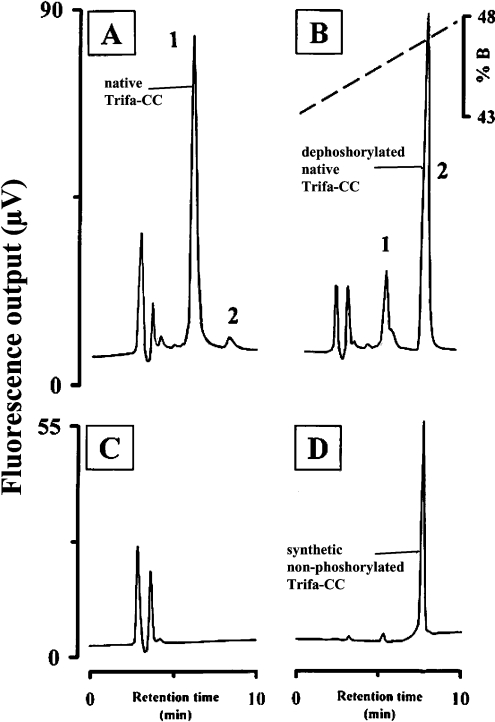
Comparison of the retention time of synthetic Trifa-CC with that of native Trifa-CC (i.e. peak 1 material from a crude corpus cardiacum extract of T. fascicularis) on the RP-HPLC column revealed that both eluted at the same time (Figure 3A versus 3B); co-injection of both materials resulted in co-elution as shown by an increase in the relative fluorescence at 10.9 min (peak 1 plus synthetic Trifa-CC; Figure 3C). In another series of experiments, alkaline phosphatase was used to demonstrate the dephosphorylation of synthetic Trifa-CC, as well as that of native Trifa-CC (peak 1 material). First, to demonstrate unequivocally that the alkaline phosphatase was active under the conditions employed, synthetic Trifa-CC was incubated with the enzyme for 90 min, the mixture was then applied to an RP-HPLC column and a new peak emerged after a retention time that corresponded to the synthetic nonphosphorylated Trifa-CC (results not shown). With this positive result to hand, native Trifa-CC was also exposed to alkaline phosphatase and aliquots of five gland-equivalents were removed at different time-points for separation by RP-HPLC. Figure 4 shows the results from the native peptide immediately after the start of the incubation with alkaline phosphatase (Figure 4A), at 90 min later (Figure 4B) and the chromatogram from a control incubation which contained only the alkaline phosphatase incubation medium and no peptide (Figure 4C). From the latter, no fluorescence peak was demonstrated, whereas the native phosphorylated material (peak 1 at 6.0 min) plus a very small fluorescence peak at 8.3 min (peak 2) were apparent shortly after mixing the native Trifa-CC peptide and the enzyme (Figure 4A). This peptide producing peak 2 had the same retention time as the synthetic nonphosphorylated form of Trifa-CC (Figure 4D) and increased proportionately in fluorescence (at the expense of peak 1 material; Figure 4B) as the incubation period increased. Hence, peak 2 in Figure 4 clearly represents the dephosphorylated Trifa-CC peptide and, thereby, confirms that the correct amino acid sequence was assigned to the minor peak of the T. fascicularis corpus cardiacum extract.
Figure 3. Co-elution of native and synthetic Trifa-CC.
Analyses were performed as described in Figure 1 except that the gradient ran from 35 to 55% B within 40 min at a flow rate of 1 ml·min−1. The fluorescence traces (276 nm excitation, 350 nm emission) are depicted for (A) a crude extract of five pairs of corpora cardiaca from T. fascicularis, (B) 3 pmol of synthetic Trifa-CC and (C) co-injection of a crude extract of five pairs of corpora cardiaca from T. fascicularis and 3 pmol of synthetic Trifa-CC.
Figure 4. Dephosphorylation of native Trifa-CC from T. fascicularis by alkaline phosphatase.
Analyses were performed as in Figure 1, except that the gradient ran from 43 to 53% B within 20 min at a flow rate of 1 ml·min−1. The fluorescence traces (276 nm excitation and 350 nm emission) are depicted for (A) an incubation of native Trifa-CC with alkaline phosphatase immediately after the start of reaction and (B) after 90 min of incubation. (C) The trace from injection of the incubation medium (with enzyme but without peptide). (D) The trace from an injection of 10 pmol of synthetic nonphosphorylated Trifa-CC.
Hence, we have unequivocally identified a novel octapeptide in the corpus cardacium of T. fascicularis which is unique on two accounts: (i) it does not possess all the classical characteristics of the AKH/RPCH family and (ii) it contains a third post-translational modification, a phosphorylation process which has not been found, to our knowledge, for any other small neuropeptides in vertebrates or invertebrates. We therefore discuss these two unique features and the implications thereof.
Although Trifa-CC has the classical blocked termini of AKH peptides (i.e. a pyroglutamic acid residue at the N-terminus and a carboxylamide at the C-terminus) and a tryptophan residue at position eight, it lacks the second aromatic amino acid which is typically found at position four in AKHs. Instead, in Trifa-CC, phenylalanine or tyrosine at position four is substituted by a methionine residue (Table 2). Considering the genetic code, a single point mutation cannot explain a change from the classical phenylalanine (UUU or UUC) to methione (AUG); at least two mutation steps have to be involved to effect this substitution. When compared with other known members of the AKH/RPCH family, the closest in primary structure are three members that differ from Trifa-CC by three amino acids: Micvi-CC found in a termite [31] and Phymo-AKH-III found in certain grasshoppers [32] have the typical phenylalanine (instead of methionine) at position four and show substitutions at positions six (proline instead of phosphothreonine) and seven (asparagine or tryptophan instead of glycine) (Table 2), whereas Psein-AKH which occurs mostly in damselflies [33] differs in positions two (valine instead of isoleucine), four (phenylalanine instead of methionine) and six (proline instead of phosphothreonine) (Table 2). Two other peptides, denoted Schgr-AKH-II and Grybi-AKH and mostly found in long- and short-horned grasshoppers [34], also differ in three positions (2, 4 and 5) but have a threonine, albeit nonphosphorylated, at position six (Table 2). It would be interesting to test whether the latter two peptides could be substrates for phosphorylation when incubated in vitro with a corpus cardacium extract from the protea beetle, since such an extract should have the necessary enzymatic machinery to enable phosphorylation.
Table 2. Comparison of the primary sequence of the novelel peptide Trifa-CC with those of AKHs that differ by three amino acid residues.
*Amino acid substitutions are indicated by bold letters. Note that the Thr residue is only phosphorylated in Trifa-CC. For references, see text.
| Peptide | Sequence | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trifa-CC | pE | I | N | M | T | pT | G | W | amide |
| Micvi-CC | pE | I | N | F | T | P | N | W | amide |
| Phymo-AKH-III | pE | I | N | F | T | P | W | W | amide |
| Psein-AKH | pE | V | N | F | T | P | G | W | amide |
| Schgr-AKH-II | pE | L | N | F | S | T | G | W | amide |
| Grybi-AKH | pE | V | N | F | S | T | G | W | amide |
The second unique modification in Trifa-CC is the phosphorylation of the threonine residue at position six. This phosphorylation was unequivocally demonstrated by the lack of a PTH-amino acid signal during Edman degradation sequencing, by detailed multiple ESI-MSn fragmentation of the isolated peptide, by co-elution of synthetic Trifa-CC with native Trifa-CC and, lastly, by specific dephosphorylation of isolated native Trifa-CC by alkaline phosphatase and co-elution of the product with synthetic nonphosphorylated Trifa-CC. The phosphorylation of proteins is well known and phosphoproteins are thought to represent up to approx. 20% of the proteome. Such phosphoproteins are very important regulators in cell biology, playing critical roles in cell signalling and the control of cellular processes which include cell cycle, apoptosis, growth and differentiation (reviewed in [35]). The post-translational phosphorylation of these proteins is achieved by protein kinases which are instrumental in signal transduction. The present work; however, is rather unique: Trifa-CC is not a large protein but a small peptide that is phosphorylated. Moreover, the mode of action of other members of the AKH family of peptides is proposed to proceed via binding to a G-protein-coupled receptor and, finally, involves various protein kinases (reviewed in [5]). We hypothesize that Trifa-CC also acts as a neurohormone and is responsible for the activation of a protein kinase on the one hand (like other AKHs), but, in addition, this neuropeptide itself must undergo phosphorylation by a protein kinase. At present, we do not know why such a complex regulatory system has evolved in the beetle T. fascicularis.
Biological parameters
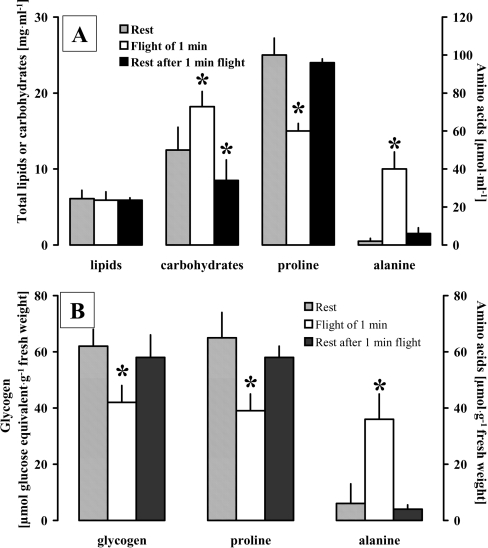
The next step was to investigate some functional aspects of this novel phosphorylated octapeptide, Trifa-CC, by performing conspecific bioassays and subjecting protea beetles to flight performance studies in order to establish which substrate is mainly used during such strenuous activity. Protea beetles were collected from the field and used in these experiments regardless of their gender; the age and physiological condition of these animals could not be established. Because wild populations cannot be physiologically standardized as easily as laboratory-reared insects, very variable concentrations of metabolites were measured in the haemolymph (see the high S.D.s calculated in Table 1 and Table 3). Despite this variability, the data are significant and unequivocal. Metabolic substrates in the haemolymph and flight muscles of protea beetles were determined after they performed a short 1 min flight under lift-producing conditions to demonstrate which substrate the beetle uses during flight. Whereas no significant change in the concentration of lipids occurred in the haemolymph, the concentrations of carbohydrates and alanine increased significantly during the flight period and levels of proline decreased (Figure 5). In the flight muscles, the concentrations of both glycogen and proline were significantly decreased, and the concentration of alanine increased during flight (Figure 5). The period of 60 min of rest after a 1 min flight was (for most substrates) sufficient time to reverse the changes that had occurred during flight, so that resting levels of the metabolites were reached again. Only the levels of carbohydrates in the haemolymph were significantly lower after this 60 min rest period than in resting beetles (Figure 5). Clearly, carbohydrates and proline are the major fuels to power the contraction of the flight muscles in the protea beetle; this is also the case in other beetles (reviewed in [36]). These two fuels have also previously been identified as flight substrates in P. sinuata where flight metabolism and its regulation by an AKH peptide was investigated in detail previously [36].
Table 3. Hypertrehalosaemic and hyperprolinaemic activity of synthetic Trifa-CC from T. fascicularis, a non-phosphorylated form of Trifa-CC and the locust neuropeptide Locmi-HrTH in the protea beetle.
Protea beetles, T. fascicularis, of both genders were injected with 10 μl of the indicated substance, and haemolymph concentrations of total carbohydrates, proline and alanine were determined. *, 2% solvent B (as described in the Experimental section). Values are means±S.D., the significance of the difference between values before and after injection is indicated by †P<0.05 (paired Student's t test); whereas the significance of the difference from the control injection is indicated by ‡P<0.05 (A11A with post-hoc Dunnett test).
| Concentration in haemolymph | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbohydrates (mg·ml−1) | Proline (μmol·ml−1) | Alanine (μmol·ml−1) | ||||||||
| Treatment | n | 0 min | 90 min | Concn. change | 0 min | 90 min | Concn. change | 0 min | 90 min | Concn. change |
| Control injection* | 11 | 10.5±5.4 | 10.4±5.2 | −0.1±0.7 | 90.2±6.0 | 90.2±5.5 | 0±1.5 | 4.5±2.2 | 4.5±2.1 | 0±0.5 |
| Trifa-CC (phosphorylated) | ||||||||||
| 10 pmol | 11 | 15.3±6.5 | 21.0±12.2 | 5.7±7.9†‡ | 92.0±14.5 | 110.3±11.6 | 18.3±8.0†‡ | 4.1±1.6 | 1.1±0.5 | −3.0±1.6†‡ |
| 2 pmol | 7 | 10.5±4.2 | 16.6±6.2 | 6.1±2.5†‡ | 89.2±10.4 | 95.9±10.0 | 6.7±2.0†‡ | 4.6±2.5 | 2.2±0.9 | −2.4±1.9†‡ |
| Trifa-CC (non-phosphorylated) | ||||||||||
| 10 pmol | 11 | 11.0±4.2 | 13.9±6.8 | 2.9±4.1†‡ | 90.2±10.2 | 101.9±10.1 | 11.7±6.4†‡ | 5.0±3.8 | 2.4±1.6 | −2.6±2.7†‡ |
| 2 pmol | 7 | 12.3±4.7 | 15.6±3.1 | 3.3±5.4 | 90.0±11.7 | 95.3±9.7 | 5.3±2.9† | 4.2±1.3 | 2.6±1.0 | −1.6±0.6† |
| Locmi-HrTH | ||||||||||
| 100 pmol | 8 | 4.6±3.0 | 4.7±3.6 | 0.1±1.8 | 91.2±10.2 | 94.3±11.8 | 3.1±3.1† | 4.5±1.4 | 3.0±1.0 | −1.5±1.0† |
| 10 pmol | 7 | 14.0±3.1 | 15.0±3.3 | 1.0±2.1 | 91.1±9.9 | 93.3±8.5 | 2.2±4.3 | 3.9±1.3 | 3.5±1.0 | −0.4±1.5 |
Figure 5. Changes in selected energy substrates during flight in T. fascicularis.
(A) Concentrations of total lipids, total carbohydrates and the amino acids proline and alanine in the haemolymph are given as means±S.D. during rest, after 1 min of flight and after 60 min of rest after 1 min of flight. (B) Concentrations of glycogen, proline and alanine in flight muscles are given as means±S.D. during rest, after 1 min of flight and after 60 min of rest following 1 min of flight. Significant differences: *, P<0.05 for flight and rest after flight, compared with rest; (A), paired t-test; (B), A11A with post-hoc Dunnett test.
Conspecific bioassays using Trifa-CC revealed the following in the current study: injection of various amounts of synthetic Trifa-CC into T. fascicularis resulted in an increase in the concentration of carbohydrates and proline in the haemolymph and a decrease in the concentration of alanine (Table 3). These changes were significantly different from those elicited by control injections of 2% solvent B (as used in RP-HPLC, because the synthetic peptide was dissolved in that medium; Table 3). The nonphosphorylated Trifa-CC peptide was also active in mobilizing carbohydrates and proline: the effect, however, was slightly weaker than when using phosphorylated Trifa-CC and only in one case (an increase of proline after injection of 10 pmol of nonphosphorylated Trifa-CC) was this significantly different from the phosphorylated peptide (P<0.05, ANOVA with post-hoc Fisher's LSD test). To ascertain that the protea beetle reacted specifically to the injected Trifa-CC peptides and that this change in metabolite concentration was not randomly caused by any small peptide, the decapeptide Locmi-HrTH was injected into a group of protea beetles. This peptide had no activity in T. fascicularis at a physiological dose of 10 pmol and was only slightly active (but not significantly so, with respect to control injections) when an unphysiologically high dose of 100 pmol was injected (Table 3). Synthetic Trifa-CC and its nonphosphorylated form were also assayed for biological activity in heterologous systems. Up to 500 pmol was injected into L. migratoria and P. americana, but neither lipids (in the locust) nor carbohydrates (in the cockroach) were elevated in the haemolymph (results not shown).
In previous studies of P. sinuata, the peptide Melme-CC (which is also present in the protea beetle in the current study) was shown to regulate proline metabolism via activation of a lipase, and the second messengers Ca2+ and cAMP are involved in this process [37]. In the present study using T. fascicularis, we show that the peptide Trifa-CC and its nonphosphorylated form are also able to increase proline concentrations; by deduction, this means that cAMP has been increased because it is the second messenger involved in this signalling pathway. It could be argued that Trifa-CC itself does not bind to a receptor but is first dephosphorylated before it can bind to a G-protein-coupled receptor and initiate the signalling cascade that ultimately results in the observed functional activity in T. fascicularis and P. sinuata. Tentatively, we argue differently. The time-course of cAMP activation in the fat-body of P. sinuata has been studied in detail and a maximal increase after AKH injection is measured after 1 min [38]. Assuming that the activation of cAMP occurs in a similar time-frame as for Trifa-CC, then it is most unlikely that sufficient dephosphorylation of Trifa-CC has taken place in this short time span. Therefore we are confident that the phosphorylated peptide, Trifa-CC, and not its dephosphorylated form, is primarily binding to an AKH receptor in the fat body of T. fascicularis. One cannot, of course, completely exclude the possiblity that circulating phosphatases may be present but even so, the half-life of Trifa-CC in the haemolymph of the protea beetle must be less than 1 min to be converted into the dephosphorylated, active form that binds to the receptor and activates cAMP. A new series of experiments in which physiological amounts of Trifa-CC are incubated in vitro with haemolymph may provide unequivocal results as to the rate of dephosphorylation of Trifa-CC.
The reality may be that Trifa-CC is responsible for the regulation of proline metabolism but why is Trifa-CC not mobilizing these metabolites in locusts and cockroaches? It has been shown clearly for a number of insects that AKH molecules that lack a phenylalanine at position four, having alanine or even the aromatic tryptophan instead, have no biological activity [39]. Thus the methionine at position four in Trifa-CC (and not the phosphothreonine at position six) is implicated in the reason why binding to the AKH receptor is prevented. Although methionine is a hydrophobic amino acid (as is phenylalanine), methionine contains sulphur and has a different shape to phenylalanine. This may have a decisive influence on the solution-structure of Trifa-CC which, in turn, is so important for receptor binding. All previous studies on the solution-conformation of AKH peptides using CD [40] or NMR [41] methods are in favour of a β-turn between residues 4–8, but are at variance with each other for the N-terminus where β-sheet, “extended structure” and “extended PII conformation” as defined in [42] have been proposed. The tightness of the β-turn is influenced by the presence of phenylalanine (tighter turn) or tyrosine (weaker turn) as revealed by conformational studies on a few AKH peptides using NMR constrained molecular dynamics [43]. Having methionine at this position may even result in no turn being formed. Since such a turn-conformation is essential for AKH receptor-binding in locusts and cockroaches [1], this may explain the absence of activity of Trifa-CC in these species.
In conclusion, the present study revealed that Trifa-CC and Melme-CC are, apparently, regulating the provision of fuels (carbohydrates and proline) in the protea beetle T. fascicularis. It is not surprising that two neuropeptides are designed to have the same task. This is quite common and there are even insects that are known to produce three AKHs with seemingly similar or identical actions [4]. To date, however, none of these peptides have been identified as being post-translationally modified by phosphorylation.
Online Data
Acknowledgments
The authors thank Dr Heather Marco (Department of Zoology, University of Cape Town) for improving the English text, Dr Roland Kellner (Merck KGaA, Darmstadt, Germany) for contributing the Edman sequencing data, Dr David King (Department of Molecular and Cellular Biology, University of California, Berkely, CA, U.S.A.) for invaluable logistical help (to K.D.C.) with the synthesis of the phosphorylated peptide and the curator of Kirstenbosch National Botanical Garden (Cape Town, South Africa) for permission to collect protea beetles. This study was financially supported by the National Research Foundation, Pretoria, South Africa (2053806) and by a staff award from the University of Cape Town (both to G.G.). Financial support came also via ENTU AV CR (AV0Z50070508) to P.S.
References
- 1.Gäde G. The revolution in insect neuropeptides illustrated by the adipokinetic hormone/red pigment-concentrating hormone family of peptides. Z. Naturforsch. 1996;51:607–617. doi: 10.1515/znc-1996-9-1001. [DOI] [PubMed] [Google Scholar]
- 2.Gäde G. The explosion of structural information on insect neuropeptides. In: Herz W., Kirby G. W., Moore R. E., Steglich W., Tamm C., editors. Progress in the Chemistry of Organic Natural Products. Wien: Springer; 1997. pp. 1–128. [PubMed] [Google Scholar]
- 3.Gäde G., Marco H. G. Structure, function and mode of action of select arthropod neuropeptides. In: Atta-ur-Rahmann, editor. Studies in Natural Product Chemistry (Bioactive Natural Products) Vol. 33. The Netherlands: Elsevier Science Publishers; 2005. pp. 69–139. [Google Scholar]
- 4.Gäde G. Regulation of intermediary metabolism and water balance of insects by neuropeptides. Annu. Rev. Entomol. 2004;49:93–113. doi: 10.1146/annurev.ento.49.061802.123354. [DOI] [PubMed] [Google Scholar]
- 5.Gäde G., Auerswald L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen. Comp. Endocrinol. 2003;132:10–20. doi: 10.1016/s0016-6480(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 6.Rao K. R. Crustacean pigmentary-effector hormones: chemistry and functions of RPCH, PDH, and related peptides. Am. Zool. 2001;41:364–379. [Google Scholar]
- 7.Gäde G., Auerswald L., Šimek P., Marco H. G., Kodrík D. Red pigment-concentrating hormone is not limited to crustaceans. Biochem. Biophys. Res. Commun. 2003;309:967–973. doi: 10.1016/j.bbrc.2003.08.107. [DOI] [PubMed] [Google Scholar]
- 8.Gäde G., Hoffmann K. H., Spring J. H. Hormonal regulation in insects: Facts, gaps, and future directions. Physiol. Rev. 1997;77:963–1032. doi: 10.1152/physrev.1997.77.4.963. [DOI] [PubMed] [Google Scholar]
- 9.Gäde G., Marco H. G., Šimek P., Marais E. The newly discovered insect order Mantophasmatodea contains a novel member of the adipokinetic hormone family of peptides. Biochem. Biophys. Res. Commun. 2005;330:598–603. doi: 10.1016/j.bbrc.2005.02.185. [DOI] [PubMed] [Google Scholar]
- 10.Rayne R. C., O'Shea M. Reconstitution of adipokinetic hormone synthesis in vitro indicates steps in prohormone processing. Eur. J. Biochem. 1994;219:781–789. doi: 10.1111/j.1432-1033.1994.tb18558.x. [DOI] [PubMed] [Google Scholar]
- 11.Gäde G., Kellner R., Rinehart K. L., Proefke M. L. A tryptophan-substituted member of the AKH/RPCH family isolated from a stick insect corpus cardiacum. Biochem. Biophys. Res. Commun. 1992;189:1303–1309. doi: 10.1016/0006-291x(92)90215-7. [DOI] [PubMed] [Google Scholar]
- 12.Hofsteenge J., Müller D. R., Debeer T., Löffler A., Richter W. J., Vliegenthart J. F. G. New-type of linkage between a carbohydrate and protein-C-glycosylation of a specific tryptophan residue in human RNase. Biochemistry. 1994;33:13524–13530. doi: 10.1021/bi00250a003. [DOI] [PubMed] [Google Scholar]
- 13.Gäde G., Janssens M. P. E. Cicadas contain novel members of the AKH/RPCH family peptides with hypertrehalosaemic activity. Biol. Chem. Hoppe Seyler. 1994;375:803–809. doi: 10.1515/bchm3.1994.375.12.803. [DOI] [PubMed] [Google Scholar]
- 14.Raina A., Pannell L., Kochansky J., Jaffe H. Primary structure of a novel neuropeptide isolated from the corpora cardiaca of periodical cicadas having adipokinetic and hypertrehalosemic activities. Insect Biochem. Mol. Biol. 1995;25:929–932. doi: 10.1016/0965-1748(95)00032-q. [DOI] [PubMed] [Google Scholar]
- 15.Veenstra J. A., Hagedorn H. H. Isolation of two AKH-related peptides from cicadas. Arch. Insect Biochem. 1995;29:391–396. doi: 10.1002/arch.940290406. [DOI] [PubMed] [Google Scholar]
- 16.Zebe E., Gäde G. Flight metabolism in the African fruit beetle, Pachnoda sinuata. J. Comp. Physiol. 1993;163:107–112. [Google Scholar]
- 17.Gäde G. Hyperglycaemia or hypertrehalosaemia? The effect of insect neuropeptides on haemolymph sugars. J. Insect Physiol. 1991;37:483–487. [Google Scholar]
- 18.Gäde G. Isolation and structure elucidation of neuropeptides of the AKH/RPCH family in long-horned grasshoppers (Ensifera) Biol. Chem. Hoppe Seyler. 1992;373:1169–1178. doi: 10.1515/bchm3.1992.373.2.1169. [DOI] [PubMed] [Google Scholar]
- 19.Gäde G., Goldsworthy G. J., Kegel G., Keller R. Single step purification of locust adipokinetic hormones I and II by reversed-phase high-performance liquid-chromatography, and amino-acid composition of the hormone II. Hoppe Seylers Z. Physiol. Chem. 1984;365:393–398. doi: 10.1515/bchm2.1984.365.1.393. [DOI] [PubMed] [Google Scholar]
- 20.Gäde G. Isolation of the hypertrehalosaemic factors I and II from the corpus cardiacum of the Indian stick insect, Carausius morosus, by reversed-phase high-performance liquid chromatography, and amino-acid composition of factor II. Biol. Chem. Hoppe Seyler. 1985;366:195–199. doi: 10.1515/bchm3.1985.366.1.195. [DOI] [PubMed] [Google Scholar]
- 21.Gäde G., Hilbich C., Beyreuther K., Rinehart K. L. Sequence analyses of two neuropeptides of the AKP/RPCH family from the lubber grasshopper, Romalea microptera. Peptides. 1988;9:681–688. doi: 10.1016/0196-9781(88)90107-6. [DOI] [PubMed] [Google Scholar]
- 22.Kodrík D., Socha R., Šimek P., Zemek R., Goldsworthy G. J. A new member of the AKH/RPCH family that stimulates locomotory activity in the firebug, Pyrrhocoris apterus (Heteroptera) Insect Biochem. Mol. Biol. 2000;30:489–498. doi: 10.1016/s0965-1748(00)00025-4. [DOI] [PubMed] [Google Scholar]
- 23.Siegert K. J. Locust corpora cardiaca contain an inactive adipokinetic hormone. FEBS Lett. 1999;447:237–240. doi: 10.1016/s0014-5793(99)00299-9. [DOI] [PubMed] [Google Scholar]
- 24.King D. S., Field C. G., Field G. B. A cleavage method which minimizes side reactions following Fmoc solid-phase peptide synthesis. Int. J. Pept. Protein Res. 1990;36:255–266. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 25.Gäde G. A unique charged tyrosine-containing member of the adipokinetic hormone red-pigment-concentrating hormone peptide family isolated and sequenced from two beetle species. Biochem. J. 1991;275:671–677. doi: 10.1042/bj2750671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gäde G. Further characteristics of adipokinetic and hyperglycaemic factor(s) of stick insects. J. Insect Physiol. 1980;26:351–360. [Google Scholar]
- 27.Zöllner N., Kirsch K. Über die quantitative Bestimmung von Lipoiden (Mikromethode) mittels der vielen natürlichen Lipoiden (allen bekannten Plasmalipoiden) gemeinsamen sulfophosphovanillin reaktion. Z. Ges. Exp. Med. 1962;135:545–561. [Google Scholar]
- 28.Spik G., Montreuil J. Deux causes d'erreur dans les dosages colorimetriques des oses neutres totaux. Bull. Soc. Chim. Biol. 1964;46:739–749. [PubMed] [Google Scholar]
- 29.Auerswald L., Schneider P., Gäde G. Proline powers pre-flight warm-up in the African fruit beetle Pachnoda sinuata (Cetoniinae) J. Exp. Biol. 1998;201:1651–1657. doi: 10.1242/jeb.201.10.1651. [DOI] [PubMed] [Google Scholar]
- 30.Gäde G., Lopata A., Kellner R., Rinehart K. L. Primary structures of neuropeptides isolated from the corpora cardiaca of various cetonid beetle species determined by pulsed-liquid phase sequencing and tandem fast-atom-bombardment mass-spectrometry. Biol. Chem. Hoppe-Seyler. 1992;373:133–142. doi: 10.1515/bchm3.1992.373.1.133. [DOI] [PubMed] [Google Scholar]
- 31.Liebrich W., Kellner R., Gäde G. Isolation and primary structures of neuropeptides of the AKH/RPCH family from various termite species. Peptides. 1995;16:559–564. doi: 10.1016/0196-9781(95)00012-9. [DOI] [PubMed] [Google Scholar]
- 32.Siegert K. J., Kellner R., Gäde G. A third active AKH is present in the pyrgomorphid grasshoppers Phymateus morbillosus and Dictyophorus spumans. Insect Biochem. Mol. Biol. 2000;30:1061–1067. doi: 10.1016/s0965-1748(00)00081-3. [DOI] [PubMed] [Google Scholar]
- 33.Gäde G., Marco H. G. The adipokinetic hormones of Odonata: A phylogenetic approach. J. Insect Physiol. 2005;51:333–341. doi: 10.1016/j.jinsphys.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Gäde G., Marco H. G., Desutter-Grandcolas L. A phylogenetic analysis of the adipokinetic neuropeptides of Ensifera. Physiol. Entomol. 2003;28:283–289. [Google Scholar]
- 35.Zeller M., König S. Mass spectrometric phosphorylation analysis. Anal. Bioanal. Chem. 2004;378:898–909. doi: 10.1007/s00216-003-2391-2. [DOI] [PubMed] [Google Scholar]
- 36.Gäde G., Auerswald L. Beetles' choice-proline for energy output: control by AKHs. Comp. Biochem. Physiol. 2002;132:117–129. doi: 10.1016/s1096-4959(01)00541-3. [DOI] [PubMed] [Google Scholar]
- 37.Auerswald L., Siegert K. J., Gäde G. Activation of triacylglycerol lipase in the fat body of a beetle by adipokinetic hormone. Insect Biochem. Mol. Biol. 2005;35:461–470. doi: 10.1016/j.ibmb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Auerswald L., Gäde G. Cyclic AMP mediates the elevation of proline by AKH peptides in the cetoniid beetle, Pachnoda sinuata. Biochim. Biophys. Acta. 2000;1495:78–89. doi: 10.1016/s0167-4889(99)00134-2. [DOI] [PubMed] [Google Scholar]
- 39.Gäde G., Hayes T. K. Structure-activity relationships for Periplaneta americana hypertrehalosemic hormone I: the importance of side chains and termini. Peptides. 1995;16:1173–1180. doi: 10.1016/0196-9781(95)02008-k. [DOI] [PubMed] [Google Scholar]
- 40.Cusinato O., Drake A. F., Gäde G., Goldsworthy G. J. The molecular conformations of representative arthropod adipokinetic peptides determined by circular dichroism spectroscopy. Insect Biochem. Mol. Biol. 1998;28:43–50. [Google Scholar]
- 41.Zubrzycki I. Z., Gäde G. Conformational study on a representative member of the AKH/RPCH neuropeptide family, Emp-AKH, in the presence of SDS micelle. Eur. J. Entomol. 1999;96:337–340. [Google Scholar]
- 42.Siligardi G., Drake A. F. The importance of extended conformations and, in particular, the P-II conformation for the molecular recognition of peptides. Biopolymers. 1995;37:281–292. doi: 10.1002/bip.360370406. [DOI] [PubMed] [Google Scholar]
- 43.Nair M. M., Jackson G. E., Gäde G. Conformational study of insect adipokinetic hormones using NMR constrained molecular dynamics. J. Comput. Aid. Mol. Des. 2001;15:259–270. doi: 10.1023/a:1008123604588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.