Abstract
The frequency of cytomegalovirus (CMV)-specific CD4+ T lymphocytes was determined in CMV-seropositive rhesus macaques with or without simian immunodeficiency virus (SIV) infection by using the sensitive assays of intracellular cytokine staining and gamma interferon ELISPOT. Both techniques yielded 3- to 1,000-fold-higher frequencies of CMV-specific CD4+ T lymphocytes than traditional proliferative limiting dilution assays. The median frequency of CMV-specific CD4+ T lymphocytes in 23 CMV-seropositive SIV-negative macaques was 0.63% (range, 0.16 to 5.8%). The majority of CMV-specific CD4+ T lymphocytes were CD95pos and CD27lo but expressed variable levels of CD45RA. A significant reduction (P < 0.05) in the frequency of CMV-specific CD4+ T lymphocytes was observed in pathogenic SIV-infected macaques but not in macaques infected with live attenuated strains of SIV. CMV-specific CD4+ T lymphocytes were not detected in six of nine pathogenic SIV-infected rhesus macaques. CMV DNA was detected in the plasma of four of six of these macaques but in no animal with detectable CMV-specific CD4+ T lymphocytes. In pathogenic SIV-infected macaques, loss of CMV-specific CD4+ T lymphocytes was not predicted by the severity of CD4+ T lymphocytopenia. Neither was it predicted by the pre-SIV infection frequencies of CD45RAneg or CCR5pos CMV-specific CD4+ T lymphocytes. However, the magnitude of activation, as evidenced by the intensity of CD40L expression on CMV-specific CD4+ T lymphocytes pre-SIV infection, was three- to sevenfold greater in the two macaques that subsequently lost these cells after SIV infection than in the two macaques that retained CMV-specific CD4+ T lymphocytes post-SIV infection. Future longitudinal studies with these techniques will facilitate the study of CMV pathogenesis in AIDS.
Although the widespread use of highly active antiretroviral therapy has resulted in a significant decline in the incidence of all opportunistic infections in AIDS, cytomegalovirus (CMV) continues to be an important pathogen in immunosuppressed human immunodeficiency virus (HIV)-infected individuals (20). CMV-specific immune responses, particularly CD8+ cytotoxic T lymphocytes (CTL), are essential for preventing reactivation of latent CMV infection in immunosuppressed individuals and for recovery from CMV disease (26, 42). Since the host immune response is primarily responsible for containing CMV replication and preventing CMV disease, an understanding of how lentiviruses target CMV-specific immunity is likely to provide useful insights into the pathogenesis of opportunistic infections in AIDS.
The SIV-rhesus macaque animal model is currently the leading animal model of AIDS and recapitulates many features of human HIV infection, including the occurrence of CMV as a major opportunistic viral pathogen (29). There are many similarities in the natural history and course of simian and human CMV infection. CMV infection is widely prevalent in captive rhesus macaques; maternal antibodies to CMV wane around 1 year of age, and seroconversion following natural infection occurs soon thereafter (15, 39). Moreover, CMV infection is asymptomatic in immunocompetent rhesus macaques (32), and CMV disease has only been reported in immunosuppressed animals (3).
Since activated CD4+ T lymphocytes are specifically targeted and depleted during the course of HIV or SIV infection (10, 38), loss of CMV-specific CD4+ T lymphocytes is likely to be an important mechanism underlying the occurrence of CMV disease in AIDS. The requirement of CD4+ T lymphocytes for effective viral clearance has been elegantly demonstrated in the murine animal model for a wide variety of pathogens, and several mechanisms have been described. One important mechanism is the help provided by CD4+ T lymphocytes for the generation or maintenance of CTL. Primary CTL responses to herpes simplex virus and influenza A virus are not generated in the absence of CD4+ T lymphocytes (12, 33). In chronic lymphocytic choriomeningitis virus infection, although primary CTL responses are unaffected in the absence of CD4+ T lymphocytes, memory CTL responses gradually decline and lead to recrudescence of viremia (4, 22, 40). In this model, the loss of memory CTL responses results both from deletion of CTL, as well as from surviving memory CTL being rendered functionally unresponsive (44). A direct antiviral effector function of CD4+ T lymphocytes has also been described (6, 21). In murine gammaherpesvirus infection, loss of control of virus replication in CD4-deficient mice is not associated with decline in the number or functionality of murine gammaherpesvirus-specific CTL (30). Instead, the antiviral effect appears to be mediated by a B-cell-independent, direct effector function of CD4+ T lymphocytes which is dependent on gamma interferon (IFN-γ) production (6). Finally, CD4+ T lymphocytes appear to be required for the production of virus-specific neutralizing antibodies, as in the instance of vesicular stomatitis virus infection (21). In humans, evidence for a protective role of CD4+ T lymphocytes comes from studies in CMV and HIV infection. In bone marrow transplant recipients, maintenance of adoptively transferred CMV CTL is dependent on the presence of an intact CMV-specific CD4+ T lymphocyte response (42). Strong CD4 T helper responses to p55gag antigen are detected in HIV-infected long-term nonprogressors and are associated with control of HIV viremia (27).
The development of sensitive techniques such as enzyme-linked immunospot (ELISPOT) and flow cytometric detection of intracellular cytokines (ICC) to delineate antigen-specific cells at the single cell level has provided new insights into pathogen-specific immune responses (23). In the present study, we adapted these techniques to determine the nature of the CMV-specific CD4+ T lymphocyte response in rhesus macaques with or without SIV infection. Our results show that the ELISPOT and ICC assays are 3- to 1,000-fold more sensitive than the traditional proliferative limiting dilution assay (LDA) for measurement of CMV-specific CD4+ T lymphocytes. Rhesus macaques infected with pathogenic SIV for a median duration of 4.5 months had significantly decreased frequencies of CMV-specific CD4+ T lymphocytes in their peripheral blood. In these animals, loss of CMV-specific CD4+ T lymphocytes was not predicted by the extent of CD4+ T lymphocytopenia. Instead, it was associated with increased CMV and SIV viral loads. Pre-SIV infection frequencies of CD45RAneg or CCR5pos CMV-specific CD4+ T lymphocytes did not predict their depletion subsequent to SIV infection. However, the extent of activation after in vitro antigen-specific stimulation, as manifested by the intensity of CD40L expression on CMV-specific CD4+ T lymphocytes, was greater in macaques that subsequently lost these cells after SIV infection.
MATERIALS AND METHODS
Animals
Rhesus macaques with and without SIV infection were included in the study. Animals were housed in the specific pathogen free or conventional colony of the New England Regional Primate Research Center in accordance with federal and institutional guidelines for animal care (1). Rhesus macaques were screened for naturally acquired CMV infection by testing sera for antibodies to rhesus CMV. In SIV-infected rhesus macaques with undetectable antibodies to CMV, sera archived prior to SIV infection were tested in order to distinguish CMV-seronegative animals from CMV-seropositive animals that had lost CMV antibodies after SIV infection. Among SIV-infected macaques, only CMV-seropositive animals were included in the study.
CMV ELISA.
Serum from rhesus macaques was screened for antibodies reacting to rhesus CMV by enzyme-linked immunoassay (ELISA). Rhesus CMV antigen coating the ELISA plates was prepared from a previously described clinical isolate of rhesus CMV (15). CMV-infected primary monkey fibroblast cells that had progressed to complete cytopathic effect were harvested and subjected to low-speed centrifugation at 1,500 rpm for 10 min to eliminate cell debris. The supernatant was filtered through a 0.45-μm-pore-size filter and subjected to ultracentrifugation at 17,000 rpm for 3 h at 4°C. The virus pellet was resuspended in buffer TNE (20 mM Tris, pH 7.5; 100 mM NaCl; 1 mM EDTA) and purified by chromatography on a 10-ml column of Sepharose 4B (CL-4B-200; Sigma, St. Louis, Mo.) at room temperature with buffer TNE as the column buffer. The eluted virus was collected in microcentrifuge tubes and spun cold in a microcentrifuge at 13,000 rpm for 1 h. The virus pellet was resuspended in 150 μl of 0.5% Triton X-100 in phosphate-buffered saline (PBS), and the protein concentration was determined on a spectrophotometer. The optimal concentration of antigen for coating the ELISA plate was determined by titration with a positive control serum.
Test serum was added to antigen coated wells and incubated for 1 h at room temperature. Wells were washed with PBS containing 0.05% Tween 20 and subsequently incubated with alkaline phosphatase-labeled antibody to the Fc fragment of human immunoglobulin G (IgG; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) for 1 h at room temperature. After being washed, the wells were incubated with substrate solution (p-nitrophenylphosphate in diethanolamine) in the dark for 30 min at room temperature. The reaction was stopped by addition of 5% EDTA, and the absorbance at 410 nm was determined on an ELISA reader.
Antibodies.
Monoclonal antibodies (MAb) used for costimulation in the ICC assays included antihuman CD28 MAb (clone 28.2; BD Pharmingen, San Diego, Calif.) and antihuman CD49d MAb (clone 9F10; Pharmingen), each at a final concentration of 10 μg/ml. Affinity-purified antibody F(ab′)2 fragments of goat anti-mouse IgG(H+L) (Kirkegaard & Perry) were used for cross-linking the costimulatory antibodies. Fluorochrome-conjugated antihuman MAbs were obtained from BD Pharmingen unless otherwise indicated. These included CD4 allophycocyanin (APC), CD69 phycoerythrin (PE) or APC, CD27 PE, CD45RA peridinin chlorophyll protein (PerCP; custom conjugated at Chromaprobe, Inc., Mountain View, Calif.), CD95 PE (Caltag Labs, Burlingame, Calif.), CD154 PE (clone TRAP1), CCR5 PE (clone 3A9), tumor necrosis factor alpha (TNF-α), fluorescein isothiocyanate (FITC), or PE (clone MAb11), and IFN-γ FITC or PE (clone B27 or clone 4S.B3).
Stimulation of CD4+ T lymphocytes.
Rhesus CMV antigen for stimulation of CD4+ T lymphocytes was prepared from primary monkey fibroblast lines infected with a clinical isolate of rhesus CMV (15). After infection had progressed to >90% cytopathic effect, the cells were subjected to multiple freeze-thaw cycles in order to release cell-associated virus, followed by sonication and centrifugation at 2,000 rpm for 15 min. The virus-containing supernatant was heat-inactivated at 56°C for 30 min, and the protein concentration was determined on a spectrophotometer (GeneQuant II; Pharmacia Biotech, Cambridge, United Kingdom). Control antigen prepared from uninfected primary monkey fibroblast lines was used at the same protein concentration as the CMV antigen. For superantigen stimulation, staphylococcal enterotoxin A (SEA) and staphylococcal enterotoxin B (SEB) were used together, each at a final concentration of 100 ng/ml (Sigma).
ICC staining assay.
Our assay represents a modification of the technique developed by Waldrop et al. to measure CMV-specific CD4+ T lymphocytes in human subjects (41). Peripheral blood mononuclear cells (PBMC) were stimulated with antigen (CMV or superantigen) in the presence of the costimulatory antibodies anti-CD28 and anti-CD49d which were cross-linked with affinity-purified F(ab′)2 fragments of goat anti-mouse (GAM) IgG for optimal costimulation. Polystyrene tubes (12 by 75 mm) precoated with 2.5 μg of GAM IgG/ml in 50 mM TrisCl (pH 8.6), followed by incubation with 10 μg each of anti-CD28 and anti-CD49d/ml, were used for stimulation. PBMC were stimulated with antigen for 6 h and Brefeldin A (Sigma) at 10 μg/ml was added for the final 4 h of stimulation. At the end of stimulation, cells were surface stained with conjugated anti-CD4 MAb for 30 min at 4°C. In some experiments, cells were also surface stained with conjugated MAbs to CD45RA, CD27, CD95, or CCR5. Cells were subsequently fixed and permeabilized with successive incubations in FACS Lysing Solution (Becton Dickinson Immunocytometry Systems [BDIS]) and FACS Permeabilizing Solution (BDIS). Permeabilized cells were incubated with conjugated anti-CD69 or anti-CD154 (CD40L) and with anti-cytokine MAbs for 30 min at 4°C, washed, and kept overnight at 4°C in 2% paraformaldehyde. Two hundred thousand events were collected on a FACSCalibur (BDIS), and the data were analyzed by CELLQuest. Antigen-specific cells were defined as CD4+ T lymphocytes that coexpressed CD69 and the cytokine of interest after antigen-specific stimulation.
IFN-γ ELISPOT assay.
PBMC suspended in RPMI containing 10% FBS (R-10 medium) were placed in 96-well microtiter plates coated with anti-monkey IFN-γ MAb MD-1 (U-Cytech, Utrecht, The Netherlands) (34). PBMC were plated in duplicate at twofold dilutions ranging from 5 × 105 cells/well to 0.3 × 105 cells/well. Cells were stimulated overnight with rhesus CMV antigen or control antigen at 37°C in a 5% CO2 incubator. After 16 to 24 h, cells were removed by extensive washing and the wells were serially incubated with a biotinylated anti-monkey IFN-γ detector antibody (U-Cytech) followed by gold-labeled anti-biotin IgG (U-Cytech); finally, an activator mix (U-Cytech) was added that allowed the formation of silver salt precipitates at the site of gold clusters (34). Spots were counted with a KS ELISPOT Automated Reader System (Carl Zeiss, Inc., Thornwood, N.Y.) by using KS ELISPOT 4.2 software (performed by Zellnet, New York, N.Y.).
CD8+ and CD4+ T lymphocyte separation.
In order to determine the phenotype of IFN-γ-producing cells, ELISPOT assays were also carried out with purified CD4+ and CD8+ T-lymphocyte populations fractionated by magnetic bead separation by using a negative selection procedure as previously described (15). Negatively selected CD8+ and CD4+ T-lymphocyte populations were >90% pure. Fractionated T lymphocytes were suspended in R-10 medium and used the same day in ELISPOT assays.
Proliferative LDA.
Fresh PBMC were plated in 96-well U-bottom plates in 24 replicate wells at cell concentrations ranging between 1,000 and 50,000 cells/well in the presence of CMV antigen or control antigen and then incubated at 37°C in a CO2 incubator. [3H]thymidine (1 μCi/well) was added on the fifth day, and cells were harvested 16 to 20 h later. Proliferation in individual wells with CMV antigen was scored positive if the counts per minute (cpm) exceeded three standard deviations (SDs) of the mean cpm in wells with control antigen at the same cell number. Precursor frequencies were calculated by using maximum-likelihood analysis, using software developed by Spyros A. Kalams, Massachusetts General Hospital.
Measurement of CMV DNA in plasma by real-time PCR.
CMV DNA was quantitated in plasma by using real-time PCR. DNA was extracted from a fixed volume of plasma by using the QIAmp DNA Mini kit (Qiagen, Inc., Valencia, Calif.). Primers and probe for amplification of rhesus CMV DNA sequences by real-time PCR were designed by using Primer Express software (Perkin-Elmer, Foster City, Calif.). The CMV primers amplify a 108-bp amplicon in the exon 1 region of the immediate-early gene of rhesus CMV (2). The forward primer (5′-GTTTAGGGAACCGCCATTCTG-3′) corresponds to residues 719 to 739, the reverse primer (5′-GTATCCGCGTTCCAATGCA-3′) corresponds to residues 826 to 808, and the probe (5′-FAM-TCCAGCCTCCATAGCCGGGAAGG-TAMRA-3′) corresponds to residues 784 to 806. The 1× PCR mix consisted of 5 to 10 ng of DNA, 300 nM each primer, 100 nM probe, 2 mM MgCl2, 200 μM each dATP, dCTP, and dGTP, 400 μM dUTP, 0.01 U of Amperase UNG/μl, 0.025 U of Taq polymerase/μl, and reaction buffer containing the passive reference dye ROX in a 50-μl final reaction volume loaded into a 96-well plate. The PCR was run on an ABI Prism 7700 Sequence Detection System (Perkin-Elmer) under the following thermal cycler conditions: an initial cycle of 2 min at 50°C, followed by 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 s and then annealing and/or extension for 1 min at 60°C.
This technique detects rhesus CMV with a linear dynamic range from 101 to 106 copies in the presence of genomic DNA. CMV DNA was quantitated by using a standard consisting of a plasmid containing the entire rhesus CMV immediate-early gene (kindly provided by Peter Barry, University of California at Davis). Tenfold dilutions of the standard plasmid ranging between 1 to 105 copies were made in genomic DNA extracted from a CMV-seronegative animal and run in duplicate during each PCR run. The threshold cycle or CT values of PCR amplification of the standards were used to generate a standard curve for quantitation of CMV DNA. The standard curve, which was included in every PCR run, was highly reproducible, with a correlation coefficient ranging between 0.98 and 1.0.
Presence of CMV disease.
The diagnosis of CMV disease was made on the basis of histological evidence of CMV in an organ(s) at necropsy. The characteristic feature on hematoxylin-eosin stain of cytomegalic cells with amphophilic intranuclear inclusions was confirmed to be CMV by immunohistochemistry by using a polyclonal rabbit antibody to the rhesus CMV immediate-early 1 protein (kindly provided by Peter Barry, UC Davis).
Measurement of SIV RNA in plasma.
Blood collected in tubes containing EDTA was spun at 2,000 rpm for 10 min within 3 h of drawing blood, and plasma was stored at −80°C for quantitation of SIV RNA. SIV RNA was quantitated by using a real-time reverse transcription-PCR assay as previously described (31).
Statistical analysis.
Statistical analysis was carried out by using StatView (Abacus Concepts, Inc., Berkeley, Calif.). Fisher's protected least-significant-difference post hoc test was used for making pairwise comparisons of the frequency of CMV-specific CD4+ T lymphocytes between different groups of animals. The relationship between ELISPOT assay, ICC assay, and proliferative LDA for quantitation of CMV-specific CD4+ T-cell frequency was determined by simple regression analysis. The nonparametric Mann-Whitney U test was used to determine whether differences in frequency of CMV-specific CD4+ T lymphocytes determined by the three methods were significant. In the pathogenic SIV-infected macaques, differences in frequency of CMV-specific CD4+ T lymphocytes between animals with high or low SIV and CMV viremia were evaluated by the nonparametric Mann-Whitney U test.
RESULTS
Determination of optimal conditions for detection of CMV-specific CD4+ T lymphocytes in rhesus macaques by the flow cytometric ICC assay.
A variety of variables were tested to determine the optimal experimental conditions necessary for flow cytometric detection of antigen-specific CD4 cells in rhesus macaques. The optimal conditions for inducing production of TNF-α in PBMC of rhesus macaques were initially determined by stimulation with the superantigens SEA and SEB in the absence or presence of antihuman MAbs to the costimulatory molecules CD28, CD49d, and CD5.
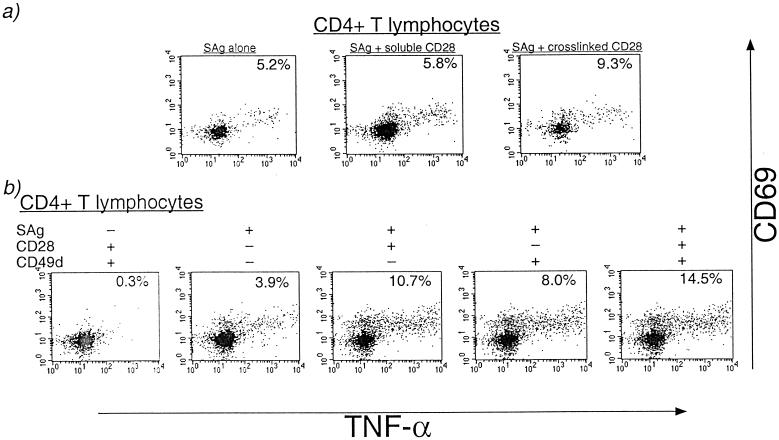
Cross-linked, but not soluble, costimulatory anti-CD28 MAb resulted in a roughly twofold enhancement of the frequency of TNF-α-secreting cells after superantigen stimulation (Fig. 1a). The use of two costimulatory antibodies, anti-CD28 in combination with anti-CD49d or anti-CD5, resulted in an additional three- to fourfold enhancement of the specific cytokine signal and did not result in increased background in the absence of specific stimulation (Fig. 1b and data not shown). The costimulatory antibodies enhanced antigen-specific cytokine secretion in a dose-dependent manner, and the optimal concentrations of CD28 and CD49d MAbs were each determined to be 10 μg/ml (data not shown). In all subsequent experiments, unless otherwise stated, cross-linked anti-CD28 and anti-CD49d MAbs were used together at 10 μg/ml each for costimulation. Other experimental conditions that were found to be essential for obtaining low-noise and highly specific cytokine signals included the use of freshly isolated PBMC that were either used the same day or cryopreserved immediately for later use, antigen-specific stimulation conducted in tubes slanted 5° above horizontal as described by Waldrop et al. (41), the use of FITC-conjugated anti-cytokine MAb along with PE-conjugated CD69 MAb, and the optimal concentration of CMV antigen. CMV-specific cytokine signals were reduced >90% in PBMC when kept overnight prior to stimulation or when there was an overnight delay prior to separation of PBMC from whole blood (data not shown). In cryopreserved PBMC, the frequency of CMV-specific CD4+ T lymphocytes was roughly 70% of that observed in freshly isolated and stimulated PBMC (data not shown). For the purpose of this analysis, all frequencies of CMV-specific CD4+ T lymphocytes were determined on PBMC that had been isolated and cryopreserved on the day of blood collection.
FIG. 1.
Optimization of flow cytometric conditions for detecting cytokine-secreting CD4+ T lymphocytes in rhesus macaques. PBMC were stimulated with the superantigens SEA and SEB (100 ng/ml each) for 6 h in the presence of Brefeldin A (10 μg/ml). Gated CD4+ T lymphocytes are shown. The percentage of CD4+ T lymphocytes coexpressing CD69 and TNF-α is shown in the right upper quadrant of each plot. (a) Comparison of soluble versus cross-linked antihuman CD28 MAb for costimulation of superantigen (SAg)-stimulated PBMC. Cross-linking was provided by affinity-purified F(ab′)2 fragments of GAM IgG as described in Materials and Methods. (b) Effect of costimulation with MAbs to CD28 and/or CD49d on TNF-α secretion after superantigen stimulation.
Quantitation of CMV-specific CD4+ T lymphocytes by ICC.
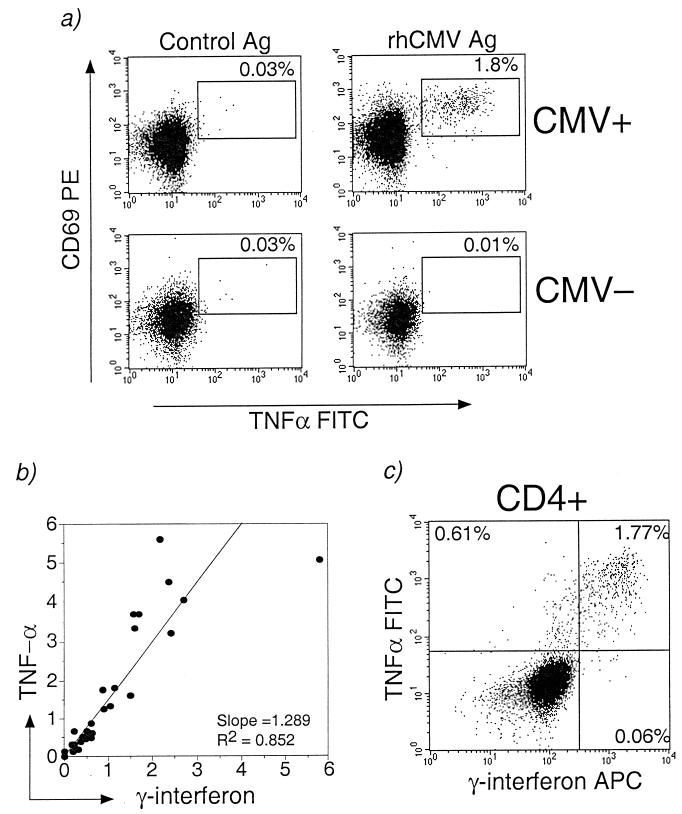
Using optimal conditions for ICC assays as described above, we were able to detect recently activated (CD69+) CD4+ T lymphocytes secreting IFN-γ or TNF-α in CMV-seropositive rhesus macaques after stimulation with whole-virus rhesus CMV antigen (Fig. 2a). Cytokine-secreting CD4+ T lymphocytes were not detected after stimulation with control antigen in CMV-seropositive macaques, nor were they detected after CMV stimulation in CMV-seronegative macaques (Fig. 2a). The cytokine response of CD4+ T lymphocytes was major histocompatibility complex (MHC) class II restricted, since it was decreased >80% when PBMC were incubated with 50 μg of antibodies to MHC class II DR, DP, or DQ (BD Pharmingen)/ml for 30 min at 37°C prior to CMV stimulation (data not shown). Both IFN-γ and TNF-α were detected after CMV-specific stimulation. Although the frequency of TNF-α-secreting CMV-specific CD4+ T lymphocytes exceeded the frequency of CMV-specific IFN-γ-secreting CD4+ T lymphocytes in most animals, the production of both cytokines was highly correlated, with an R2 value of 0.85 (Fig. 2b). In separate experiments, we demonstrated that >90% of the CMV-specific IFN-γ-secreting CD4+ T lymphocytes also secreted TNF-α (Fig. 2c).
FIG. 2.
Detection of CMV-specific CD4+ T lymphocytes in rhesus macaques by flow cytometry using the ICC assay. (a) Representative dot plots gated on CD4+ T lymphocytes showing coexpression of CD69 and TNF-α in PBMC from a CMV-seropositive rhesus macaque after CMV antigen (top right panel) but not after control antigen stimulation (top left panel). The percentage of CD4+ T lymphocytes coexpressing CD69 and TNF-α is shown. Control antigen consisted of a sonicate preparation of uninfected primary monkey fibroblast lines. No TNF-α secretion was detected in CD4+ T lymphocytes from a CMV-seronegative macaque stimulated with CMV antigen (bottom right panel). (b) Positive correlation between the percentages of CMV-specific CD4+ T lymphocytes secreting TNF-α or IFN-γ in individual animals. (c) Dot plot showing coexpression of IFN-γ and TNF-α by CMV-specific CD4+ T lymphocytes.
Using this technique, we determined the frequency of CMV-specific CD4+ T lymphocytes in 46 rhesus macaques (Table 1). In 23 CMV-seropositive SIV-naive rhesus macaques with a median age of 12 years (range, 2.7 to 22 years), the frequency of IFN-γ-secreting CMV-specific CD4+ T lymphocytes ranged between 0.16% and 5.8% (median, 0.63%) and varied less than twofold over a 6-month period (data not shown). The ICC assay was highly sensitive and specific, since in eight CMV-seronegative rhesus macaques, <0.04% CD69+ IFN-γ-secreting CD4+ T lymphocytes were detected after CMV-specific stimulation (range, 0.0 to 0.04%; median frequency, 0.01%). The lower limit of CMV-specific detection determined as three SDs above the mean frequency of CD69+ IFN-γ+ CD4+ T lymphocytes in the CMV-seronegative macaques was 0.08%. The ICC assay was also highly reproducible for enumerating CMV-specific CD4+ T lymphocytes in rhesus macaques. In CMV-seropositive rhesus macaques, the assay had a low intraassay and interassay variation. The intraassay coefficient of variation for replicates of the same sample assayed in the same experiment ranged between 0.01 and 0.24 (mean ± SD, 0.13 ± 0.09). The interassay coefficient of variation for aliquots of the same sample assayed at two different timepoints 3 to 6 months apart ranged between 0.10 and 0.51 (mean ± SD, 0.29 ± 0.14).
TABLE 1.
Characteristics of rhesus macaques grouped on the basis of CMV serology and SIV infection status
| Animal parameter | CMV seropositive
|
CMV seronegative (SIV−) | ||
|---|---|---|---|---|
| SIV− | WT SIV+a | Attenuated SIV+b | ||
| Number | 23 | 9 | 6 | 8 |
| Age (yr) | ||||
| Median | 12 | 4.8 | 7.5 | 2.5 |
| Range | 2.7-22 | 2.5-5.7 | 3.4-12.7 | 2-4 |
| % CD4 counts | ||||
| Median | 29.5 | 5.6 | 36.8 | 34.8 |
| Range | 18.6-65.8 | 1.8-59.5 | 19.1-49.8 | 20.6-49.0 |
| Duration of SIV infection (mo) | ||||
| Median | NAc | 4.5 | 72.5 | NA |
| Range | 3.5-26 | 15-126 | ||
| Level of SIV RNA (copies/ml) in plasma | ||||
| Median | NA | 1.1 × 107 | NDd | NA |
| Range | 5 × 105-8 × 108 | |||
Animals infected with pathogenic SIVmac239 or SIVmac251. WT, wild type.
Animals infected with live attenuated strains of SIV (as described in Results).
NA, not applicable.
ND, not detected.
Comparison of ICC assay, ELISPOT assay, and proliferative LDA for enumeration of CMV-specific CD4+ T lymphocytes in rhesus macaques.
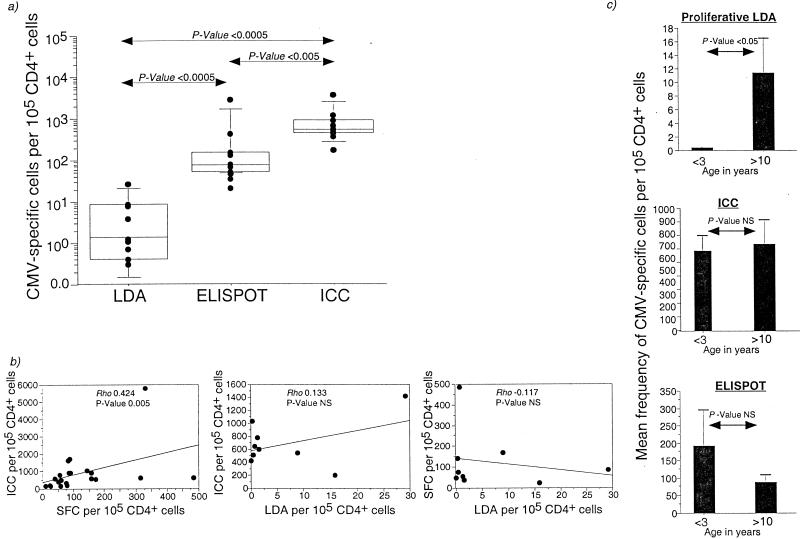
The frequency of CMV-specific CD4+ T lymphocytes determined by IFN-γ ICC assay, IFN-γ ELISPOT assay, and proliferative LDA was compared in 10 CMV-seropositive rhesus macaques (Fig. 3a). The frequency of CMV-specific CD4+ T cells detected by ICC assay was 1 to 3 logs greater than that determined by proliferative LDA (P = 0.0002; Mann-Whitney U test), while frequencies determined by IFN-γ ELISPOT assay were 3- to 100-fold greater than those obtained by proliferative LDA (P = 0.0002; Mann-Whitney U test). The frequency of CMV-specific CD4+ T lymphocytes determined by IFN-γ ICC assay was 1.3- to 16.3-fold higher than that obtained by IFN-γ ELISPOT assay (P = 0.005; Mann-Whitney U test).
FIG. 3.
Comparison between ICC assay, ELISPOT assay, and proliferative LDA for quantitation of CMV-specific CD4+ T lymphocytes. (a) Box plot showing frequency of CMV-specific CD4+ T lymphocytes determined by proliferative LDA, ELISPOT assay, and ICC assay in 10 CMV-seropositive rhesus macaques. Horizontal bars show median values, boxes indicate 75% confidence intervals, and error bars show 95% confidence intervals. P values were determined by the nonparametric Mann-Whitney U test. (b) Regression analysis showing the relationship between the IFN-γ ICC and IFN-γ ELISPOT assays (n = 21; left panel), the IFN-γ ICC assay and proliferative LDA (n = 9; middle panel), and the IFN-γ ELISPOT assay and proliferative LDA (n = 9; right panel). SFC, spot-forming cells. SIV-infected macaques were excluded from this analysis. The correlation coefficient and P values were determined by the Spearman rank correlation coefficient test. (c) Age-related differences in the proliferative LDA, ICC assay, and ELISPOT assay for enumeration of CMV-specific CD4+ T lymphocytes in rhesus macaques. Data on nine SIV-negative, CMV-seropositive animals are shown (<3 years, n = 4; >10 years, n = 5). Error bars show the standard error. P values were determined by the nonparametric Mann-Whitney U test.
As expected, we observed a significant positive correlation between the frequency of CMV-specific CD4+ T lymphocytes determined by IFN-γ ICC and IFN-γ ELISPOT assays (Fig. 3b). However, there was no correlation between the frequency of CMV-specific CD4+ T lymphocytes determined by the IFN-γ ICC assay and proliferative LDA (Fig. 3b). Neither did we observe a correlation between IFN-γ ELISPOT assay and proliferative LDA (Fig. 3b). The lack of relationship between the assays with different readouts, i.e., the proliferation after long-term stimulation as opposed to cytokine-secretion after short-term stimulation, appeared to be age related (Fig. 3c). In 4 of 4 juvenile macaques of <3 years of age, CMV-specific CD4+ T lymphocytes were not detected by proliferative LDA, although they were detected at levels comparable to adult macaques when measured by ELISPOT or ICC assays (Fig. 3c). We observed a significant positive correlation between the age of an animal and the frequency of CMV-specific CD4+ T lymphocytes determined by proliferative LDA (R2-0.43; P value, 0.03; data not shown), but not by IFN-γ ELISPOT or IFN-γ ICC assays (data not shown).
Heterogeneity in phenotype of CMV-specific CD4+ T lymphocytes among SIV-naive CMV-seropositive rhesus macaques.
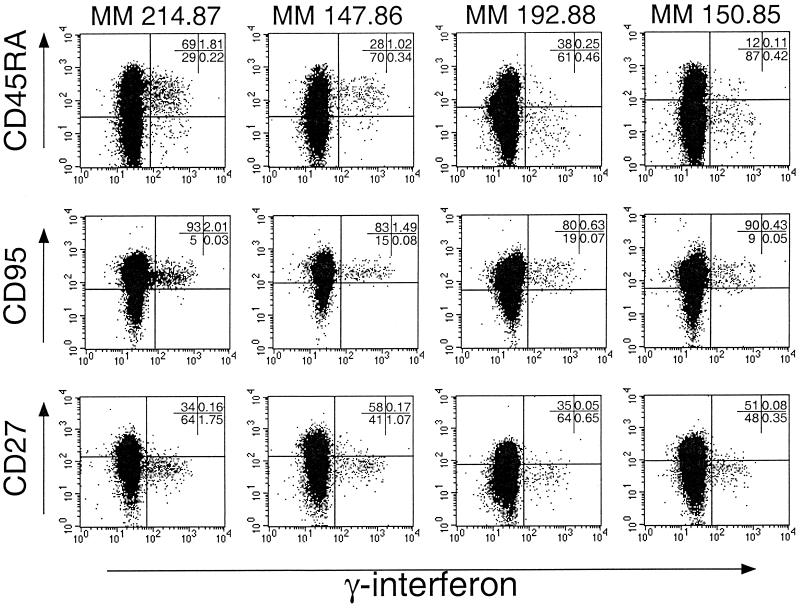
Since antigen-specific cells are visualized by the ICC assay, we used it to characterize the phenotype of CMV-specific CD4+ T lymphocytes in nine SIV-naive CMV-seropositive rhesus macaques (Table 2). CMV-specific CD4+ T lymphocytes were examined with regard to the surface expression of CD45RA, CD27, and CD95, markers commonly employed to characterize memory cells (8, 9). The expression of CD45RA on CMV-specific CD4+ T lymphocytes varied considerably among animals, and two polar patterns were observed (Fig. 4 and Table 2). One pattern demonstrated by animals MM 214.87 and MM 147.86 (Fig. 4) consisted of CMV-specific CD4+ T lymphocytes being predominantly CD45RApos, while the other pattern demonstrated by MM 192.88 and MM 150.85 consisted of CMV-specific CD4+ T lymphocytes being predominantly CD45RAlo or CD45RAneg (Fig. 4). The differential expression of CD45RA on CMV-specific CD4+ T lymphocytes did not appear to be related to differences in the time of acquisition of natural CMV infection, since all of the examined animals were >8 years of age and group housed and thus likely to have seroconverted by the age of 1 year. In contrast to the expression of CD45RA, the expression of CD27 and CD95 on CMV-specific CD4+ T lymphocytes was homogeneous in all of the animals (Table 2 and Fig. 4). In two of two animals, the expression of CD45RA, CD27, and CD95 on CMV-specific CD4+ T lymphocytes was stable over a 3- to 6-month period.
TABLE 2.
Phenotype of CMV-specific and total CD4+ T lymphocytes in the peripheral blood of SIV-negative CMV-seropositive rhesus macaques
| Animal no. | Age (yr) | % CMV-specific CD4+ T lymphocytes that are:
|
% Total CD4+ T lymphocytes that are:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD45RAhi | CD27hi | CD95pos | CD45RAhi CD27lo/neg | CD45RAlo/neg CD27hi | CD45RAlo/neg CD27lo/neg | CD45RAhi | CD27hi | CD95pos | CD45RAhi CD27lo/neg | CD45RAlo/neg CD27hi | CD45RAlo/neg CD27lo/neg | ||
| MM 158.86 | 14 | 37.9 | 3.5 | NTa | 24.1 | 0.0 | 72.4 | 56.6 | 18.8 | NT | 39.9 | 16.7 | 32.9 |
| MM 226.86 | 14 | 20.9 | NT | NT | NT | NT | NT | 7.3 | NT | NT | NT | NT | NT |
| MM 150.85 | 15 | 23.8 | 19.4 | 85.1 | 19.0 | 10.9 | 64.9 | 16.8 | 47.8 | 89.6 | 12.0 | 42.4 | 40.2 |
| MM 190.85 | 15 | 13.8 | NT | NT | NT | NT | NT | 16.6 | NT | NT | NT | NT | NT |
| MM 147.86 | 14 | 80.2 | 10.4 | 95.7 | 71.4 | 2.9 | 15.6 | 30.9 | 52.1 | 82.9 | 19.2 | 43.9 | 24.1 |
| MM 214.87 | 13 | 89.2 | 4.4 | 97.6 | 82.4 | 0.4 | 10.0 | 70.3 | 27.1 | 93.8 | 49.9 | 12.2 | 17.1 |
| MM 226.88 | 12 | 68.8 | NT | NT | NT | NT | NT | 20.2 | NT | NT | NT | NT | NT |
| MM 192.88 | 12 | 34.2 | 7.6 | 89.8 | 33.5 | 3.2 | 60.8 | 38.6 | 35.3 | 79.9 | 22.9 | 12.7 | 47.6 |
| MM 245.91 | 9 | 22.0 | 32 | NT | 22 | 22.0 | 56.0 | 42.8 | 35.1 | NT | 38.7 | 24.7 | 31.1 |
NT, not tested.
FIG. 4.
Expression of CD45RA, CD95, and CD27 on the surface of CMV-specific CD4+ T lymphocytes. Representative data from four SIV negative, CMV-seropositive adult rhesus macaques are shown. The dot plots shown are gated on CD4+ T lymphocytes.
Cross-sectional analysis of the frequency of CMV-specific CD4+ T lymphocytes in normal and SIV-infected rhesus macaques.
Depletion of CD4+ T lymphocytes is a hallmark of pathogenic lentiviral infection, and loss of pathogen-specific CD4+ T lymphocytes is likely to be an important mechanism for the emergence of opportunistic infections. We examined the effect of SIV infection on CMV-specific CD4+ T lymphocytes in a cross-sectional analysis by using the ICC assay to compare the frequency of CMV-specific IFN-γ secreting CD4+ T lymphocytes in normal and SIV-infected rhesus macaques (Table 1 and Fig. 5). In addition to 23 SIV-naive CMV-seropositive rhesus macaques, CMV-specific CD4+-T-lymphocyte frequencies were determined in nine CMV-seropositive macaques infected with pathogenic SIV strains (SIVmac251 or SIVmac239), and six CMV-seropositive macaques infected with live attenuated strains of SIV (Table 1). The strains of live attenuated SIV used to inoculate the macaques included SIVΔnef (n = 2), SIVΔ3x (n = 2), SIVΔ3 (n = 1), and SIVΔ4 (n = 1) which have been previously described (13, 14).
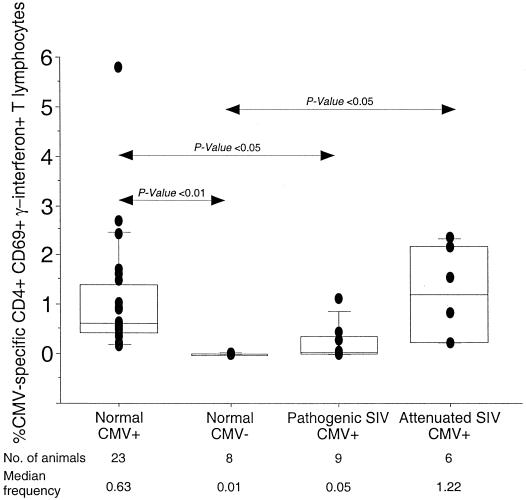
FIG. 5.
Cross-sectional analysis of the frequency of CMV-specific CD4+ T lymphocytes in rhesus macaques in the absence or presence of SIV infection. Horizontal bars show median values, boxes indicate 75% confidence intervals, and error bars show 95% confidence intervals. The P value was determined by the nonparametric Mann-Whitney U test.
CMV-seropositive rhesus macaques infected with pathogenic SIVmac239 or SIVmac251 for a median duration of 4.5 months (range, 3.5 to 26 months) had a 12-fold-lower median frequency of CMV-specific CD4+ T lymphocytes than did SIV uninfected CMV-seropositive rhesus macaques (P < 0.05; Fig. 5). In contrast, the frequency of CMV-specific CD4+ T lymphocytes in rhesus macaques infected with live attenuated strains of SIV was comparable to that observed in normal macaques (Fig. 5). While CMV-specific CD4+ T lymphocytes were detected in 23 of 23 CMV-seropositive SIV-naive macaques and in 6 of 6 macaques infected with live attenuated strains of SIV, they were detected in only 3 of 9 macaques infected with pathogenic SIV (Fig. 5). The difference in frequency of CMV-specific CD4+ T lymphocytes between normal and pathogenic SIV-infected rhesus macaques was unlikely to be age-related since we did not observe a relationship between age and frequency of CMV-specific IFN-γ-secreting CD4+ T lymphocytes in normal CMV-seropositive rhesus macaques.
Predictors of loss of CMV-specific CD4+ T lymphocytes in pathogenic SIV-infected rhesus macaques.
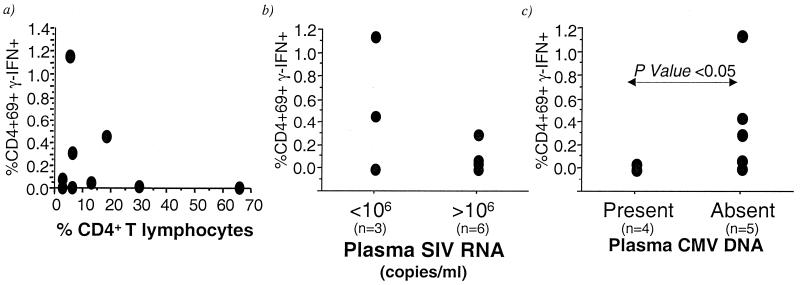
In HIV-infected humans, CMV disease is a late manifestation of AIDS and generally occurs when the peripheral CD4 count drops below 50/μl (28). We investigated whether CD4+ T lymphocytopenia, high SIV viral loads, or CMV viremia were factors associated with the depletion of CMV-specific CD4+ T lymphocytes in pathogenic SIV-infected macaques. At the time of the cross-sectional analysis, SIV-infected macaques with undetectable CMV-specific CD4 cells had been infected for a median duration of 4.5 months (range, 3.5 to 26 months), while the median duration of SIV infection in the three macaques with detectable CMV-specific CD4 cells was 26 months (range, 3.5 to 26 months). We did not observe any relationship between the degree of CD4+ T lymphocytopenia and loss of CMV-specific CD4+ T lymphocytes in the nine pathogenic SIV-infected rhesus macaques (Fig. 6a). Notably, one SIVmac251-infected rhesus macaque with profound and sustained CD4+ T lymphocytopenia for >1 year never lost its CMV-specific CD4+ cells. At the time that this animal finally succumbed to AIDS (45 days since the time point used in the cross-sectional analysis), 2.1% of its CD4+ T lymphocytes were still CMV specific (data not shown). Since macaques infected with live attenuated strains of SIV had undetectable SIV viremia and normal frequencies of CMV-specific CD4+ T lymphocytes, we investigated whether lower SIV viral loads in pathogenic SIV-infected macaques correlated with the persistence of CMV-specific CD4+ T lymphocytes. SIV-infected macaques with lower levels of SIV RNA in plasma (<106 copies/ml) had a higher mean frequency of CMV-specific CD4+ T lymphocytes compared to macaques with a level of SIV RNA in plasma of >106 copies/ml (Fig. 6b), although this difference did not reach statistical significance (P = 0.12; Mann-Whitney U test). Similarly, an inverse correlation observed between plasma SIV RNA and frequency of CMV-specific CD4+ T lymphocytes was not statistically significant (ρ = −0.37; P = 0.30). Finally, levels of SIV RNA plasma were higher in four pathogenic SIV-infected macaques with detectable CMV DNA in plasma (median, 1.3 × 108; range, 3.1 × 106 to 8 × 108) compared to 5 macaques with undetectable levels of CMV DNA in plasma (median, 5 × 105; range, 4.6 × 102 to 2.1 × 107), although this difference also failed to reach statistical significance (P = 0.09; Mann-Whitney U test).
FIG. 6.
Relationship between the frequency of CMV-specific CD4+ T lymphocytes and peripheral blood CD4 counts (a), levels of SIV RNA in plasma (b), and levels of CMV DNA in plasma (c) in CMV-seropositive rhesus macaques with pathogenic SIV infection.
Finally, we investigated the relationship between loss of CMV-specific CD4+ T lymphocytes and the CMV viral load. CMV DNA in plasma was detected by real-time PCR in four of six pathogenic SIV-infected rhesus macaques with undetectable CMV-specific CD4+ T lymphocytes in their peripheral blood (Fig. 6c). It was not detected in three of three pathogenic SIV-infected macaques with detectable CMV-specific CD4+ T lymphocytes (Fig. 6c). Nor was it detected in any of the SIV-naive rhesus macaques or in macaques inoculated with live attenuated strains of SIV (data not shown). Pathogenic SIV-infected macaques with undetectable CMV DNA in plasma had significantly higher frequencies of CMV-specific CD4+ T lymphocytes than animals with detectable CMV DNA in plasma (P = 0.03; Mann-Whitney U test; Fig. 6c). Three of four animals with detectable CMV DNA in plasma had evidence of CMV disease by the time they died of AIDS (Table 3). Of these, two macaques with >1,000 copies of CMV DNA/ml of plasma died within a week of analysis and had histological evidence of CMV pneumonitis (Table 3). Two macaques with <1,000 copies of CMV DNA/ml of plasma died >9 months later, and one had evidence of CMV arteritis at necropsy (Table 3).
TABLE 3.
CMV DNA level in plasma in four pathogenic SIV-infected rhesus macaques with undetectable CMV-specific CD4+ T lymphocytes
| Animal no. | Mean CMV DNA copy no./ml of plasmaa | Time (days) from ICC assay to death | Presence (+) or absence (−) of CMV disease at necropsy |
|---|---|---|---|
| MM 285.95 | 275 | 326 | + |
| MM 265.95 | 376 | 296 | − |
| MM 249.97 | 3,328 | 7 | + |
| MM 239.95 | 381,585 | 0 | + |
At time of ICC assay.
Phenotype of CMV-specific CD4+ T lymphocytes pre-SIV infection as a predictor of subsequent loss post-SIV infection.
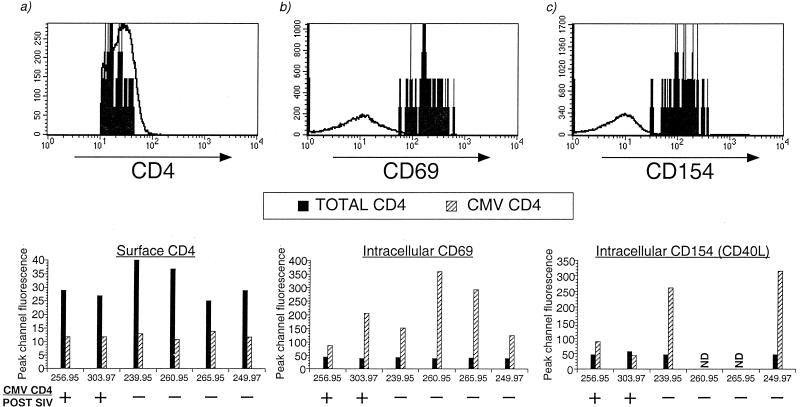
Since SIV and HIV can selectively deplete different memory populations of CD+ T lymphocytes (35, 37, 43), we examined whether differences in the phenotype of CMV-specific cells, including the expression of CD45RA, CCR5, and activation markers, could account for differential susceptibility to the loss of CMV-specific CD4+ T lymphocytes after SIV infection. In six of nine pathogenic SIV-infected macaques, PBMC archived prior to SIV infection were available to be examined by the ICC assay. Of these, two animals had detectable CMV-specific CD4+ T lymphocytes and four had lost their CMV-specific CD4+ T lymphocytes after SIV infection (Table 4). Similar to SIV-naive animals, a heterogeneous pattern of CD45RA expression was observed on the surface of CMV-specific CD4+ T lymphocytes, which was indistinguishable between the two animal groups (Table 4). Up to 18% of CMV-specific CD4+ T lymphocytes in the peripheral blood expressed CCR5 on their surface (Table 4). Macaques that had lost their CMV-specific CD4+ T lymphocytes after SIV infection did not have a higher frequency of CCR5 positive CMV-specific CD4+ cells in peripheral blood (Table 4). Since SIV and HIV preferentially replicate in activated cells, we also examined whether the degree of activation of CMV-specific CD4+ T lymphocytes after in vitro CMV stimulation differed between animals. CMV-specific CD4+ T lymphocytes from all six macaques had lower levels of surface CD4 expression than the total population of CD4+ T lymphocytes (Fig. 7a). We did not observe a significant difference in the degree of CD69 upregulation on CMV-specific CD4+ T lymphocytes between animals that retained or lost their CMV-specific CD4+ cells after SIV infection (Fig. 7b). In four animals, we also examined whether there were differences in the pattern of CD154 (CD40L) expression on CMV-specific CD4+ T lymphocytes. After CMV stimulation, CD154 (CD40L) was upregulated in 73 to 90% of the CMV-specific IFN-γ-secreting CD4+ T lymphocyte population (Table 4). Interestingly, the peak fluorescent intensity of CD154 (CD40L) on CMV-specific CD4+ cells from two animals that had lost their CMV-specific CD4+ T lymphocytes after SIV infection was three- to sevenfold higher than that observed in two animals that did not lose their CMV-specific CD4+ T lymphocytes after SIV infection (Fig. 7c). Although the small number of animals precludes a definitive conclusion, this observation suggests the possibility that preexisting differences in the degree of activation of antigen-specific CD4 cells between animals may be a factor contributing to susceptibility to destruction after SIV infection.
TABLE 4.
Pre-SIV infection phenotype of CMV-specific CD4+ T lymphocytes in six pathogenic SIV-infected rhesus macaques
| Animal no. | Presence (+) or absence (−) of CMV-CD4 post-SIV infection | % CMV-specific CD4+ T lymphocytes expressing:
|
% Total CD4+ T lymphocytes expressing:
|
||||
|---|---|---|---|---|---|---|---|
| CD45RA | CCR5 | CD154 | CD45RA | CCR5 | CD154 | ||
| MM 256.95 | + | 53 | 11.4 | 90 | 29 | 9.2 | 1.0 |
| MM 303.97 | + | 12 | 18.2 | 83 | 14 | 8.4 | 0.7 |
| MM 239.95 | − | 59 | 18.4 | 90 | 41 | 11.2 | 1.7 |
| MM 260.95 | − | 33 | 8.5 | NDa | 53 | 11.2 | ND |
| MM 265.95 | − | 29 | 0 | ND | 46 | 2.8 | ND |
| MM 249.97 | − | 19 | 16.2 | 73 | 16 | 15.1 | 0.83 |
ND, not done.
FIG. 7.
Level of expression of CD4, CD69, and CD154 (CD40L) on CMV-specific CD4+ T lymphocytes in six rhesus macaques that were subsequently inoculated with pathogenic SIVmac251. Data on pre-SIV infection samples shown. Representative overlay histograms are shown in the top panel for expression of surface CD4 (a), intracellular CD69 (b), and intracellular CD154 (c) on CMV-specific CD4+ T lymphocytes (solid histograms) and total CD4+ T lymphocytes (open histograms). The bottom panels show the peak channel fluorescence of CD4 (a), CD69 (b), and CD154 (c) on CMV-specific and total CD4+ T lymphocytes in individual rhesus macaques. ND, no data.
DISCUSSION
In this study we characterized CMV-specific CD4+ T-lymphocyte responses in the peripheral blood of rhesus macaques with or without SIV infection by using techniques that detect cytokine-secreting antigen-specific T lymphocytes at the single-cell level. In SIV-uninfected, naturally CMV-infected rhesus macaques, 0.16 to 5.8% of the circulating pool of CD4+ T lymphocytes were CMV specific. Similar or higher frequencies were observed in macaques that had been infected for >8 years with live attenuated strains of SIV. In contrast, the majority of animals infected with pathogenic SIV had no detectable peripheral CMV-specific CD4+ T lymphocytes. In these animals, loss of CMV-specific CD4+ T lymphocytes was associated with an increase in CMV viral load, suggesting that CD4+ T lymphocytes contribute to in vivo control of CMV replication.
The absence of CMV-specific CD4+ T lymphocytes in the majority of pathogenic SIV-infected macaques is in contrast to observations in HIV-infected human subjects. By the ICC assay, CMV-specific CD4+ T lymphocytes are detected at normal to higher frequencies in HIV-infected individuals, despite the presence of significant CD4+ T lymphocytopenia or high HIV viral loads (24, 41). In our study, although the frequency of CMV-specific CD4+ T lymphocytes in healthy, SIV-naive CMV-seropositive rhesus macaques was comparable to that reported in human subjects, CMV-specific CD4+ cells were absent in the majority of pathogenic SIV-infected macaques. The discrepancy between the results in human subjects and the simian model may be a species-specific difference or may be due to differences in the stage of disease or to differences in the biology of HIV and SIV infection. Unlike the relatively prolonged course of asymptomatic HIV infection, SIV typically produces AIDS in macaques within 1 year of infection. Although it is possible that the animals were surveyed late in the course of infection at a time when they had already developed AIDS, it is unlikely to account for the discrepancy since, in HIV-infected individuals, there was no correlation between the frequency of CMV-specific CD4+ T lymphocytes and the stage of HIV disease (41). Further, differences in duration of SIV infection or the severity of CD4+ T lymphocytopenia did not differentiate macaques that lost their CMV-specific CD4+ T lymphocytes from those that continued to have detectable CMV-specific CD4+ cells after SIV infection. Recently, there have been reports of decreased CMV-specific CD4+ T lymphocytes in HIV-infected individuals with active CMV end-organ disease (11, 18). In our study, increased CMV viral load was detected in four of six pathogenic SIV-infected rhesus macaques with undetectable CMV-specific CD4+ T lymphocytes, and three of them progressed to develop CMV disease, an observation which is consistent with the human data. Finally, since CMV-specific CD4+ T lymphocytes were quantitated by enumeration of IFN-γ-secreting cells, we have not excluded the possibility that, in pathogenic SIV-infected macaques, CMV-specific CD4+ cells were undetectable because they had switched phenotype and were secreting Th2 cytokines or interleukin-10. Such a scenario has been described in HCV infection in human subjects, where the cytokine-secreting profile of HCV-specific CD4+ T cells was qualitatively different between nonviremic and chronically infected patients (7).
Although the decline in HIV viremia after institution of HAART (highly active antiretroviral therapy) is generally associated with significant gains in pathogen-specific immune responses, persistent deficits in CMV-specific CD4+ T lymphocytes despite adequate control of HIV viremia have been described, suggesting a selective destruction of CMV-specific cells (17). In our study, although two-thirds of the pathogenic SIV-infected rhesus macaques had lost their CMV-specific CD4+ T lymphocytes, we observed a subset, and in particular one macaque, that maintained a high percentage of CMV-specific CD4+ T lymphocytes. This animal had severe CD4+ T lymphocytopenia, and thus its total frequency of CMV-specific CD4+ T lymphocytes was low. Among host factors contributing to differential target cell susceptibility in HIV or SIV infection, we investigated whether the phenotype or activation status of CMV-specific CD4+ T lymphocytes predisposed to selective loss of these cells during the course of SIV infection. We detected marked heterogeneity of CD45RA expression on CMV-specific CD4+ T lymphocytes in healthy SIV-negative rhesus macaques. This observation is not surprising since memory CD4 cells with a spectrum of CD45RA and CD45RO expression have been described in vivo (8). In antigen-specific CD8+ T lymphocytes, heterogeneity of CD45RA expression is associated with differential effector function (5). Among the pathogenic SIV-infected macaques, the pre-SIV phenotype of the CMV-specific CD4+ T lymphocytes displayed the same CD45RA heterogeneity as was observed in the SIV-uninfected macaque colony. We did not detect a difference in the frequency of CD45RAneg or CD45RApos CMV-specific CD4+ T lymphocytes between macaques that lost or did not lose these cells after SIV infection. Similarly, CCR5 was expressed in <20% of CMV-specific CD4 cells pre-SIV infection, and there were no differences between the SIV-infected animals to account for the preservation of CMV-specific CD4+ cells in selected animals. Since a higher frequency of CCR5-positive CD4+ T lymphocytes is detected among gut-associated lymphocytes, the first cells to be targeted by SIV (36, 37), it is possible that macaques that lost their CMV-specific CD4+ T lymphocytes had higher frequencies of CCR5 positive CMV-specific CD4+ cells in their guts. Interestingly, albeit in a very small number of animals, we observed a difference in CD40L expression between animals. Pre-SIV, the intensity of CD40L expression on CMV-specific CD4+ T lymphocytes was three- to sevenfold higher in two macaques that lost their CMV-specific CD4+ cells, in contrast to two animals that continued to have detectable CMV-specific CD4+ cells. The small number of animals precludes any definite conclusions but suggests that variation in the degree of activation of antigen-specific CD4+ cells between animals may be a factor contributing to differential susceptibility to SIV infection. Prospective studies on larger numbers of animals will be required to determine whether upregulation of CCR5 and other memory or activation markers on CMV-specific CD4+ T lymphocytes selectively predisposes animals to destruction of their CMV-specific CD4+ cells after SIV infection.
In the present study, we successfully adapted the ICC and ELISPOT assays to detect CMV-specific CD4+ T lymphocytes in rhesus macaques. Although the ICC assay can detect frequencies of antigen-specific cells that are 1 to 2 logs higher than those reported in the earlier literature by LDA, there are few published studies showing a head-to-head comparison of all three assays (16). As expected, comparison with traditional proliferative assays showed the cytokine assays to be more sensitive. However, to our surprise, although a strong positive correlation was observed between the ELISPOT and ICC assays, we did not observe a correlation between the ICC assay and the proliferative LDA. The lack of correlation appeared to be age dependent. Juvenile rhesus macaques had high frequencies of CMV-specific CD4+ cells detected by the cytokine assays, although the proliferative LDAs scored negative. The discrepancy between the proliferative and cytokine assays may reflect the differences inherent in the assay system, in that a positive readout in the proliferative LDA is dependent not only on the initial frequency of antigen-specific cells but also on their ability to expand exponentially over a 5- to 6-day stimulation period (23). Another possibility is that CMV-specific CD4+ T lymphocytes are heterogeneous with respect to their proliferative and cytokine-secreting abilities. In primary CMV infection in renal transplant recipients, CMV-specific CD4 cells were predominantly CD45RA+, CD45RO+, and Ki-67+, a phenotype remarkably different from quiescent infection (25). It is likely that these phenotypic differences are associated with different functional abilities of memory CD4 cells. Thus, juvenile rhesus macaques may have a higher proportion of activated CMV-specific CD4+ cells which score poorly in traditional proliferative assays either due to apoptosis in long-term stimulation cultures or due to a decreased proliferative ability. A dichotomy between cytokine-secreting and proliferative ability has been described in chimeric mice with antigen-specific precursor frequencies high enough to impose constraints on proliferation; in this model noncycling antigen-specific CD4+ T cells can synthesize IFN-γ (19).
In conclusion, we have adapted the precise techniques of ELISPOT and intracellular cytokine staining to enumerate and analyze CMV-specific CD4+ T lymphocytes in rhesus macaques. Using these techniques we report the novel finding of a decreased frequency of CMV-specific CD4+ T lymphocytes in association with increased CMV viremia in SIV-infected rhesus macaques. The ability to determine the phenotype of antigen-specific CD4+ cells by flow cytometry has facilitated the characterization of CMV-specific CD4+ T lymphocytes in rhesus macaques and enabled study of factors predisposing to their selective loss after SIV infection. These techniques provide a powerful tool for future prospective studies on CMV pathogenesis in the rhesus macaque animal model of AIDS.
Acknowledgments
This study was supported by Public Health Service grants RR00168 and AI43890 (A.K.) and AI45314 (R.P.J.).
We gratefully acknowledge Peter Barry for providing plasmid with the rhesus CMV immediate-early gene insert and polyclonal rabbit anti-rhesus CMV IE1 antibody; Danny Silva for the preparation of rhesus CMV antigen; Dan Shaffer for the design of primers and probe for the rhesus CMV real-time PCR; Andrew Lackner and Ron Desrosiers for the use of blood samples from SIV-infected monkeys; Jeff Lifson for the measurement of plasma SIV RNA; Spyros Kalams for providing the software used to calculate precursor frequencies by limiting dilution analysis; and Silvia Janetzki from Zellnet, New York, N.Y., for analysis of the ELISPOT plates.
REFERENCES
- 1.Anonymous. 1996. Guide for the care and use of laboratory animals, p. 86-123. The Institute of Laboratory Animal Resources, National Research Council, Washington, D.C.
- 2.Barry, P. A., D. J. Alcendor, M. D. Power, H. Kerr, and P. A. Luciw. 1996. Nucleotide sequence and molecular analysis of the rhesus cytomegalovirus immediate-early gene and the UL121-117 open reading frames. Virology 215:61-72. (Erratum, 218:296.) [DOI] [PubMed] [Google Scholar]
- 3.Baskin, G. B. 1987. Disseminated cytomegalovirus infection in immunodeficient rhesus monkeys. Am. J. Pathol. 129:345-352. [PMC free article] [PubMed] [Google Scholar]
- 4.Battegay, M., D. Moskophidis, A. Rahemtulla, H. Hengartner, T. W. Mak, and R. M. Zinkernagel. 1994. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 68:4700-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, G., P. Shankar, C. Lange, H. Valdez, P. R. Skolnik, L. Wu, N. Manjunath, and J. Lieberman. 2001. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood 98:156-164. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, J. P., R. D. Cardin, K. C. Branum, and P. C. Doherty. 1999. CD4+ T cell-mediated control of a γ-herpesvirus in B cell-deficient mice is mediated by IFN-γ. Proc. Natl. Acad. Sci. USA 96:5135-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godkin, A., N. Jeanguet, M. Thursz, P. Openshaw, and H. Thomas. 2001. Characterization of novel HLA-DR11-restricted HCV epitopes reveals both qualitative and quantitative differences in HCV-specific CD4+ T-cell responses in chronically infected and non-viremic patients. Eur. J. Immunol. 31:1438-1446. [DOI] [PubMed] [Google Scholar]
- 8.Hamann, D., P. A. Baars, B. Hooibrink, and R. W. van Lier. 1996. Heterogeneity of the human CD4+ T-cell population: two distinct CD4+ T-cell subsets characterized by coexpression of CD45RA and CD45RO isoforms. Blood 88:3513-3521. [PubMed] [Google Scholar]
- 9.Hamann, D., P. A. Baars, M. H. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazenberg, M. D., D. Hamann, H. Schuitemaker, and F. Miedema. 2000. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat. Immunol. 1:285-289. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson, M. A., R. Schrier, J. M. McCune, F. J. Torriani, G. N. Holland, J. J. O'Donnell, W. R. Freeman, and B. M. Bredt. 2001. Cytomegalovirus (CMV)-specific CD4+ T lymphocyte immune function in long-term survivors of AIDS-related CMV end-organ disease who are receiving potent antiretroviral therapy. J. Infect. Dis. 183:1399-1404. [DOI] [PubMed] [Google Scholar]
- 12.Jennings, S. R., R. H. Bonneau, P. M. Smith, R. M. Wolcott, and R. Chervenak. 1991. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell. Immunol. 133:234-252. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, R. P., R. L. Glickman, J. Q. Yang, A. Kaur, J. T. Dion, M. J. Mulligan, and R. C. Desrosiers. 1997. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J. Virol. 71:7711-7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. S. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur, A., M. D. Daniel, D. Hempel, D. Lee-Parritz, M. S. Hirsch, and R. P. Johnson. 1996. Cytotoxic T-lymphocyte responses to cytomegalovirus in normal and simian immunodeficiency virus-infected rhesus macaques. J. Virol. 70:7725-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keane, N. M., P. Price, S. F. Stone, M. John, R. J. Murray, and M. A. French. 2000. Assessment of immune function by lymphoproliferation underestimates lymphocyte functional capacity in HIV patients treated with highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 16:1991-1996. [DOI] [PubMed] [Google Scholar]
- 17.Komanduri, K. V., J. Feinberg, R. K. Hutchins, R. D. Frame, D. K. Schmidt, M. N. Viswanathan, J. P. Lalezari, and J. M. McCune. 2001. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J. Infect. Dis. 183:1285-1289. [DOI] [PubMed] [Google Scholar]
- 18.Komanduri, K. V., M. N. Viswanathan, E. D. Wieder, D. K. Schmidt, B. M. Bredt, M. A. Jacobson, and J. M. McCune. 1998. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat. Med. 4:953-956. [DOI] [PubMed] [Google Scholar]
- 19.Laouar, Y., and I. N. Crispe. 2000. Functional flexibility in T cells: independent regulation of CD4+ T cell proliferation and effector function in vivo. Immunity 13:291-301. [DOI] [PubMed] [Google Scholar]
- 20.Ledergerber, B., M. Egger, V. Erard, R. Weber, B. Hirschel, H. Furrer, M. Battegay, P. Vernazza, E. Bernasconi, M. Opravil, D. Kaufmann, P. Sudre, P. Francioli, A. Telenti, and The Swiss HIV Cohort Study. 1999. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy. JAMA 282:2220-2226. [DOI] [PubMed] [Google Scholar]
- 21.Maloy, K. J., C. Burkhart, T. M. Junt, B. Odermatt, A. Oxenius, L. Piali, R. M. Zinkernagel, and H. Hengartner. 2000. CD4+ T cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability. J. Exp. Med. 191:2159-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picker, L. J., and V. C. Maino. 2000. The CD4+ T cell response to HIV-1. Curr. Opin. Immunol. 12:381-386. [DOI] [PubMed] [Google Scholar]
- 24.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518-525. [DOI] [PubMed] [Google Scholar]
- 25.Rentenaar, R. J., L. E. Gamadia, N. van DerHoek, F. N. van Diepen, R. Boom, J. F. Weel, P. M. Wertheim-van Dillen, R. A. van Lier, and I. J. ten Berge. 2000. Development of virus-specific CD4+ T cells during primary cytomegalovirus infection. J. Clin. Investig. 105:541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reusser, P., S. R. Riddell, J. D. Meyers, and P. D. Greenberg. 1991. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 78:1373-1380. [PubMed] [Google Scholar]
- 27.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 28.Salmon-Ceron, D., M. C. Mazeron, S. Chaput, N. Boukli, B. Senechal, N. Houhou, C. Katlama, S. Matheron, A. M. Fillet, J. Gozlan, C. Leport, V. Jeantils, F. Freymuth, and D. Costagliola. 2000. Plasma cytomegalovirus DNA, pp65 antigenaemia and a low CD4 cell count remain risk factors for cytomegalovirus disease in patients receiving highly active antiretroviral therapy. AIDS 14:1041-1049. [DOI] [PubMed] [Google Scholar]
- 29.Simon, M. A., L. V. Chalifoux, and D. J. Ringler. 1992. Pathologic features of SIV-induced disease and the association of macrophage infection with disease evolution. AIDS Res. Hum. Retrovir. 8:327-337. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1998. Virus-specific CD8+ T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc. Natl. Acad. Sci. USA 95:15565-15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retrovir. 14:183-189. [DOI] [PubMed] [Google Scholar]
- 32.Swack, N. S., and G. D. Hsiung. 1982. Natural and experimental simian cytomegalovirus infections at a primate center. J. Med. Primatol. 11:169-177. [PubMed] [Google Scholar]
- 33.Tripp, R. A., S. R. Sarawar, and P. C. Doherty. 1995. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of the H-2lAb gene. J. Immunol. 155:2955-2959. [PubMed] [Google Scholar]
- 34.van der Meide, P. H., R. J. Groenestein, M. C. de Labie, J. Heeney, P. Pala, and M. Slaoui. 1995. Enumeration of lymphokine-secreting cells as a quantitative measure for cellular immune responses in rhesus macaques. J. Med. Primatol. 24:271-281. [DOI] [PubMed] [Google Scholar]
- 35.van Noesel, C. J., R. A. Gruters, F. G. Terpstra, P. T. Schellekens, R. A. van Lier, and F. Miedema. 1990. Functional and phenotypic evidence for a selective loss of memory T cells in asymptomatic human immunodeficiency virus-infected men. J. Clin. Investig. 86:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 37.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogel, P., B. J. Weigler, H. Kerr, A. G. Hendrickx, and P. A. Barry. 1994. Seroepidemiologic studies of cytomegalovirus infection in a breeding population of rhesus macaques. Lab. Anim. Sci. 44:25-30. [PubMed] [Google Scholar]
- 40.von Herrath, M. G., M. Yokoyama, J. Dockter, M. B. Oldstone, and J. L. Whitton. 1996. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 70:1072-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waldrop, S. L., C. J. Pitcher, D. M. Peterson, V. C. Maino, and L. J. Picker. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Investig. 99:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 43.Willerford, D. M., M. J. Gale, R. E. Benveniste, E. A. Clark, and W. M. Gallatin. 1990. Simian immunodeficiency virus is restricted to a subset of blood CD4+ lymphocytes that includes memory cells. J. Immunol. 144:3779-3783. [PubMed] [Google Scholar]
- 44.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]