Abstract
The organotypic raft culture system has allowed the study of the differentiation-dependent aspects of the human papillomavirus (HPV) life cycle. However, genetic strategies to more completely understand the HPV life cycle are limited. The generation of chimeric viruses has been a useful tool in other virus systems to analyze infection and replication. To investigate the specificity of the interaction of nonstructural genes of one HPV type with the structural genes of another HPV type, we have replaced the L2 and L1 open reading frames (ORFs) of HPV type 18 (HPV18) with the L2 and L1 ORFs of HPV type 16 (HPV16). The resulting HPV18/16 chimeric construct was introduced into primary keratinocytes, where it was stably maintained episomally at a range of 50 to 100 copies of HPV genomic DNA, similar to that typically found in HPV-infected cells in vivo. The integrity of the HPV18/16 genomic DNA chimera was demonstrated. Upon differentiation in raft cultures, late viral functions, including viral DNA amplification, capsid gene expression, and virion morphogenesis, occurred. Chimeric HPV18/16 virions were purified from the raft cultures and were capable of infecting keratinocytes in vitro. Additionally, infection was specifically neutralized with human HPV16 virus-like particle (VLP)-specific antiserum and not with human HPV18 VLP-specific antiserum. Our data demonstrate that the nonstructural genes of HPV18 functionally interact with the structural genes of HPV16, allowing the complete HPV life cycle to occur. We believe that this is the first report of the propagation of chimeric HPV by normal life cycle pathways.
The life cycle of human papillomaviruses (HPV) is intimately connected to the differentiation program of host epithelial tissues (14, 20, 22, 36). The use of an organotypic (raft) epithelial culture system has allowed for the development of an in vitro culture system capable of reproducing the complete HPV life cycle, including the propagation of infectious viral particles (20, 22). The raft culture system has been used to describe in detail the steps in the HPV life cycle (2, 8, 11, 18, 25-30), including the kinetics and spatial patterns of HPV gene expression (18, 25-29). Flores et al. used the raft culture system to begin a genetic analysis of the HPV life cycle by using an E7-deficient HPV type 16 (HPV16) genome (7). They found that this genome, while being maintained episomally, failed to amplify its DNA and expressed reduced levels of the L1 capsid protein. That study was done by using a spontaneously immortalized keratinocyte cell line (1). Attempts have been made to use a genetic approach to study the HPV life cycle by using primary keratinocytes (15, 39). These studies found that the majority of mutations examined, both in noncoding and in coding regions, were unstable in their ability to maintain the viral DNA (vDNA) in an episomal state.
It has been reported that the interaction of the HPV nonstructural proteins, in particular, E2, with the structural capsid proteins, L1 and L2, is important for viral morphogenesis (5, 13). We designed experiments to investigate whether the nonstructural genes from one HPV type could functionally interact with the structural genes of another HPV type, allowing the complete viral life cycle to occur, with the production of infectious progeny. To explore the probability of the functional interaction and the relatedness of the nonstructural and structural genes of two different HPV types, we used the genetic approach of making a chimeric virus.
The generation of chimeric viruses has been a useful genetic tool in other virus systems to analyze viral infectivity, replication, transformation, and virulence factors (3, 4, 6, 9, 10, 12, 16, 17, 23, 24, 33, 34, 37, 38, 40, 41, 43). Chimeric viruses are commonly used to compare genes from one virus with the homologous genes from a related virus to determine the similarities and differences of these genes. A chimeric virus system can be used to assign a particular viral phenotype to a specific gene or sequence. Another use of a chimeric virus system is to ascertain the commonalities of related viral genes. A chimeric virus is typically generated by replacing a gene sequence from one virus with the similar sequence from a related but different virus. Information gained from these kinds of studies includes defining mechanisms of viral replication and pathology and identifying common mechanisms for therapeutic targeting. In addition, chimeric viruses containing altered and optimized capsid protein epitopes could be generated and used as a tool in viral vaccine development.
We have replaced the L1 and L2 capsid protein open reading frames (ORFs) from HPV type 18 (HPV18) with the L1 and L2 capsid protein ORFs from HPV16. The resulting HPV18/16 chimeric construct was introduced into primary keratinocytes and then allowed to grow and differentiate in raft cultures. The HPV18/16 chimeric genomic DNA immortalized primary keratinocytes, was maintained in an episomal state, and induced a transformed phenotype in raft cultures. The episomal HPV18/16 genomic DNA maintained the integrity of the chimeric structure. Late viral functions of vDNA amplification and capsid gene expression appeared normal in raft cultures. The expression of the HPV16-specific structural gene, L1, was demonstrated, and HPV18/16 virus was produced. Finally, early transcript expression and neutralization with HPV16 virus-like particle (VLP)-specific antiserum demonstrated the propagation of infectious virus. This is the first report of the propagation of chimeric HPV virions by normal life cycle pathways.
MATERIALS AND METHODS
Plasmid construction.
HPV18 DNA, a generous gift from Harold zur Hausen, was cloned into pBluescript SK(+) [pBSSK(+)] (Stratagene, La Jolla, Calif.) at the unique HPV18 EcoRI site within the E1 ORF, a unique site within the HPV18 upstream regulatory region (URR). In order to substitute the HPV18 L2 and L1 ORFs with the HPV16 L2 and L1 ORFs, pBSHPV18 was digested at the unique AatII site within the E2 ORF, and the digested DNA was purified with a Centrisep column (Princeton Separations, Inc., Adelphia, N.J.). The purified DNA was further digested with AflII, and the resulting 6,647-bp fragment containing only HPV18 early gene and vector sequences was gel purified by using a QIAex gel extraction kit (Qiagen, Inc., Valencia, Calif.). Using oligonucleotides (sequences are shown in Table 1) named 18 AatII and 18 BglII B (The Midland Certified Reagent Co., Midland, Tex.), a 720-bp PCR product containing HPV18 DNA from the AatII site at nucleotide (nt) 3542 to the start codon of the HPV18 L2 ORF at nt 4246 was generated. The 5′ end of the PCR product contained an AatII restriction site, and the 3′ end contained a BglII restriction site. Using oligonucleotides (Table 1) named 18 AflII and 18 BglII F (The Midland Certified Reagent Co.), a 593-bp PCR product containing HPV18 DNA from the stop codon of the HPV18 L1 ORF at nt 7137 to the AflII restriction site at nt 7730 was generated. The 5′ end of this PCR product contained a BglII restriction site, and the 3′ end contained an AflII restriction site. The 720- and 593-bp products were purified by using a QIAquick PCR purification kit (Qiagen). The 720-bp product was digested with AatII, and the 593-bp product was digested with AflII. The AflII/AatII-digested 6,647-bp product was then ligated to the 720- and 593-bp PCR products, and the resulting ligation product was digested with BglII, purified, and religated. The resulting construct, pBSHPV18ΔL2/L1, contains HPV18 sequences on a pBSSK(+) backbone with the L2 and L1 ORFs removed and replaced with a BglII site.
TABLE 1.
Oligonucleotide primers used for construction of the HPV18/16 chimera
| Oligonucleotide name | Oligonucleotide sequence (5′ to 3′)a |
|---|---|
| 18 AatII | CGG CCA GAC GTC GGC TGC TAC ACG |
| 18 BgIII B | GCT AGC AGA TCT ACT TTT ATT ACA AAA ATA CAA AAA GC |
| 18 AfIII | GTA TGC AAT TAG CTT AAG TAA AAA CAA AC |
| 18 BgIII F | GCT AGC AGA TCT TAT GTG TGT GTG TAT ATA TAT ATA CAT |
| 16 L2 BgIII F | GCT AGC AGA TCT ATG CGA CAC AAA CGT TCT GCA AAA CG |
| 16 L1 BgIII B | GCT AGC AGA TCT TTA CAG CTT ACG TTT TTT GCG TTT AGC AG |
| 16 L1 EcoRIM1 | CA TAC ATA CAT TCT ATG AAC TCC ACT ATT TTG GAG |
| 16 L1 EcoRIM2 | CTC CAA AAT AGT GGA GTT CAT AGA ATG TAT GTA TG |
The EcoRI site is underlined, and the mutated nucleotide is shown in bold type.
Oligonucleotides (Table 1) named 16 L2 BglII F and 16 L1 BglII B were used to generate a 2.9-kb PCR product containing the HPV16 L2 and L1 ORFs. The DNA template was derived from CaSKi cell HPV16 L2 and L1 DNAs previously cloned into pUC18 (Promega, Madison, Wis.). Both the 5′ and 3′ ends of the 2.9-kb PCR product contained a BglII restriction site. The 2.9-kb PCR product was digested with BglII and purified.
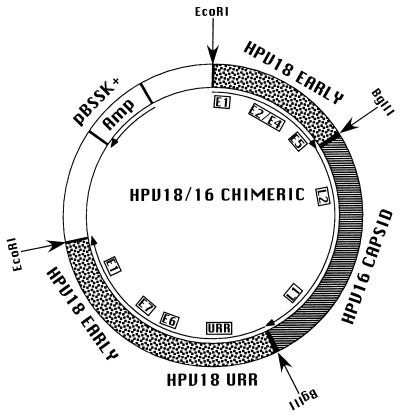
Plasmid pBSHPV18ΔL2/L1 was digested with BglII, dephosphorylated, and ligated to the BglII-digested HPV16 L2-L1 2.9-kb PCR product. The resulting plasmid contained the complete URR and early genes of HPV18 and the late genes, L2 and L1, of HPV16. The HPV16 L1 ORF contained a single EcoRI restriction site. The electroporation protocol requires the release of the HPV genomic DNA from the vector DNA sequences. Since the HPV18 genomic DNA was cloned into the vector DNA at an EcoRI site, it was necessary to mutate the EcoRI site contained within the HPV16 L1 ORF at nt 6819. Site-directed mutagenesis was performed by using a Quick Change site-directed mutagenesis kit (Stratagene) according to manufacturer directions. To alter the EcoRI site of the HPV16 L1 protein without altering the amino acid sequence, oligonucleotides (Table 1) named 16 L1 EcoRIM1 and 16 L1 EcoRIM2 were used. The EcoRI restriction site GAATTA was changed to GAACTC, altering the Asn code used from AAT to AAC. Sequence and restriction endonuclease analyses confirmed the single nucleotide change in the mutated EcoRI restriction site. The final plasmid product was designated pHPV18/16 (Fig. 1).
FIG. 1.
HPV18/16 chimeric recombinant plasmid. Speckled areas represent HPV18 early genes and the URR. The striped area represents HPV16 late genes. The approximate positions of the HPV ORFs are shown in insets. White areas represent the pBSSK(+) vector; the position of the Ampr gene is shown. HPV18 was cloned into pBSSK(+) at the unique EcoRI site. The HPV18 late gene ORFs were removed and replaced with a unique BglII site. The HPV16 late gene ORFs were inserted into this unique BglII site.
pCR18-L1 was produced by TA cloning (Invitrogen, San Diego, Calif.) a 1,701-bp PCR product generated by amplification with primers corresponding to nt 5426 to 5452 (5′) and nt 7198 to 7173 (3′) of the HPV18 genome. pHPV16-RL1 was produced by first cloning the HPV16 genomic DNA into vector pBSSK(+) at the unique BamHI site at nt 6151 within the L1 ORF, making plasmid pBSHPV16. pBSHPV16 was then digested with EcoRI. EcoRI digested pBSHPV16 in three places, once in the pBSSK(+) polylinker and twice in the HPV16 sequence, at nt 6819 and 7454. The 3,618-bp fragment containing pBSSK(+) vector sequences and HPV16 L1 ORF nt 6151 to 6819 was gel purified and recircularized, creating pHPV16-RL1.
Cell and raft cultures.
Primary human foreskin keratinocytes (HFK) were purchased from BioWhittaker (Walkersville, Md.). Keratinocytes were grown in monolayer cultures by using KGM-2 growth medium (BioWhittaker). The HCK18:1Bj cell line (22) and keratinocyte cell lines stably maintaining HPV18/16 DNA following electroporation were grown in monolayer cultures by using E medium (unless otherwise stated, our E medium lacks epidermal growth factor [EGF]) in the presence of mitomycin C-treated J2 3T3 feeder cells (19, 20, 22).
Raft cultures were grown as previously described (19, 20, 22). Briefly, cell lines were seeded onto rat tail type 1 collagen matrices containing J2 3T3 feeder cells not treated with mitomycin C. After cell attachment and growth to confluence, collagen matrices were lifted onto stainless steel grids. Once lifted to the air-liquid interface, raft cultures were fed by diffusion from below with E medium. Raft cultures were treated with 10 μM 1,2-dioctanoyl-sn-glycerol (C8; Sigma Chemical Co., St. Louis, Mo.) in E medium every other day. Viral gene expression has been shown to peak after 10 to 12 days in the raft system (25-29); therefore, raft cultures were allowed to stratify and differentiate for 12 days.
Electroporation of primary keratinocytes.
pHPV18/16 plasmid DNA was digested with EcoRI, linearizing the vDNA and separating it from the vector DNA. For each electroporation, we mixed 10 μl of EcoRI-digested pHPV18/16 plasmid DNA (1 μg/μl) and 4.25 μl of sonicated and denatured salmon sperm DNA (10 μg/μl) in a 1.5-ml Eppendorf tube. Keratinocytes (5 × 106) in a volume of 250 μl of E medium containing 10% fetal bovine serum (FBS) and 5 mM N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid were added to each DNA mixture. DNA-keratinocyte solution was transferred to an electroporation cuvette and electroporated by using a Gene Pulser (Bio-Rad Laboratories, Hercules, Calif.) set at 210 V and 960 μF. The electroporated cell solution was then layered onto 10 ml of E medium containing 10% FBS and centrifuged at 25 × g for 10 min. The medium was removed, the cell pellets were resuspended in E medium containing 10% FBS, and the suspensions were added to 10-cm tissue culture plates containing mitomycin C-treated J2 3T3 feeder cells. On the following day, 5 ng of EGF/ml was added to the culture medium. Cultures were fed every other day for 7 days with E medium containing 10% FBS and 5 ng of EGF/ml. After 7 days, cells were fed with E medium containing 5% FBS and 5 ng of EGF/ml until the keratinocytes grew to confluence, after which EGF was omitted from the medium.
Southern (DNA) blot hybridization.
Total cellular DNA was isolated as previously described (20, 22, 25); 5-μg samples were digested with either EcoRI or BglII or left undigested and then electrophoresed in an 0.8% agarose gel. Southern blot hybridization was performed as previously described (20, 22, 25). Blots were probed first with an HPV18 complete genomic probe and then were stripped and probed with an HPV16 complete genomic probe. Stripping was accomplished by placing the blots in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-1% sodium dodecyl sulfate and boiling for 1 h.
Histochemical analyses.
Raft cultures were grown for 12 days, harvested, fixed in 10% buffered formalin, and embedded in paraffin; 4-μm sections were prepared. Sections were stained with hematoxylin and eosin as described previously (18, 20, 21, 29, 30, 42). Immunostaining was done by using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif.) (18, 20, 30, 42). HPV16 VLP-specific antiserum, a generous gift from John T. Schiller, was used for immunostaining (1:500) (31, 32). HCK18:1Bj raft cultures were used as positive and negative controls.
RPAs.
Antisense HPV16 and HPV18 L1 RNA probes were synthesized by using constructs pCR18-L1 and pHPV16-RL1. Total RNA was extracted from raft cultures by using TRIzol reagent according to manufacturer instructions (Gibco BRL, Bethesda, Md.) and was treated with DNase I to remove copurifying vDNA and cellular DNA. Antisense RNA probes were synthesized as previously described (29). pCR18-L1 was digested with AflIII, which yielded a 496-nt probe predicted to protect 420 nt of the HPV18 L1 mRNA. pHPV16-RL1 was digested with SspI to yield a 340-nt probe predicted to protect 267 nt of the HPV16 L1 mRNA. Full-length probes were purified and eluted as described previously (29). RNase protection assays (RPAs) were performed by using an RPAII kit according to manufacturer instructions (Ambion Inc., Austin, Tex.). Ten micrograms of total RNA or yeast RNA was hybridized to 8 × 104 cpm of either the HPV16 or the HPV18 probe at 42°C for 16 h. Unhybridized RNA was digested with RNase Cocktail Mix, which contained 500 U of RNase A/ml and 20,000 U of RNase T1/ml (Ambion). Samples were electrophoresed on a 7 M urea-5% polyacrylamide gel, followed by autoradiography.
HPV particle isolation and electron microscopy.
Virions were isolated by first scraping the raft culture epithelium off the collagen with a scalpel and then washing the tissue with 5 ml of 0.15 M NaCl. Tissue was ground in a mortar with sea sand, resuspended in 15 ml of buffer 1 (1 M NaCl, 0.05 M Na2HPO4 [pH 8.0]), and centrifuged at 4°C for 10 min at 8,000 × g. The supernatant was collected and stored on ice; the pellet was reextracted with 20 ml of buffer 1 and centrifuged at 4°C for 10 min at 8,000 × g. The pellet was discarded, and the supernatants from both centrifugations were pooled and centrifuged at 4°C for 1 h at 130,000 × g. Following centrifugation, the supernatant was discarded, and the pellet was resuspended in 15 ml of buffer 2 (0.05 M NaCl, 0.1 M EDTA, 0.05 M Na2HPO4 [pH 7.4]) and centrifuged at 4°C for 10 min at 8,000 × g. The supernatant was collected and stored on ice; the pellet was reextracted with 15 ml of buffer 2 and centrifuged at 4°C for 10 min at 8,000 × g. The supernatants from these last two centrifugations were pooled and centrifuged at 4°C for 1 h at 130,000 × g. After removal of the supernatant, the pellet was resuspended in 2.5 ml of phosphate-buffered saline and stored at −20°C as virus stocks. Electron microscopy was performed on a portion of the purified virus preparation as previously described (20, 22).
HPV infection and neutralization.
The HPV18/16 infectivity and neutralization studies were done with an immortalized human keratinocyte cell line (kindly provided by Norbert Fusenig) based on the in vitro system described by Smith et al. (35). HaCaT cells were grown to 50% confluence in 96-well flat-bottom tissue culture plates and cultured in Dulbecco's modified Eagle's medium containing (final concentrations) 10% fetal bovine serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 4,500 ng of glucose/liter, l-glutamine, and pyridoxine hydrochloride but no sodium pyruvate. At the time of infection, the medium was aspirated and 50 μl of HPV18/16 diluted to 1:200 was added. For antibody-mediated neutralization, the final dilutions of polyclonal serum used were 1:200, 1:400, 1:800, and 1:1,600. Twenty-five microliters of diluted serum was added to the cells, and the plates were rocked back and forth. Virus diluted in a volume of 25 μl, for a final virus concentration of 1:200, was added immediately. Dilutions of virus and serum were made with Opti-Mem (Gibco BRL) and incubation for 1 h at 37°C, with occasional rocking to mix. An additional 100 μl of Dulbecco's modified Eagle's medium was then added to each well, and the plates were incubated at 37°C in 5% CO2 for 4 days.
The human sera used in this study was obtained from individuals shown to be PCR negative for HPV16 and HPV18 prior to immunization with either HPV16 VLPs or HPV18 VLPs. Pre- and postimmunization sera from a total of three individuals immunized with either 16 VLPs or 18 VLPs were tested.
Detection of the spliced HPV18 E1∧E4 message and cellular β-actin mRNA ∧ by RT-PCR.
HPV18/16 infection of HaCaT cells after 4 days of culturing was demonstrated by reverse transcriptase PCR (RT-PCR) amplification of the spliced HPV18 E1∧E4 mRNA species (35). RNA was extracted from the cells by using the Qiagen RNeasy 96-well method as described by the manufacturer. Efficient and sensitive detection of HPV18 E1∧E4 in this cell culture system required the use of nested PCR primer sets. The locations of the primers used in these studies and their nucleotide sequences are shown in Table 2. A Gibco Quantitative Platinum one-step RT-PCR kit was used with the first set of primers, H18-F1, H18-R1, BA-U1, and BA D2, and the conditions were as follows: 50°C for 30 min for the RT reaction; 95°C for 5 min, 95°C for 15 s, 65°C for 30 s, and 72°C for 30 s for 35 cycles of PCR; 72°C for 7 min; and a 4°C hold. A portion of this reaction (10 μl from the 50-μl reaction) was then used in a second set of PCRs with nested primers H18-F2 and H18-R2 and the following temperature profile: 95°C for 1.3 min, 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s for 35 cycles; 72°C for 7 min; and a 4°C hold. The final amplimer sizes were 733 bp for β-actin and 521 bp for HPV18 E1∧E4. The final concentration of deoxynucleotide triphosphates during cDNA synthesis and PCR was 0.2 mM, and the final concentration of primers was 0.2 μM.
TABLE 2.
RT-PCR primers
| Primer description | Location in nested set | Genomic nucleotides | Nucleotide sequence (5′ to 3′) |
|---|---|---|---|
| HPV 18 | |||
| H18-F1 | Upstream, outside | 530-553 | CAACCGAGCACGACAGGAACGAC |
| H18-R1 | Downstream, outside | 3583-3604 | CAGGTCCACAATGCTGCTTCTC |
| H18-F2 | Upstream, inside | 554-574 | TCCAACGACGCAGAGAAACAC |
| H18-R2 | Downstream, inside | 3560-3580 | GAGTCCACAGTGTCCAGGTC |
| β-Actin | |||
| BA-U1 | Upstream, outside | 1578-1587 and 2029-2039 | GATGACCCAGATCATGTTTG |
| BA-D2 | Downstream, outside | 2735-2744 and 2857-2876 | GGAGCAATGATCTTGATCTTC |
Negative control PCRs were routinely performed to confirm the absence of contamination events. The PCR products were separated by 2% agarose-ethidium gel electrophoresis and photographed with Polaroid film.
RESULTS
Construction of an HPV18/16 chimeric genomic plasmid.
To investigate whether the nonstructural genes of one HPV type could functionally interact with the structural genes of a second HPV type during the complete viral life cycle, we constructed a recombinant plasmid consisting of the URR and the nonstructural early gene ORFs of HPV18 and the structural late genes ORFs of HPV16 (Fig. 1). We used a plasmid containing the complete HPV18 genome cloned at the unique EcoRI site (nt 2440) within the E1 ORF (22). The HPV18 L2 and L1 ORFs were removed and replaced with a novel BglII site, resulting in pBSHPV18ΔL2/L1. The HPV16 L2-L1 ORF sequence was amplified by using PCR primers which added BglII restriction sites to both ends. The amplified HPV16 L2-L1 ORF sequence containing BglII at its ends was ligated to pBSHPV18ΔL2/L1 at its unique BglII site. We designated the resulting chimeric plasmid pHPV18/16.
Development of cell lines maintaining episomal copies of HPV18/16 chimeric genomic DNA.
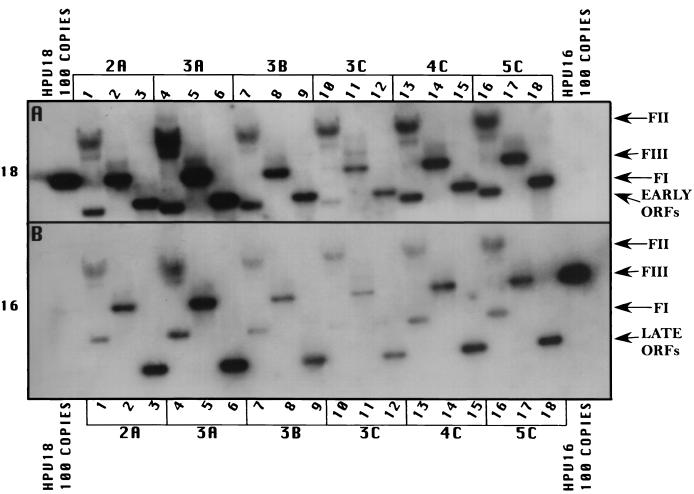
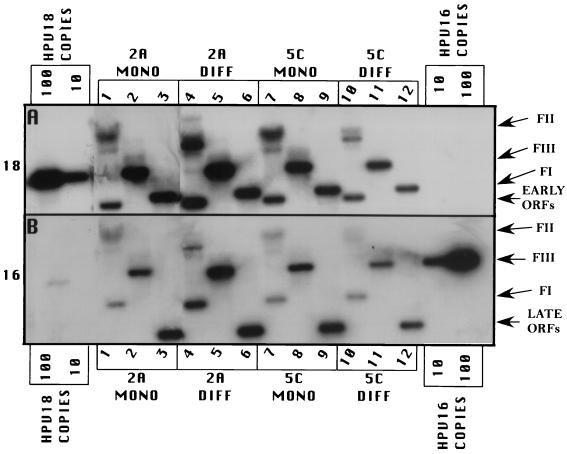
In order to develop cell lines maintaining episomal copies of HPV18/16 chimeric DNA, we used an electroporation technique previously developed by our laboratory to establish cell lines maintaining episomal copies of wild-type HPV18 (22). The pHPV18/16 chimeric plasmid was digested with EcoRI, linearizing and separating the HPV18/16 genomic DNA from the vector DNA. Several different batches of primary HFK were electroporated with the linearized HPV18/16 chimeric DNA. Cells from an individual electroporation were pooled and expanded into cell lines. Recircularization and episomal maintenance of the HPV18/16 chimeric genome were confirmed by Southern blot hybridization (Fig. 2). All cell lines maintained HPV18/16 chimeric genomic DNA episomally, most averaging approximately 50 to 100 copies per cell (Fig. 2, lanes 3, 6, 9, 12, 15, and 18), as determined with copy number controls. These results demonstrated that exchanging the HPV18 L2 and L1 ORFs with the L2 and L1 ORFs of HPV16 had no effect on the ability of the genomic vDNA to recircularize and replicate in an episomal state.
FIG. 2.
Southern (DNA) blot hybridization of chimeric HPV18/16 DNA-electroporated HFK cell lines grown in monolayer cultures. Six independently derived cell lines, 2A, 3A, 3B, 3C, 4C, and 5C, were analyzed for the episomal maintenance, copy number, and integrity of the chimeric HPV18/16 genomic DNA. (A) The blot was probed with an HPV18-specific probe. (B) The blot was stripped and reprobed with an HPV16-specific probe. Samples in lanes 1, 4, 7, 10, 13, and 16 were undigested. Samples in lanes 2, 5, 8, 11, 14, and 17 were digested with EcoRI, a single cutter of the HPV18/16 genome. Samples in lanes 3, 6, 9, 12, 15, and 18 were digested with BglII to separate the HPV18 sequences (early ORFs) from the HPV16 sequences (late ORFs). HPV18 genomic 100-copy-number standards (left side) and HPV16 genomic 100-copy-number standards (right side) are shown. Arrows indicate form I DNA (FI), form II DNA (FII), form III DNA (FIII), early ORFs (HPV18), and late ORFs (HPV16).
Total DNA was isolated from cells after several weeks in cultures, during which time a minimum of 20 population doublings occurred, demonstrating stable vDNA replication and episomal maintenance. Additionally, in these cell lines we were unable to detect the presence of integrated HPV DNA, which would have appeared as a band migrating slowly above the nicked or form II DNA on the Southern blot, coincident with high-molecular-weight cellular DNA; this result validated the efficiency of our system for establishing cell lines with episomally replicating HPV DNA. The number of episomally maintained HPV18/16 chimeric genomes was similar to what we usually observe with wild-type HPV18, which is, on average, higher than what we usually see with other HPV types. The efficiency of HPV18/16 chimeric vDNA for developing cell lines capable of stable episomal maintenance was 100%, similar to what we achieve with wild-type HPV18, whereas other HPV types develop cell lines capable of stable episomal maintenance about 50% of the time.
Southern blotted DNA was probed with HPV18 radiolabeled probes (Fig. 2A). The blots then were stripped and reprobed with HPV16 radiolabeled probes (Fig. 2B) to demonstrate the presence of both HPV18 and HPV16 DNAs. Southern blotted DNA was further digested with EcoRI to linearize the genomic vDNA (Fig. 2, lanes 1, 4, 7, 10, 13, and 16) and BglII to separate the HPV16 sequences from the HPV18 sequences (Fig. 2, lanes 2, 5, 8, 11, 14, and 17). Blots probed with HPV18 or HPV16 and digested with EcoRI demonstrated a linear band consistent with a length of 7,803 nt, the size of the HPV18/16 genomic DNA. Blots probed with HPV18 and digested with BglII showed a band consistent with a length of 4,887 nt (Fig. 2A, lanes 2, 5, 8, 11, 14, and 17), the size of the URR and early region of HPV18. Blots probed with HPV16 and digested with BglII showed a band consistent with a length of 2,916 nt (Fig. 2B, lanes 2, 5, 8, 11, 14, and 17), representing the L2 and L1 ORFs of HPV16. Blots containing undigested DNA (Fig. 2, lanes 3, 6, 9, 12, 15, and 18) depicted supercoiled (FI) and nicked (FII) genomic DNAs when probed with either HPV18 or HPV16. This analysis shows that the vDNA was recircularized and was being maintained episomally at 50 to 100 copies per cell and that the chimeric structure was preserved.
Morphology and L1 expression in HPV18/16 cell lines grown in raft cultures.
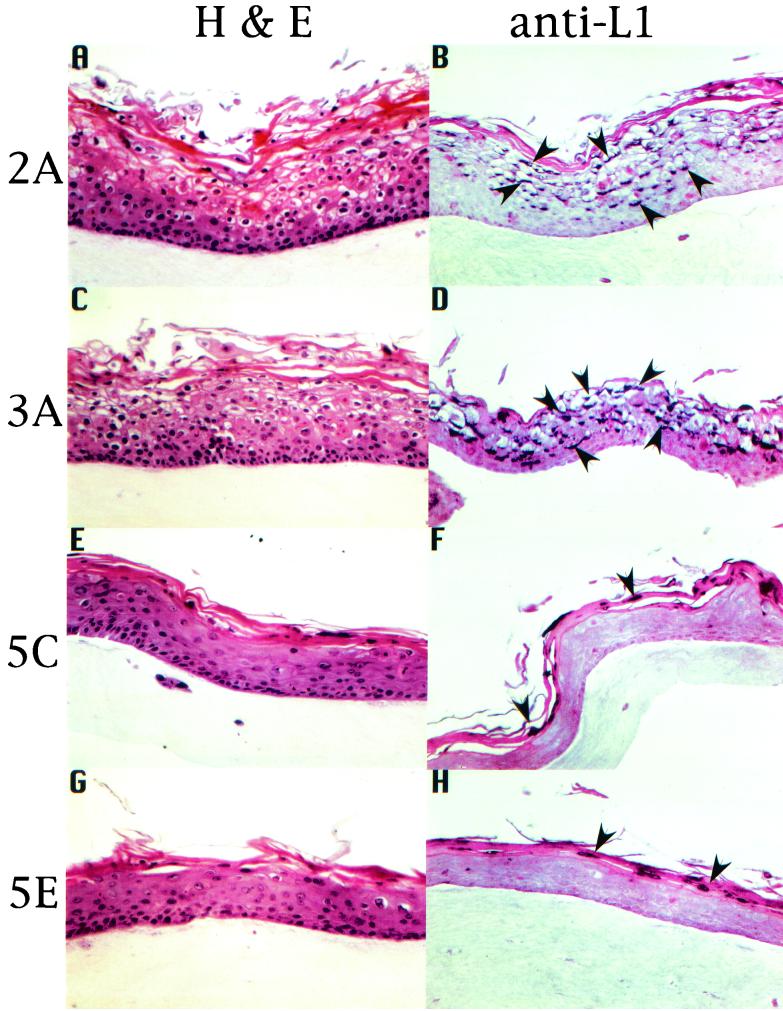
A few HPV18/16 chimeric cell lines were selected for further analysis. We were interested in examining the effect of HPV18/16 on tissue differentiation and morphology. HPV18/16 chimeric cell lines were allowed to grow as stratified and differentiated epithelial tissues in raft cultures. Raft culture tissue sections were stained with hematoxylin and eosin to observe tissue morphology. Figure 3 shows the results for four representative cell lines, HPV18/16:2A, HPV18/16:3A, HPV18/16:5C, and HPV18/16:5E. Two general patterns were observed. The first pattern displayed numerous koilocytes across the upper strata of the tissue, parakeratosis, and a greatly disturbed cornified layer (Fig. 3, HPV18/16:2A and HPV18/16:3A); the second pattern displayed only an occasional koilocyte, intermittent parakeratotic bodies, and a more normal-looking cornified layer (Fig. 3, HPV18/16:5C and HPV18/16:5E). These two patterns were observed throughout the tissues. The first pattern (Fig. 3, HPV18/16:2A and HPV18/16:3A) clearly demonstrated more of the characteristics that one would associate with a productive infection (36).
FIG.3.
IHC analysis of chimeric HPV18/16-infected tissues. HPV18/16-infected, fully stratified and differentiated raft culture tissues were stained with hematoxylin and eosin (A, C, E, and G) or immunostained with an HPV16 L1 VLP polyclonal antiserum (B, D, F, and H). Arrowheads indicate examples of L1 protein expression staining positive within tissue thin sections. Four independently derived cell lines, 2A, 3A, 5C, and 5E, are shown representing the two phenotypic patterns observed. The first phenotypic pattern (2A and 3A) exhibits a more disturbed differentiation program reminiscent of cervical intraepithelial neoplasia type I or II and a high level of L1 expression. The second phenotypic pattern (5C and 5E) exhibits a much less disturbed differentiation program and a low level of L1 expression.
To determine whether the HPV18/16 raft culture tissues were capable of reproducing the complete HPV life cycle, we investigated whether late steps in the viral life cycle occurred in the differentiated raft culture tissues. To do this, we initially investigated the ability of the raft culture tissues to express the major viral capsid protein, L1. Immunohistochemistry (IHC) analysis was performed to demonstrate the expression of the L1 capsid protein (Fig. 3). The expression of the L1 capsid protein was detected by using antiserum raised against HPV16 VLPs (31, 32). In tissues displaying numerous koilocytes, parakeratosis, and a greatly disturbed cornified layer, high levels of L1 capsid protein were clearly expressed in the nuclei of cells of the intermediate layers (Fig. 3). Tissues with only occasional koilocytes had L1 capsid protein expression detected by IHC analysis to a similar magnitude across the upper strata. Raft culture tissues grown from HCK18:1Bj, an HPV18-positive cell line, were found negative for the expression of L1 capsid protein by use of HPV16 VLP-specific antiserum (data not shown). These data suggest that there is a correlation between the tissue morphology and cytopathic effects (CPE) of a viral infection and the ability to express viral structural genes.
RPAs to identify the specific expression of the HPV16 L1 capsid protein in HPV18/16 chimeric raft cultures.
Southern blot analysis definitively confirmed that the chimeric nature of the viral genomes was maintained in the cell lines that we developed. IHC analysis provided evidence that the HPV16 L1 capsid protein was efficiently expressed from the chimeric vDNA. To provide further evidence that the major HPV16 L1 capsid protein was being expressed and to compare the levels of expression between the two morphological tissue patterns observed, RPAs were performed. Two cell lines, HPV18/16:2A and HPV18/16:5C, representing the two tissue morphology patterns, were chosen for this analysis. Total RNA was harvested from HPV18/16:2A and HPV18/16:5C raft culture tissues allowed to grow and differentiate for 10 days at the air-liquid interface. For a positive control of HPV18 L1 expression, we used the HCK18:1Bj cell line, capable of undergoing the complete viral life cycle, including the synthesis of infectious HPV18 particles (22). HCK18:1Bj cells were also allowed to grow and differentiate for 10 days in raft cultures, and then total RNA was harvested.
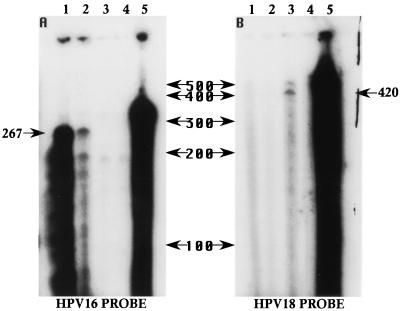
RNA samples were analyzed by RPAs with antisense RNA probes specific to internal regions of the HPV16 and HPV18 L1 ORFs. The HPV16 and HPV18 L1-specific probes were made to protect fragments of 267 and 420 nt, respectively. In both HPV18/16 chimeric cell lines, 267-nt fragments were protected when the HPV16 L1-specific probe was used, whereas the HPV18 L1-specific probe did not produce a protected fragment when RNA from the chimeric virus tissues was used (Fig. 4). The film was overexposed to demonstrate the difference in expression between the two cell lines representing the two tissue morphology patterns observed. The HPV18 L1-specific probe protected a 420-nt fragment only when RNA from HCK18:1Bj raft culture tissue was used (Fig. 4). Like the results observed in the IHC analysis, the RPA results showed that HPV18/16:2A raft cultures produced much higher levels of HPV16 L1 than did HPV18/16:5C raft cultures. These data again suggest a direct relationship between tissue CPE and the expression of viral capsid proteins.
FIG. 4.
RPAs quantifying HPV L1 transcripts. Chimeric HPV18/16- and HPV18-infected cell lines were fully stratified and differentiated in raft cultures. Ten micrograms of DNase I-treated total RNA or yeast RNA was analyzed. The locations and sizes of RNA Century Markers (Ambion) are shown between panels A and B in nucleotides. (A) Samples were probed with an HPV16 L1-specific probe (340 nt) which was predicted to specifically protect an mRNA fragment 267 nt in length. Lane 1 contains RNA from chimeric HPV18/16:2A raft culture tissue. Lane 2 contains RNA from chimeric HPV18/16:5C raft culture tissue. Lane 3 contains RNA from HPV18-infected raft culture tissue (HCK18:1Bj cell line). Lane 4 contains yeast RNA samples digested with RNase to show probe specificity. Lane 5 contains yeast RNA samples left undigested to show the sizes of the input probes. (B) Samples were probed with an HPV18 L1-specific probe (496 nt) which was predicted to specifically protect a fragment 420 nt in length. Lane 1 contains RNA from chimeric HPV18/16:2A raft culture tissue. Lane 2 contains RNA from chimeric HPV18/16:5C raft culture tissue. Lane 3 contains RNA from HPV18-infected raft culture tissue (HCK18:1Bj cell line). Lane 4 contains yeast RNA samples digested with RNase to show probe specificity. Lane 5 contains yeast RNA samples left undigested to show the sizes of the input probes.
Amplification of viral genomic DNA in HPV18/16 cell lines grown in raft cultures.
It appeared that the tissue phenotype was directly related to the competency for expressing late viral life cycle functions, such as viral capsid protein expression. Another important step in the life cycle of HPV is the amplification of its genomic DNA in differentiating cells of the host tissue in preparation for encapsidation by the capsid proteins. To investigate HPV DNA amplification, total tissue DNA was harvested from HPV18/16:2A and HPV18/16:5C raft cultures and analyzed by Southern blot analysis. Southern blots were probed with HPV18-specific probes, stripped, and reprobed with HPV16-specific probes. Samples of DNA were analyzed undigested (Fig. 5, lanes 1, 4, 7, and 10), EcoRI digested to linearize the chimeric viral genomes (Fig. 5, lanes 2, 5, 8, and 11), and BglII digested to separate the HPV18 URR and early gene sequences from the HPV16 L2 and L1 sequences (Fig. 5, lanes 3, 6, 9, and 12). The bands revealed were true to the chimeric nature of the virus. EcoRI-digested blots probed with either HPV18 or HPV16 demonstrated a linear band approximately 7,800 nt in length, consistent with linearized HPV18/16 genomic DNA (Fig. 5, lanes 2, 5, 8, and 11). HPV16-probed blots showed a band approximately 2,900 nt in length, the size of the HPV16 L2 and L1 ORFs following digestion with BglII (Fig. 5, lanes 3 and 6). HPV18-probed blots showed a band approximately 4,900 nt in length, a size consistent with the HPV18 URR and early ORFs following digestion with BglII (Fig. 5, lanes 9 and 12). Whether probed with HPV18 or HPV16, blots containing undigested DNA exhibited supercoiled (FI) and nicked (FII) genomic DNAs (Fig. 5, lanes 1, 4, 7, and 10).
FIG. 5.
Southern blot hybridization analysis of chimeric HPV18/16 genome amplification in differentiating host tissue. Chimeric HPV18/16:2A cells (lanes 1 to 6) and chimeric HPV18/16:5C cells (lanes 7 to 12) were grown in monolayers or grown and differentiated in raft cultures. Lanes 1 to 3 and 7 to 9 contain DNAs from undifferentiated monolayer cultures (MONO), and lanes 4 to 6 and 10 to 12 contain DNAs from differentiated raft cultures (DIFF). Total DNA from monolayer cultures was prepared as described in the legend to Fig. 2 and Materials and Methods. Raft cultures were harvested after lifting to the air-liquid interface after 12 days of growth and differentiation. Five micrograms of total cellular DNA was separated by electrophoresis on an 0.8% agarose gel, transferred to a nylon membrane, and hybridized with a 32P-labeled probe made either from the complete HPV18 genome or from the complete HPV16 genome. Samples in lanes 1, 4, 7, and 10 were left undigested. Samples in lanes 2, 5, 8, and 11 were digested with EcoRI, a single cutter of the HPV18/16 genome. Samples in lanes 3, 6, 9, and 12 were digested with BglII to separate the HPV18 sequences (early ORFs) from the HPV16 sequences (late ORFs). HPV18 genomic 100- and 10-copy-number standards (left side) and HPV16 genomic 100- and 10-copy-number standards (right side) are shown. Arrows indicate form I DNA (FI), form II DNA (FII), form III DNA (FIII), early ORFs (HPV18), and late ORFs (HPV16).
While both cell lines had equivalent viral genomic copy numbers in monolayer cultures, it was observed that HPV18/16:2A cells amplified their vDNA during differentiation of the host tissue in raft cultures, whereas HPV18/16:5C cells did not amplify their vDNA during host tissue differentiation (Fig. 5, compare lanes 2 and 5 or lanes 8 and 11). These data strengthen the suggestion that the tissue phenotype directly relates to the efficiency with which late viral life cycle functions occur. They also suggest that a viral genome dosage effect dependent on the level of amplified viral genomes in differentiating tissues and not the maintenance copy number seen in proliferating monolayer cell cultures may be associated with CPE and structural gene expression.
Infectious chimeric HPV18/16 biosynthesis.
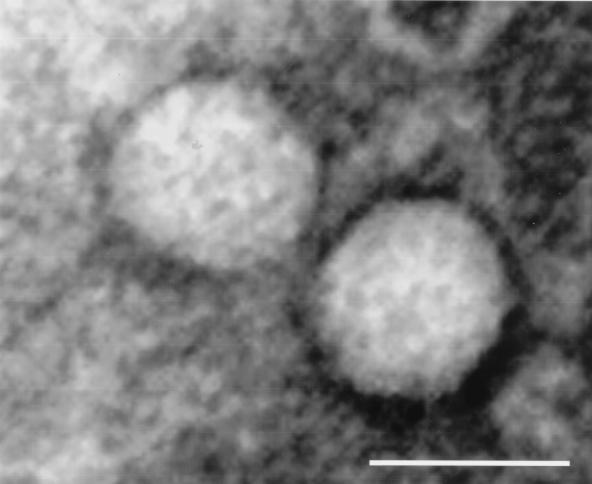
The final step in the HPV life cycle is the biosynthesis of infectious viral particles. To investigate the possibility that chimeric HPV18/16 raft culture tissues allow for the morphogenesis of virions, putative viral stocks were prepared and examined by electron microscopy. Electron microscopic examination showed the presence of viral particles of the proper shape and approximately 50 to 55 nm in diameter (Fig. 6).
FIG. 6.
Electron micrograph of chimeric HPV18/16 virions, approximately 50 nm in diameter. Viral stocks were prepared as described in Materials and Methods. Bar, 50 nm.
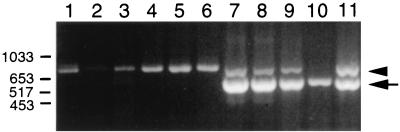
We next wanted to know whether our HPV18/16 stocks were infectious in an HaCaT cell infection assay (35). HaCaT cells were infected with dilutions of chimeric HPV18/16 stocks. Cells were harvested after 4 days, and virus infection was detected by the presence of the HPV spliced E1∧E4 transcript. Nested RT-PCR produced a 521-bp HPV18 E1∧E4 product (Fig. 7). Chimeric HPV18/16:2A was infectious to a 1:400 dilution in our HaCaT cell assay, while the HPV18/16:5C preparation did not contain detectable infectious virions.
FIG. 7.
Infectious titers of chimeric HPV18/16:5C and HPV18/16:2A. Shown is a 2% agarose gel of nested RT-PCR-amplified HPV18 E1∧E4 and β-actin. Lane 1, negative control (no virus). Lanes 2 to 6, HPV18/16:5C at 1:10, 1:50, 1:100, 1:200, and 1:400, respectively. Lanes 7 to 11, HPV18/16:2A at 1:10, 1:50, 1:100, 1:200, and 1:400, respectively. β-Actin is indicated by the arrowhead, and HPV18 E1∧E4 is indicated by the arrow. Molecular size markers in base pairs are indicated on the left.
Neutralization analyses.
After determining that our HPV18/16 stocks were infectious, we wanted to determine whether antiserum raised against HPV16 L1 VLPs was capable of neutralizing viral infectivity. HaCaT cells were incubated in the presence of dilutions of pre- and postimmune HPV16 L1 VLP or HPV18 L1 VLP human sera and chimeric HPV18/16. Three separate sets of HPV16 L1 VLP or HPV18 VLP human sera were used. Cells were harvested after 4 days, and virus infection was again detected by the presence of a 521-bp HPV18 E1∧E4 nested RT-PCR product. The 96-well neutralization assay showed that HPV18/16 was specifically neutralized by HPV16 VLP polyclonal antiserum (Fig. 8A) and was not neutralized by preimmune or HPV18 L1 VLP antiserum (Fig. 8B).
FIG. 8.
Neutralization of chimeric HPV18/16. Shown is a 2% agarose gel of nested RT-PCR-amplified HPV 18 E1∧E4 and β-actin. Arrowheads indicate β-actin and arrows indicate HPV18 E1∧E4 amplified products. Molecular size markers in base pairs are indicated to the left of each gel. The HPV18/16 dilution was 1:200. (A) Pre- and postimmune sera were each diluted 1:200, 1:400, 1:800, and 1:1,600. Lanes 1 to 4, preimmune serum 1. Lanes 5 to 8, postimmune HPV16 VLP serum 1. Lanes 9 to 12, preimmune serum 2. Lanes 13 to 16, postimmune HPV16 VLP serum 2. Lanes 17 to 20, preimmune serum 3. Lanes 21 to 24, postimmune HPV16 VLP serum 3. (B) Pre- and postimmune sera were each diluted 1:200, 1:400, 1:800, and 1:1,600. Lanes 1 to 4, preimmune serum 1. Lanes 5 to 8, postimmune HPV18 VLP serum 1. Lanes 9 to 12, preimmune serum 2. Lanes 13 to 16, postimmune HPV18 VLP serum 2. Lanes 17 to 20, preimmune serum 3. Lanes 21 to 24, postimmune HPV18 VLP serum 3.
DISCUSSION
Using HPV chimeric genomic DNA as a genetic tool, we have investigated the effect on the viral life cycle of exchanging the structural genes between two different HPV types while maintaining the nonstructural genes. We created a genomic construct containing the URR and early (nonstructural) genes of HPV18 combined with the late (structural) genes of HPV16. When this HPV chimeric genomic DNA was introduced into keratinocytes, it was maintained in an episomal state at approximately 50 to 100 copies per cell. Southern blot analysis demonstrated that the chimeric genotype of the vDNA was stable. When HPV18/16-infected cells were allowed to fully stratify and differentiate, they behaved in a manner similar to that of wild-type HPV18-infected cells in our raft culture system (22). When HPV18/16-containing cells were allowed to fully stratify and differentiate in raft cultures, chimeric genomic DNA was amplified, capsid genes were expressed, and complete virion morphogenesis occurred. Diagnostic restriction digestion and Southern blotting demonstrated that the cells contained vDNA composed of the URR and early genes of HPV18 and the late genes of HPV16. Chimeric HPV18/16 raft cultures specifically expressed HPV16 L1 capsid genes. The virions produced in raft cultures were shown to specifically express HPV18 early genes and HPV16 late genes. The infectivity of the viral stocks was neutralized by antibodies raised against HPV16 VLPs and not by antibodies raised against HPV18 VLPs. These data demonstrate that the nonstructural genes of HPV18 can interact with the structural genes of HPV16 to efficiently reproduce the complete HPV life cycle, including the production of infectious virions.
During our studies, we observed an interesting correlation between the raft culture tissue phenotype and the ability of the tissue to produce infectious viral stocks. Raft culture tissues grown from cell lines represented by HPV18/16:2A and HPV18/16:3A exhibited numerous koilocytic cells in the intermediate layers. This finding correlated with a high level of expression of L1 transcripts, as determined by RPAs (Fig. 4), a high level of expression of L1 capsid protein, as determined by IHC analysis (Fig. 3), a high level of genomic amplification (Fig. 5), and the production of infectious progeny virus (Fig. 6 and 8). Cell lines HPV18/16:5C and HPV18/16:5E represented a second tissue phenotype. In raft cultures, this tissue phenotype was only slightly disturbed (Fig. 3), exhibiting only a rare koilocytic cell in the intermediate layers. This finding correlated with a low level of expression of L1 capsid protein, as determined by IHC analysis (Fig. 3), a low level of genomic amplification (Fig. 5), and no detectable production of infectious progeny virus (Fig. 7). Interestingly, in the undifferentiated state, all of the cell lines maintained approximately the same copy number of genomic vDNA molecules episomally per cell (Fig. 2), regardless of their phenotype in raft cultures or their ability to express late viral life cycle functions. This result indicates that neither vDNA copy number nor episomal maintenance directly correlates with the ability of a cell line to support the complete viral life cycle in stratifying and differentiating tissues. We hypothesize that the differences between the cell lines could be due to quantitative or qualitative differences in viral gene expression and/or an undefined selection process of the cells during the electroporation and immortalization process.
It has been proposed that the papillomavirus E2 protein plays a role in the selective encapsidation of papillomavirus DNA. Using immunofluorescence staining, it was observed that in the absence of other viral components, the E2 and L1 proteins were relocalized into promonocytic leukemia protein oncogenic domains when L2 was expressed (5). This L2-dependent colocalization suggests a mechanism whereby the assembly of viral particles is augmented by an increase in the local concentrations of viral gene products involved in virion morphogenesis, raising the potential of interactions with necessary viral components. Recently, a direct protein-protein interaction between E2 and L2 was demonstrated (13). Numerous studies have shown E2 binding to vDNA (14). Additionally, a direct interaction between L2 and vDNA has been shown (44). These findings suggest that papillomavirus morphogenesis occurs via the recruitment of L1 and E2 to defined regions of the nucleus by L2. E2 and/or L2 also recruit vDNA to these same defined nuclear regions. Once the concentrations of the necessary viral components are sufficient, virion morphogenesis occurs. We have shown that to induce efficient virion production in the raft culture system, the addition of a protein kinase C (PKC) activator, such as synthetic diacylglycerol, is necessary (20, 22, 29). The addition of a PKC activator allows for the production of infectious viral stocks. The mechanism whereby PKC activation stimulates virion synthesis is unresolved, although we have demonstrated that raft cultures treated with a PKC activator express higher levels of capsid proteins L1 and L2 (29). This result suggests that PKC signaling pathways stimulate capsid gene expression, increasing the internal pool of capsid proteins available for virion morphogenesis.
In this study, we examined whether an interaction between structural and nonstructural viral genes, required to carry out the complete viral life cycle, needed to be type specific. With HPV18 and HPV16, there was no type-specific constraint. The nonstructural genes of HPV18 were able to functionally interact with the structural genes of HPV16 to carry out all necessary functions of the viral life cycle leading to the production of chimeric progeny virus. As mentioned above, previously published studies suggested that an interaction between the L2 capsid protein and the E2 nonstructural protein is necessary for virion morphogenesis (4, 19, 20, 41). Our study shows that whether an interaction between L2 and E2 is required for virion morphogenesis, this interaction is not type specific. In other words, an E2 protein from one viral type (in our study, HPV18) can functionally interact with the L2 protein of another viral type (in our study, HPV16).
Another goal of this investigation was to examine the efficacy of using chimeric genetic analysis for papillomaviruses in our raft culture system. Chimeric genetic analysis systems have been used with other viruses to study viral infectivity, replication, transforming potential, immunity, and virulence factors. Commonly, chimeric viruses are used to compare genes from one virus with homologous genes from a related virus to attempt to ascertain their similarities and differences. Using a chimeric virus system, one can assign a particular phenotype to a specific gene or viral sequence. Examining chimeric viruses may reveal relatedness between exchanged sequences via the ability of the foreign sequence to provide all the needed functions and interactions for the virus to replicate and exhibit its pathological phenotype. Information gained from such studies can elucidate functional mechanisms of viral replication and pathology and identify common mechanisms for therapeutic targeting. Chimeric viruses have been successfully used to genetically analyze the biology of many DNA viruses, including chimeric JC virus, simian virus 40, BK virus, herpesviruses, and adenoviruses (3, 4, 6, 9, 10, 12, 16, 17, 23, 24, 33, 34, 37, 38, 40, 41, 43). We present here the first successful propagation of a chimeric HPV.
This system of creating chimeric HPV will allow in vivo-like examination of HPV genetics and make possible the evaluation of HPV type-specific antibody production in preventative vaccine studies. Chimeric HPV virions are a valuable resource in HPV vaccine development. Few HPV types have been successfully propagated to produce infectious viral stocks. To test the efficacy of HPV VLP vaccine candidates, viral neutralization assays have been developed. These assays are based upon the premise that vaccinated individuals will produce antibodies that will block HPV infection. Therefore, infectious virus is required. Thus, the ability to produce a source of live virus containing the capsid proteins of a specific HPV type will allow vaccine evaluation in current neutralization systems.
We have demonstrated for the first time the use of a viable chimeric virus system to study HPV genetics. We constructed an HPV18/16 chimeric plasmid containing the nonstructural genes of HPV18 and the structural genes of HPV16. Following electroporation of the chimeric vDNA into primary keratinocytes, HPV18/16 chimeric genomes were maintained episomally. HPV late functions were active in the differentiating tissues of raft cultures, including the synthesis of HPV18/16 chimeric virions. Our study establishes the ability of the nonstructural genes of HPV18 to functionally interact with the structural genes of HPV16. Antibodies directed against HPV16 VLPs and not HPV18 VLPs specifically neutralized the virions. Future studies of chimeric HPVs will allow increased examination of HPV genetics and assessment of HPV vaccine development.
Acknowledgments
We thank Kathleen Brandt, Lyn Budgeon, Danuta Huber, and Scott Hoffman for excellent technical assistance. We are grateful to the Meyers laboratory for critical reading of the manuscript.
This work was supported by an industry university research contract from Merck & Co., Inc., West Point, Pa.
REFERENCES
- 1.Allen-Hoffman, B., S. Schlosser, C. Ivarie, C. Sattler, L. Meissner, and S. O'Conner. 2000. Normal growth and differentiation in a spontaneously immortalized near-diploid keratinocyte cell line, NIKS. J. Investig. Dermatol. 114:444-455. [DOI] [PubMed] [Google Scholar]
- 2.Bedell, M. A., J. B. Hudson, T. R. Golub, M. E. Turyk, M. Hosken, G. D. Wilbanks, and L. A. Laimins. 1991. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J. Virol. 65:2254-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bollag, B., W. Chuke, and R. Frisque. 1989. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: identification of sequences important for efficient transformation. J. Virol. 63:863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel, A., J. Swenson, R. Mayreddy, K. Khalili, and R. Frisque. 1996. Sequences within the early and late promoters of archetype JC virus restrict viral DNA replication and infectivity. Virology 216:90-101. [DOI] [PubMed] [Google Scholar]
- 5.Day, P. M., R. B. Roden, D. R. Lowy, and J. T. Schiller. 1998. The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to promonocytic leukemia protein oncogenic domains. J. Virol. 72:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberle, R., B. Tanamachi, D. Black, E. Blewett, M. Ali, H. Openshaw, and E. Cantin. 1997. Genetic and functional complementation of the HSV1 UL27 gene and gB glycoprotein by simian alpha-herpesvirus homologs. Arch. Virol. 142:721-736. [DOI] [PubMed] [Google Scholar]
- 7.Flores, E. R., B. L. Allen-Hoffmann, D. Lee, and P. F. Lambert. 2000. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J. Virol. 74:6622-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frattini, M. G., H. B. Lim, and L. A. Laimins. 1996. In vitro synthesis of oncogenic human papillomaviruses requires episomal genomes for differentiation-dependent late expression. Proc. Natl. Acad. Sci. USA 93:3062-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gall, J., R. Crystal, and E. Falck-Pedersen. 1998. Construction and characterization of hexon-chimeric adenoviruses: specification of adenovirus serotype. J. Virol. 72:10260-10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gall, J., A. Kass-Eisler, L. Leinwand, and E. Falck-Pedersen. 1996. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J. Virol. 70:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grassmann, K., B. Rapp, H. Maschek, K. U. Petry, and T. Iftner. 1996. Identification of a differentiation-inducible promoter in the E7 open reading frame of human papillomavirus type 16 (HPV-16) in raft cultures of a new cell line containing high copy numbers of episomal HPV-16 DNA. J. Virol. 70:2339-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haggerty, S., D. Walker, and R. Frisque. 1989. JC virus-simian virus 40 genomes containing heterologous regulatory signals and chimeric early regions: identification of regions restricting transformation by JC virus. J. Virol. 63:2180-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heino, P., J. Zhou, and P. Lambert. 2000. Interaction of the papillomavirus transcription/replication factor, E2, and the viral capsid protein, L2. Virology 276:304-314. [DOI] [PubMed] [Google Scholar]
- 14.Howley, P. 1996. Papillomavirinae: the viruses and their replication, p. 2045-2076. In B. Fields and D. Knipe (ed.), Fields virology, 3rd ed. Raven Press, New York, N.Y.
- 15.Hubert, W. G., T. Kanaya, and L. A. Laimins. 1999. DNA replication of human papillomavirus type 31 is modulated by elements of the upstream regulatory region that lie 5′ of the minimal origin. J. Virol. 73:1835-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krasnykh, V., G. Mikheeva, J. Douglas, and D. Curiel. 1996. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 70:6839-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch, K., S. Haggerty, and R. Frisque. 1994. DNA replication of chimeric JC virus-simian virus 40 genomes. Virology 204:819-822. [DOI] [PubMed] [Google Scholar]
- 18.Mayer, T., and C. Meyers. 1998. Temporal and spatial expression of the E5a protein during the differentiation-dependent life cycle of human papillomavirus type 31b. Virology 248:208-217. [DOI] [PubMed] [Google Scholar]
- 19.Meyers, C. 1996. Organotypic (raft) epithelial tissue culture system for the differentiation-dependent replication of papillomavirus. Methods Cell Sci. 18:201-210. [Google Scholar]
- 20.Meyers, C., M. G. Frattini, J. B. Hudson, and L. A. Laimins. 1992. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science 257:971-973. [DOI] [PubMed] [Google Scholar]
- 21.Meyers, C., M. Mane, M. Kokorina, S. Alam, and P. Hermonat. 2000. Ubiquitous human adeno-associated virus type 2 autonomously replicates in differentiating keratinocytes of a normal skin model. Virology 272:338-346. [DOI] [PubMed] [Google Scholar]
- 22.Meyers, C., T. J. Mayer, and M. A. Ozbun. 1997. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J. Virol. 71:7381-7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazawa, N., P. Leopold, N. Hackett, B. Ferris, S. Worgall, E. Falck-Pedersen, and R. Crystal. 1999. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 73:6056-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Neill, F., X. Xu, and Y. Gao. 1992. Host range analysis of a chimeric simian virus 40 genome containing the BKV capsid genes. Virus Res. 25:169-187. [DOI] [PubMed] [Google Scholar]
- 25.Ozbun, M., and C. Meyers. 1998. Hum. papillomavirus type 31b E1 and E2 transcript expression correlates with vegetative viral genome amplification. Virology 248:218-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozbun, M., and C. Meyers. 1999. Human papillomavirus type 31b transcription during the differentiation-dependent viral life cycle. Curr. Top. Virol. 1:203-217. [Google Scholar]
- 27.Ozbun, M., and C. Meyers. 1998. Temporal usage of multiple promoters during the life cycle of human papillomavirus type 31b. J. Virol. 72:2715-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozbun, M., and C. Meyers. 1999. Two novel promoters in the upstream regulatory region of human papillomavirus type 31b are negatively regulated by epithelial differentiation. J. Virol. 73:3505-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozbun, M. A., and C. Meyers. 1997. Characterization of late gene transcripts expressed during vegetative replication of human papillomavirus type 31b. J. Virol. 71:5161-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozbun, M. A., and C. Meyers. 1996. Transforming growth factor beta1 induces differentiation in human papillomavirus-positive keratinocytes. J. Virol. 70:5437-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roden, R. B., H. L. Greenstone, R. Kirnbauer, F. P. Booy, J. Jessie, D. R. Lowy, and J. T. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 18 virion pseudotype. J. Virol. 70:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roden, R. B., H. L. Hubbert, R. Kirnbauer, N. D. Christensen, J. Jessie, D. R. Lowy, and J. T. Schiller. 1996. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J. Virol. 70:3298-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy, S., P. Shirley, A. McClelland, and M. Kaleko. 1998. Circumvention of immunity to the adenovirus major coat protein hexon. J. Virol. 72:6875-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawada, Y., J. Raskova, K. Fujinaga, and J. Raska, K. 1994. Identification of functional domains of adenovirus tumor-specific transplantation antigen in types 5 and 12 by viable viruses carrying chimeric E1A genes. Int. J. Cancer 57:598-603. [DOI] [PubMed] [Google Scholar]
- 35.Smith, L. H., C. Foster, M. E. Hitchcock, G. S. Leiserowitz, K. Hall, R. Isseroff, N. D. Christensen, and J. W. Kreider. 1995. Titration of HPV-11 infectivity and antibody neutralization can be measured in vitro. J. Investig. Dermatol. 105:438-444. [DOI] [PubMed] [Google Scholar]
- 36.Taichman, L., and R. LaPorta. 1986. The expression of papillomaviruses in epithelial cells, p. 109-139. In N. Salzmans and P. Howley (ed.), The Papovaviridae. The papillomaviruses, vol. 2. Plenum Press, New York, N.Y. [Google Scholar]
- 37.Tavis, J., P. Trowbridge, and R. Frisque. 1994. Converting JCV T antigen Rb binding domain to that of SV40 does not alter JCV's limited transforming activity but does eliminate viral viability. Virology 199:384-392. [DOI] [PubMed] [Google Scholar]
- 38.Telling, G., and J. Williams. 1994. Constructing chimeric type 12-type 5 adenovirus E1A genes and using them to identify an oncogenic determinant of adenovirus type 12. J. Virol. 68:877-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas, J. T., W. G. Hubert, M. N. Ruesch, and L. A. Laimins. 1999. Hum. papillomavirus type 31 oncoproteins E6 and E7 are required for the maintenance of episomes during the viral life cycle in normal human keratinocytes. Proc. Natl. Acad. Sci. USA 96:8449-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trowbridge, P., and R. Frisque. 1993. Analysis of G418-selected Rat2 cells containing prototype, variant, mutant, and chimeric JC virus and SV40 genomes. Virology 196:458-474. [DOI] [PubMed] [Google Scholar]
- 41.Vacante, D., R. Traub, and E. Major. 1989. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology 170:353-361. [DOI] [PubMed] [Google Scholar]
- 42.Visalli, R., R. Courtney, and C. Meyers. 1997. Infection and replication of herpes simplex virus type 1 in an organotypic epithelial culture system. Virology 230:236-243. [DOI] [PubMed] [Google Scholar]
- 43.Zabner, J., M. Chillon, T. Grunst, T. Moninger, B. Davidson, R. Gregory, and D. Armentano. 1999. A chimeric type 2 adenovirus vector with a type 17 fiber enhances gene transfer to human airway epithelia. J. Virol. 73:8689-8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou, J., X. Y. Sun, K. Louis, and I. H. Frazer. 1994. Interaction of human papillomavirus (HPV) type 16 capsid proteins with HPV DNA requires an intact L2 N-terminal sequence. J. Virol. 68:619-625. [DOI] [PMC free article] [PubMed] [Google Scholar]