Abstract
The cellular response to viral infection often includes activation of pathways that shut off protein synthesis and thereby inhibit viral replication. In order to enable efficient replication, many viruses carry genes such as the E3L gene of vaccinia virus that counteract these host antiviral pathways. Vaccinia virus from which the E3L gene has been deleted (VVΔE3L) is highly sensitive to interferon and exhibits a restricted host range, replicating very inefficiently in many cell types, including human fibroblast and U373MG cells. To determine whether human cytomegalovirus (CMV) has a mechanism for preventing translational shutoff, we evaluated the ability of CMV to complement the deficiencies in replication and protein synthesis associated with VVΔE3L. CMV, but not UV-inactivated CMV, rescued VVΔE3L late gene expression and replication. Thus, complementation of the VVΔE3L defect appears to depend on de novo CMV gene expression and is not likely a result of CMV binding to the cell receptor or of a virion structural protein. CMV rescued VVΔE3L late gene expression even in the presence of ganciclovir, indicating that CMV late gene expression is not required for complementation of VVΔE3L. The striking decrease in overall translation after infection with VVΔE3L was prevented by prior infection with CMV. Finally, CMV blocked both the induction of eukaryotic initiation factor 2α (eIF2α) phosphorylation and activation of RNase L by VVΔE3L. These results suggest that CMV has one or more immediate-early or early genes that ensure maintenance of a high protein synthetic capacity during infection by preventing activation of the PKR/eIF2α phosphorylation and 2-5A oligoadenylate synthetase/RNase L pathways.
The innate immune response to viral infection is mediated in part by interferons which bind to cellular receptors and activate the expression of many genes, including the protein kinase PKR and 2′5′ oligoadenylate (2-5A) synthetases (reviewed in reference 26). The products of these two genes, when activated by double-stranded RNAs (dsRNA), trigger pathways designed to shut off protein synthesis in the infected cell and thereby prevent viral replication. After dimerization and autophosphorylation, PKR phosphorylates the α subunit of eukaryotic initiation factor 2 (eIF2α) and thereby inhibits translation initiation. 2-5A synthetases catalyze the synthesis of 2′-5′ oligoadenylates, which in turn activate latent RNase L, resulting in degradation of mRNA and rRNA and inhibition of protein synthesis.
Because viral replication depends on continued protein synthesis, many viruses have evolved strategies to evade these host cell antiviral responses (8, 15, 18, 25, 26, 29, 30, 36). For example, the vaccinia virus (VV) E3L gene encodes a dsRNA-binding protein that prevents the activation of PKR and 2-5A synthetases (18). VV from which E3L has been deleted (VVΔE3L) is sensitive to the antiviral effects of interferon and exhibits a restricted host range (2). Protein synthesis and replication of VVΔE3L can be complemented by expression of wild-type E3L or other genes encoding dsRNA-binding proteins in trans or by insertion of genes from other viruses that also inhibit these antiviral responses, such as those that encode the reovirus σ3 and hepatitis C virus NS5A proteins, into the VVΔE3L genome (1, 11, 17).
Because recent studies in other viral systems have demonstrated the critical pathogenetic role of viral mechanisms that block the antiviral response pathways (6, 21, 23), we designed studies to evaluate whether human cytomegalovirus (CMV) can counteract these pathways. We hypothesized that the robust protein synthetic capacity that is characteristic of CMV-infected cells (22, 35, 37) was due in part to viral factors that thwart the host cell antiviral responses that would otherwise shut off translation. Using VVΔE3L as a means of activating the PKR and RNase L pathways in CMV-permissive cells, we discovered that CMV can prevent the shutoff of translation mediated by the PKR and 2-5A synthetase pathways.
MATERIALS AND METHODS
Cells and virus.
Human fibroblasts (HF), U373MG (a human glioblastoma cell line obtained from the ATCC), and BHK cells were maintained at 37°C in 5% CO2 in Dulbecco modified Eagle medium supplemented with 10% NuSerum (Collaborative Biomedical), penicillin-streptomycin (100 U/ml), and 2 mM l-glutamine. VV Copenhagen strain (VC-2 [38]) and VVΔE3L, the E3L-minus deletion mutant of VC-2 (vP1080 [1]), obtained from Bertram Jacobs (Arizona State University), were propagated in BHK cells, and their titers were determined. CMV Towne and CMV GFP Toledo strains, in which green fluorescent protein (GFP) expression is controlled by the cellular elongation factor 1α promoter (HV5.111 [19]; obtained from Jeff Vieira, Fred Hutchinson Cancer Research Center), were propagated in HF. Cells were infected at a multiplicity of infection of 3 to 5 PFU/cell by adding virus diluted in medium to the cells and incubating them at 37°C in 5% CO2 for 1 h. The inoculum was then aspirated, the monolayer was washed with medium, and fresh medium was added. To determine VV titers, cell monolayers were scraped and lysed by three freeze-thaw cycles. β-Galactosidase (β-Gal) activity in infected cells was measured by using the fluorogenic substrate 4-methylumbelliferyl-β-d-galactoside (MUG; Sigma) as described earlier (4).
UV inactivation of CMV.
CMV GFP Toledo was inactivated by spreading 1.25 ml of virus in a thin layer in a six-well tissue culture dish. The virus was then UV irradiated in a Stratalinker 1800 (Stratagene) for one, two, or six auto-cross-link cycles for total doses of 0.12, 0.24, or 0.72 J/cm2. GFP expression was monitored at 24 h post-CMV infection by using a Cytofluor II fluorescence plate reader (PerSeptive Biosystems).
Immunoblot analysis.
At the indicated times postinfection cells were washed with phosphate-buffered saline (PBS) and then lysed with 2% sodium dodecyl sulfate (SDS) at 65°C. Protein concentrations were determined by using o-pthaldehyde (14), and equal quantities of the cell lysates were denatured at 95°C for 5 min and separated on SDS-12% polyacrylamide gels, and the proteins were transferred to polyvinylidene difluoride transfer membrane by electroblotting. Immunoblot analysis was carried out by using the Western-Light Plus chemiluminescent detection system (Tropix, Inc.) according to the manufacturer's recommendations. CMV pp65 monoclonal antibody (clone 3A12) was purchased from Virusys Corporation. Anti-phospho-eIF2α and anti-eIF2α polyclonal antisera were purchased from Cell Signaling Technology, Inc. All antisera were used according to the manufacturer's recommendations.
Radiolabeling of virus-infected cells.
At 24 h post-VV infection, HF and U373MG cells were pulse-labeled with Tran35S-label (50 μCi/ml; ICN) for 1 h. The cells were then washed with PBS and lysed with 2% SDS at 65°C. Protein concentrations were determined, and equivalent amounts of each lysate were separated on SDS-12% polyacrylamide gels as described above. The gels were dried and visualized by autoradiography.
RNA analysis.
Whole-cell RNA was harvested by the acid guanidinuim isothiocyanate method (33) and resolved on 1% formaldehyde gels, followed by staining with SYBR Green II (Molecular Probes, Inc.).
RESULTS
CMV rescues VVΔE3L replication.
To determine whether CMV encodes a function that can counteract host cell antiviral responses, we utilized a well-characterized VV mutant, VVΔE3L (1, 3, 6, 31, 32). The interferon sensitivity and limited host cell range of VVΔE3L appear to be due to activation of the PKR and 2-5A synthetase pathways and the resulting block to protein synthesis in infected cells.
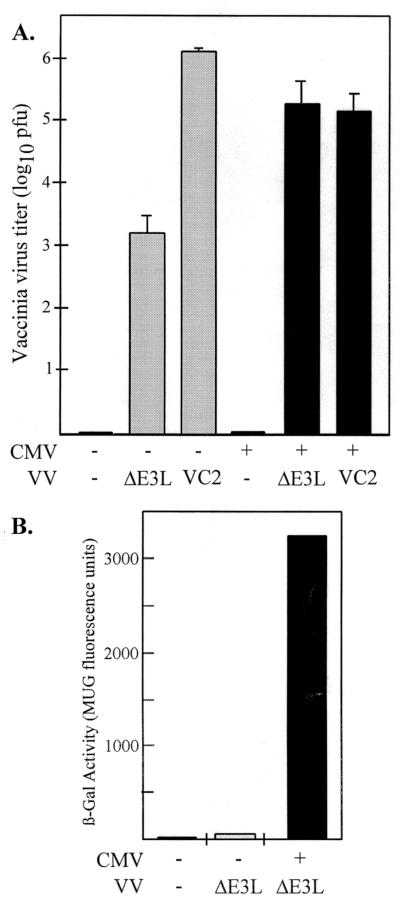
We first investigated whether CMV infection could overcome the replication defect of VVΔE3L. Twenty-four hours after HF had been either mock infected or infected with CMV, the cells were mock infected or infected with VVΔE3L or wild-type VV (VC-2) and VV production was assessed 24 h later by determining the titers of intracellular virus in BHK cells, a cell type that is permissive for VVΔE3L replication (Fig. 1A). The production of VVΔE3L was ∼1,000-fold lower than that of the wild-type VC-2 in HF, a finding similar to the observed phenotypes of these viruses in other cell types (11, 31). CMV infection resulted in a reproducible ∼100-fold increase in VVΔE3L titers. In contrast, CMV infection caused a 10-fold reduction in VC-2 replication. Thus, CMV appears to complement the VVΔE3L replication defect that results from deletion of the E3L gene.
FIG. 1.
Rescue of VVΔE3L replication and late gene expression by CMV. (A) At 24 h after triplicate wells of HF had been mock infected (−, shaded bars) or infected with CMV (+, black bars), they were mock infected (−) or infected with the indicated VV. VV titers (+ standard deviation) in freeze-thaw lysates prepared 24 h later were determined by plaque assays in BHK cells. (B) At 24 h after HF had been mock infected (−) or infected with CMV (+), they were mock-infected (−) or infected with VVΔE3L. After 24 h, the β-Gal activities were measured as described in Materials and Methods.
Because VVΔE3L contains a VV late promoter-lacZ cassette in place of the E3L gene (1), measuring the β-Gal activity in infected cells provides a means of monitoring VV late gene expression. Infection with CMV resulted in a 50-fold increase in β-Gal activity in the VVΔE3L-infected cells (Fig. 1B). Thus, CMV rescues late gene expression, as well as the replication of VVΔE3L in HF.
UV inactivation of CMV eliminates complementation of VVΔE3L replication.
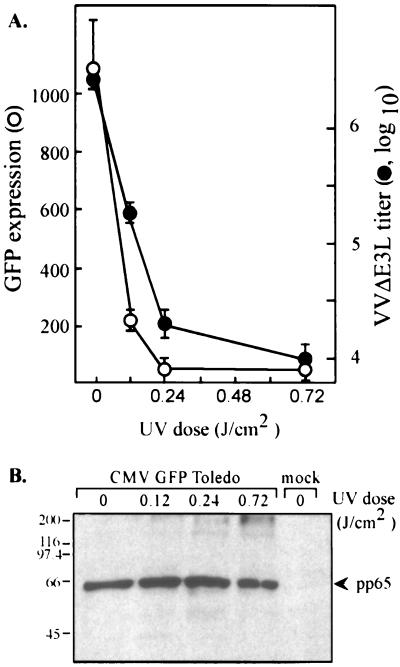
Binding of CMV, or even just a soluble form of its major envelope glycoprotein gB, to the cell surface affects the transcription of numerous cellular genes, including many genes known to be induced by interferon (5, 34, 41, 42). To evaluate the possibility that the observed complementation of VVΔE3L replication was caused by CMV binding to the cell surface or by a factor carried in by the virus, we analyzed the consequences of UV irradiation on the ability of CMV to rescue VVΔE3L replication. A recombinant CMV that expresses enhanced GFP (GFP Toledo) was used for this experiment so that we could monitor the efficacy of UV inactivation of the viral genome by fluorescence measurements. HF were infected with CMV GFP Toledo that had been treated with various doses of UV irradiation. At 2 h postinfection (hpi), mock-infected cells, CMV-infected cells, and cells infected with each UV-irradiated inoculum were harvested for pp65 immunoblot analysis. The remaining samples were analyzed for GFP expression at 24 hpi; they were then infected with VVΔE3L. At 24 h after VV infection, the titers of virus harvested from the cells were determined for BHK cells.
Treatment of GFP Toledo with 0.12 J of UV irradiation/cm2 reduced GFP expression approximately 5-fold and decreased VVΔE3L production almost 10-fold (Fig. 2A). Higher doses of UV irradiation virtually eliminated GFP expression and reduced VVΔE3L titers to the level detected in the absence of CMV coinfection. The reduction of GFP expression resulting from UV inactivation was evident by fluorescence microscopy (data not shown), as well as by plate reader measurements. CMV GFP Toledo binding to the cells was not inhibited by UV treatment, as is evident from the presence of similar amounts of cell-associated pp65 (UL83), a major CMV tegument protein, at 2 hpi at each UV dosage (Fig. 2B). The presence of higher-molecular-weight immunoreactive bands suggests that some cross-linking of pp65 occurred at the highest UV irradiation levels but that the virus was still at least able to bind to the cells. Although it is possible that UV inactivation interfered with entry or uncoating of CMV virus, these results suggest that de novo CMV gene expression is necessary in order to rescue the replication of VVΔE3L in HF.
FIG. 2.
UV inactivation of CMV prevents rescue of VVΔE3L replication. HF were mock infected or infected with CMV that had been exposed to various doses of UV irradiation in quadruplicate as described in Materials and Methods. (A) After 24 h, GFP levels were measured by using a fluorescence plate reader, and then the cells were infected with VVΔE3L. At 24 h post-VVΔE3L infection, VVΔE3L titers were measured (•). GFP levels (○) represent the mean (± the standard deviation [SD]) level of expression in triplicate wells minus the background fluorescence present in mock-infected cells. VVΔE3L titers represent the means (±SD) of VVΔE3L harvested from the same triplicate wells as were used to measure GFP expression. The x axis is set at 3.8 × 103 PFU, representing the titer of VVΔE3L produced in the absence of CMV infection. (B) At 2 h post-CMV infection, samples from the same experiment were harvested and analyzed by immunoblot assay by using antibody to the CMV tegument protein pp65. Molecular size markers are indicated on the left in kilodaltons.
CMV late gene expression is not required for complementation of VVΔE3L replication.
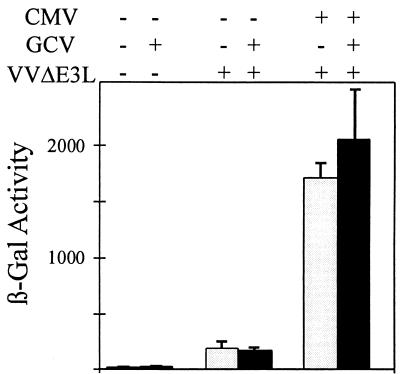
To further explore the characteristics of the CMV gene responsible for VVΔE3L complementation, the ability of CMV to rescue VVΔE3L gene expression in the presence of ganciclovir, an inhibitor of late CMV gene expression (10), was analyzed. HF cells were mock infected or infected with CMV; they were then incubated in the continuous presence or absence of ganciclovir. At 24 h post-CMV infection, the cells were either mock infected or infected with VVΔE3L or VC-2, and these infections were allowed to proceed for 24 h, after which β-Gal expression was measured. Ganciclovir reproducibly had little or no effect on the stimulation of β-Gal expression by CMV, as illustrated in Fig. 3. Stimulation of VVΔE3L replication by CMV, measured by plaque assays, was also evident in the presence of ganciclovir (data not shown). These results indicate that late CMV gene expression is not necessary for complementation of VVΔE3L. Since virion structural proteins appear not to be sufficient for the effect (Fig. 2), we conclude that a CMV immediate-early or early gene (or genes) is most likely necessary for the rescue of VVΔE3L replication.
FIG. 3.
CMV rescues VVΔE3L late gene expression even in the presence of ganciclovir. Triplicate wells of HF were mock infected (−) or infected with CMV (+). At 1 hpi, cells were fed with medium containing (+, black bars) or lacking (−, shaded bars) 30 μM ganciclovir. At 24 hpi, the cells were mock infected (−) or infected with VVΔE3L (+), and β-Gal activity (+SD) was measured 24 h later.
CMV prevents VVΔE3L-induced inhibition of protein synthesis.
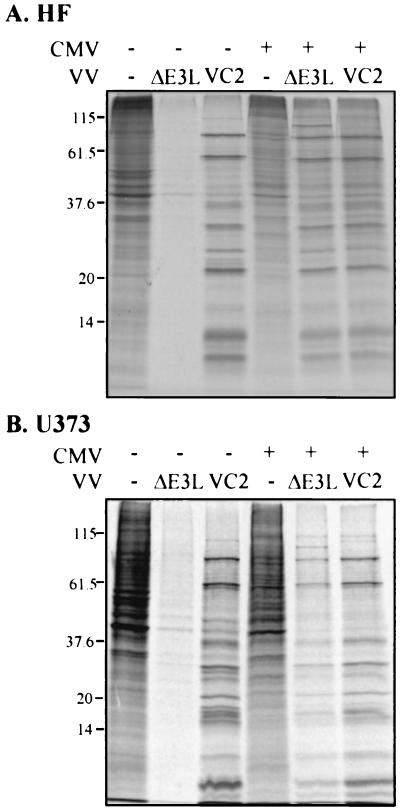
The interferon sensitivity and inefficient replication of VVΔE3L in many cell types are associated with a shutoff of both viral and cellular protein synthesis (1, 3, 11). To evaluate whether CMV can prevent the shutoff of protein synthesis by VVΔE3L, HF and U373MG cells were infected with CMV and VV, alone or in combination, and protein synthesis was monitored by metabolic labeling with [35S]methionine and autoradiography of cell lysates. VVΔE3L markedly inhibited overall protein synthesis in both CMV-permissive cell types (Fig. 4). Prior infection with CMV rescued viral protein synthesis after VVΔE3L infection, as is evident by the level of virus-specific protein expression shown in Fig. 4. As well, in this experiment, CMV increased β-Gal expression in VVΔE3L-infected U373MG cells 54-fold, similar to its effects in HF (Fig. 1B). VC-2 protein synthesis was efficient both in the presence and in the absence of CMV. Thus, CMV specifically reverses the protein synthetic defect resulting from deletion of the E3L gene.
FIG. 4.
Effects of CMV on protein synthesis in VV-infected cells. HF (A) and U373MG (B) cells were mock infected (−) or infected with CMV (+), and after 24 h were mock infected (−) or infected with the indicated VV. At 24 h post-VV infection, cells were labeled with [35S]methionine for 1 h, and then 20 μg of protein in cell extracts was analyzed by SDS-PAGE and autoradiography. Molecular size markers are indicated on the left in kilodaltons.
CMV prevents VVΔE3L-induced eIF2α phosphorylation.
The translational shutoff in VVΔE3L-infected cells is thought to result from an increase in eIF2α phosphorylation and activation of RNase L (18). Therefore, we investigated whether the ability of CMV to preserve translation after VVΔE3L infection is due to its ability to block activation of these pathways.
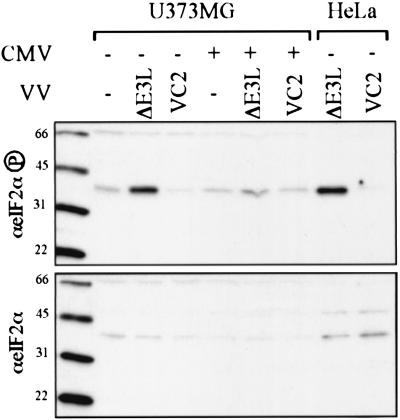
We first determined the effect of CMV infection on eIF2α phosphorylation in VVΔE3L-infected cells. At 24 h after having been mock infected or infected with CMV, U373MG cells were mock infected or infected with VC-2 or VVΔE3L. At 24 h post-VV infection, cell lysates were prepared and the level of eIF2α phosphorylation was assessed by immunoblot assay (Fig. 5). While mock-infected and VC-2-infected cells contained a small amount of phosphorylated eIF2α, infection of these cells with VVΔE3L caused a substantial increase in eIF2α phosphorylation, a finding similar to that observed after VVΔE3L infection of HeLa cells. Coinfection with CMV reduced the abundance of eIF2α phosphorylation almost to the level detected in mock-infected cells, whereas total eIF2α protein levels in these samples were similar. Comparable results were obtained after infections in HF (data not shown), although in some experiments the background level of phosphorylated eIF2α even in mock-infected HF was already high and did not further increase with VVΔE3L infection, thus precluding our ability to assess any effect of CMV. Nonetheless, in all informative experiments in both HF and U373MG cells, CMV reduced the level of eIF2α phosphorylation caused by VVΔE3L infection. These results suggest that CMV contains a gene that prevents the activation of PKR and/or another eIF2α kinase or that stimulates eIF2α dephosphorylation.
FIG. 5.
eIF2α phosphorylation after CMV and VV infection. U373MG cells were mock infected (−) or infected with CMV (+). After 24 h, the cells were mock infected (−) or infected with the indicate VV, and then lysates, prepared 24 h later, along with control extracts from VV-infected HeLa cells, were separated by SDS-PAGE and analyzed by immunoblot assay by using antibody specific for phosphorylated eIF2α (top) or total eIF2α (bottom). Molecular size markers are shown in kilodaltons to the left of each panel.
CMV prevents VVΔE3L-induced RNase L activity.
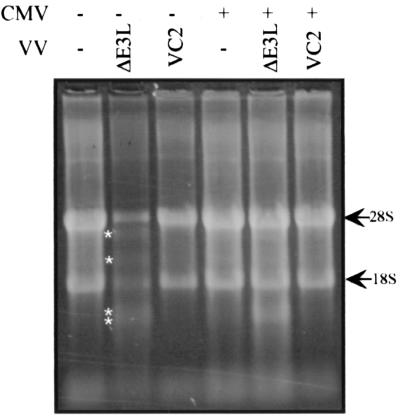
To determine whether CMV also blocks VVΔE3L-induced activation of the 2-5A synthetase pathway, we monitored rRNA cleavage in infected cells. Activation of RNase L results in cleavage of rRNA into discrete products (40). HF (Fig. 6) or U373MG cells (data not shown) that had been mock infected or infected with VC-2 contained intact 18S and 28S rRNA bands, regardless of whether the cells had been preinfected with CMV. Cells infected with VVΔE3L, however, revealed the characteristic RNase L-induced rRNA cleavage pattern, and this cleavage was substantially alleviated by prior infection with CMV, indicating that the CMV can block activation of the 2-5A synthetase pathway.
FIG. 6.
RNase L activity in CMV- and VV-infected cells. HF were mock infected (−) or infected with CMV (+). After 24 h, the cells were mock infected (−) or infected with the indicated VV. At 24 h after VV infection, RNA was harvested and ∼6 μg was visualized by formaldehyde agarose electrophoresis and SyBr green staining. RNase L cleavage products are indicated by asterisks.
DISCUSSION
The host response to viral infection often includes the production of interferons that bind to cell membrane receptors, triggering a signal transduction cascade that results in the induction of multiple genes, including 2-5A synthetases and PKR. Activation of these factors by dsRNAs leads to the shutoff of protein synthesis. Since viruses require continued protein synthesis for replication, many have evolved strategies to counteract the PKR and 2-5A synthetase pathways (26). Even when viruses succeed in preventing shutoff of translation, their mRNA may still be in competition for limiting components of the translational machinery in the cells, and thus viruses have also evolved strategies that promote selective translation of their own mRNAs while inhibiting the translation of cellular mRNAs. CMV differs from many other well-studied viruses in that cellular proteins continue to be synthesized in abundance throughout infection despite increasing viral protein synthesis (22). To ensure adequate production of both viral and cellular proteins, CMV may require extraordinary protein synthetic capacity in the cell. In fact, peaks of increased overall protein synthesis and increases in rRNA synthesis have been described during CMV infections (22, 35, 37). At a minimum, CMV likely needs to counteract any cellular antiviral mechanisms that would otherwise shut off translation. These considerations, along with provocative recent studies demonstrating a critical role of the PKR pathway in herpes simplex virus type 1 (HSV-1) pathogenesis (21), prompted us to investigate whether CMV has the ability to counteract these cellular antiviral defenses.
To evaluate whether CMV has such a mechanism, we utilized the well-characterized VV mutant VVΔE3L that activates PKR and 2-5A synthetase pathways in a variety of cell types (18). We found that VVΔE3L infection of two CMV-permissive cell types, HF and U373MG, results in a low level of viral late gene expression and replication and overall protein synthesis, and a high level of eIF2α phosphorylation and RNase L activity. Prior infection with CMV reverses each of these outcomes (Fig. 1, 4, 5, and 6). These effects of CMV are specific to VVΔE3L since CMV has little or no impact on the replication and translational properties of VC-2, the wild-type parent of VVΔE3L. Thus, CMV has a function that can thwart the host cell antiviral responses triggered by VVΔE3L.
Binding of CMV or even just its envelope glycoprotein B to the cell surface activates transcription of many cellular genes, including 2-5A synthetases and other interferon-regulated genes (5, 34, 41, 42). Similarly, entry of HSV-1 into cells activates several interferon-regulated genes that can inhibit subsequent viral replication (24). These observations suggest that a common early cellular response to herpesvirus infections is activation of components of the interferon response that may reflect an attempt by the cell to limit viral replication. Since it is possible that CMV exploits this cellular response in order to enhance viral replication, we evaluated the possibility that the ability of CMV to rescue VVΔE3L replication was a result of CMV binding to the cell membrane or to a virion-associated factor. However, UV inactivation of CMV caused a parallel reduction in CMV gene expression and rescue of VVΔE3L replication without altering the binding or entry of CMV (Fig. 2). This result suggests that viral binding or virion-associated proteins that are delivered to the cell upon entry are not mediators of enhanced replication of VVΔE3L. Rather, de novo CMV gene expression seems to be required for the rescue of VVΔE3L replication by CMV.
Our studies thus far provide few additional clues about the nature of the CMV gene or genes needed to rescue VVΔE3L replication. CMV late gene expression is not necessary for the effect since CMV rescues VVΔE3L late gene expression even in the presence of ganciclovir (Fig. 3). Although these results do not exclude the possibility that a CMV late gene has a similar function, one or more genes expressed with immediate-early or early kinetics must be able to counteract the host cell antiviral response pathways and to rescue VVΔE3L replication.
Other viruses interfere with the antiviral responses at various steps in the pathways. In fact, several viruses, including VV and HSV-1, have at least two genes that serve this function (9, 12, 16, 18, 25). In both of these viruses and in several other systems (26), a common mechanism for preventing translational shutoff appears to be sequestration of dsRNA by a virally encoded dsRNA-binding protein that thereby prevents activation of both PKR and 2-5A synthetases. The origin of the dsRNAs that serve as activators of these proteins are unknown in most cases but are thought to include replication intermediates of RNA viruses or dsRNAs that result from the transcription from both strands of DNA viruses. As well, certain mRNAs may fold into structures that activate PKR (13, 28, 39). Since CMV represses both the PKR and 2-5A synthetase pathways, we have considered the possibility that CMV encodes a dsRNA-binding protein. However, we have been unable to identify, using computer analyses, any CMV open readingframe with recognizable dsRNA-binding motifs or substantial sequence similarity to proteins known to inhibit the PKR pathway. It remains possible that CMV expresses an unconventional dsRNA-binding protein or that it induces a cellular dsRNA-binding protein. The recent finding that CMV induces transcription of p58IPK (or DNAJ-C3), a cellular inhibitor of PKR that is activated during influenza virus infection, suggests that this protein also block, PKR activity during CMV infection (7, 20). While p58IPK is not known to block the 2-5A synthetase pathway, other virally encoded or induced genes might interfere with that pathway.
Studies of other viruses have elegantly demonstrated the importance of viral regulators of the host PKR and 2-5A synthetase pathways in determining the pathogenesis of infections in animal models. For example, deletion of the E3L gene increases the 50% lethal dose by >1,000-fold compared to wild-type VV after intranasal or intracranial inoculation of mice (6). The HSV-1 γ34.5 gene appears to act as a regulatory subunit of protein phosphatase 1α that redirects the enzyme to dephosphorylate eIF2α. HSV-1 mutants that lack this gene have markedly reduced virulence in wild-type mice but are highly virulent in PKR-null mice, providing strong genetic evidence that a major role of the γ34.5 gene in vivo is to counteract the PKR pathway (21). A second site suppressor mutant of the HSV-1 γ34.5 deletion mutant was isolated and found to express a dsRNA-binding protein, the product of Us11, abnormally early in infection (9, 15, 27). Thus, it appears that repression of the PKR pathway may depend on multiple viral factors expressed at different times during infection. Interestingly, both this HSV-1 second site suppressor mutant and a VV mutant containing the carboxy-terminal portion of E3L that is sufficient for replication and interferon resistance in cell culture display reduced virulence in animals (6, 23), suggesting that blocking the antiviral response pathways in vivo is more complex than in cell culture systems.
Our studies reveal that CMV infection is clearly capable of blocking the shutoff of translation mediated by the PKR and 2-5A synthetase pathways, at least under conditions in which activation of these pathways would otherwise occur, as in the case of VVΔE3L infection. Until future studies identify and test the effects of inactivating the CMV gene or genes responsible for blocking these pathways, we cannot be certain that preventing activation of the PKR and 2-5A synthetase pathways is necessary for CMV replication. However, the fact that CMV has a mechanism for counteracting the host antiviral response pathways suggests that, as in other viral systems, the outcome of the struggle between the virus and the host for control of protein synthetic activity in the cell is a major determinant of viral replication and pathogenesis.
Acknowledgments
We thank Bert Jacobs for providing VV strains, Jeff Vieira for providing CMV GFP Toledo, and Yupeng He and Michael Katze for advice and reagents. We also thank the Biotechnology, Biocomputing, and Image Analysis Resources Department of the Fred Hutchinson Cancer Research Center for technical assistance.
This work was supported by Public Health Service grant AI-26672 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Beattie, E., K. L. Denzler, J. Tartaglia, M. E. Perkus, E. Paoletti, and B. L. Jacobs. 1995. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J. Virol. 69:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beattie, E., E. B. Kauffman, H. Martinez, M. E. Perkus, B. L. Jacobs, E. Paoletti, and J. Tartaglia. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes 12:89-94. [DOI] [PubMed] [Google Scholar]
- 3.Beattie, E., E. Paoletti, and J. Tartaglia. 1995. Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L− and E3L− mutant viruses. Virology 210:254-263. [DOI] [PubMed] [Google Scholar]
- 4.Biegalke, B. J., and A. P. Geballe. 1990. Translational inhibition by cytomegalovirus transcript leaders. Virology 177:657-667. [DOI] [PubMed] [Google Scholar]
- 5.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne, E. P., B. Wing, D. Coleman, and T. Shenk. 2001. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 75:12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassady, K. A., M. Gross, and B. Roizman. 1998. The herpes simplex virus US11 protein effectively compensates for the γ1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J. Virol. 72:8620-8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassady, K. A., M. Gross, and B. Roizman. 1998. The second-site mutation in the herpes simplex virus recombinants lacking the γ134.5 genes precludes shutoff of protein synthesis by blocking the phosphorylation of eIF-2α. J. Virol. 72:7005-7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers, J., A. Angulo, D. Amaratunga, H. Guo, Y. Jiang, J. S. Wan, A. Bittner, K. Frueh, M. R. Jackson, P. A. Peterson, M. G. Erlander, and P. Ghazal. 1999. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J. Virol. 73:5757-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, H. W., L. H. Uribe, and B. L. Jacobs. 1995. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J. Virol. 69:6605-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou, J., J.-J. Chen, M. Gross, and B. Roizman. 1995. Association of a Mr 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2α and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis, S., and J. C. Watson. 1996. In vitro activation of the interferon-induced, double-stranded RNA-dependent protein kinase PKR by RNA from the 3′ untranslated regions of human α-tropomyosin. Proc. Natl. Acad. Sci. USA 93:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geballe, A. P., and E. S. Mocarski. 1988. Translational control of cytomegalovirus gene expression is mediated by upstream AUG codons. J. Virol. 62:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He, B., J. Chou, R. Brandimarti, I. Mohr, Y. Gluzman, and B. Roizman. 1997. Suppression of the phenotype of γ134.5− herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the α47 gene. J. Virol. 71:6049-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, Y., S. L. Tan, S. U. Tareen, S. Vijaysri, J. O. Langland, B. L. Jacobs, and M. G. Katze. 2001. Regulation of mRNA translation and cellular signaling by hepatitis C virus nonstructural protein NS5A. J. Virol. 75:5090-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs, B. L. 2000. Translational control in poxvirus-infected cells, p. 951-971. In W. B. Hershey, M. B. Matthews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 19.Jarvis, M. A., C. E. Wang, H. L. Meyers, P. P. Smith, C. L. Corless, G. J. Henderson, J. Vieira, W. J. Britt, and J. A. Nelson. 1999. Human cytomegalovirus infection of Caco-2 cells occurs at the basolateral membrane and is differentiation state dependent. J. Virol. 73:4552-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, T. G., N. Tang, S. Thompson, J. Miller, and M. G. Katze. 1994. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol. Cell. Biol. 14:2331-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2674. In D. M. Knipe et al. (ed)., Fields virology, 4th ed. Lippincott/Williams and Wilkins, New York, N.Y.
- 23.Mohr, I., D. Sternberg, S. Ward, D. Leib, M. Mulvey, and Y. Gluzman. 2001. A herpes simplex virus type 1 γ34.5 second-site suppressor mutant that exhibits enhanced growth in cultured glioblastoma cells is severely attenuated in animals. J. Virol. 75:5189-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulvey, M., J. Poppers, A. Ladd, and I. Mohr. 1999. A herpesvirus ribosome-associated, RNA-binding protein confers a growth advantage upon mutants deficient in a GADD34-related function. J. Virol. 73:3375-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pe'ery, T., and M. B. Matthews. 2000. Viral translational strategies and host defense mechanisms, p. 371-424. In W. B. Hershey, M. B. Matthews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Poppers, J., M. Mulvey, D. Khoo, and I. Mohr. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 74:11215-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson, H. D., L. Manche, and M. B. Mathews. 1996. Paradoxical interactions between human delta hepatitis agent RNA and the cellular protein kinase PKR. J. Virol. 70:5611-5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider, R. J. 2000. Adenovirus inhibition of cellular protein synthesis and preferential translation of viral mRNAs, p. 901-914. In W. B. Hershey, M. B. Matthews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Shatkin, A. J. 2000. Reovirus translational control, p. 915-932. In W. B. Hershey, M. B. Matthews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Shors, S. T., E. Beattie, E. Paoletti, J. Tartaglia, and B. L. Jacobs. 1998. Role of the vaccinia virus E3L and K3L gene products in rescue of VSV and EMCV from the effects of IFN-α. J. Interferon Cytokine Res. 18:721-729. [DOI] [PubMed] [Google Scholar]
- 32.Shors, T., and B. L. Jacobs. 1997. Complementation of deletion of the vaccinia virus E3L gene by the Escherichia coli RNase III gene. Virology 227:77-87. [DOI] [PubMed] [Google Scholar]
- 33.Siebert, P. D., and A. Chenchik. 1993. Modified acid guanidinium thiocyanate-phenol-chloroform RNA extraction method which greatly reduces DNA contamination. Nucleic Acids Res. 21:2019-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmen, K. A., J. Singh, B. G. M. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stinski, M. F. 1977. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J. Virol. 23:751-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan, S.-L., M. G. Katze, and J. M. J. Gale. 2000. Translational reprogramming during influenza virus infection, p. 933-950. In W. B. Hershey, M. B. Matthews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Tanaka, S., T. Furukawa, and S. A. Plotkin. 1975. Human cytomegalovirus stimulates host cell RNA synthesis. J. Virol. 15:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tartaglia, J., M. E. Perkus, J. Taylor, E. K. Norton, J. C. Audonnet, W. I. Cox, S. W. Davis, J. van der Hoeven, B. Meignier, M. Riviere, et al. 1992. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188:217-232. [DOI] [PubMed] [Google Scholar]
- 39.Tian, B., R. J. White, T. Xia, S. Welle, D. H. Turner, M. B. Mathews, and C. A. Thornton. 2000. Expanded CUG repeat RNAs form hairpins that activate the double-stranded RNA-dependent protein kinase PKR. RNA 6:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wreschner, D. H., T. C. James, R. H. Silverman, and I. M. Kerr. 1981. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2′p)nA) in interferon-treated cells. Nucleic Acids Res. 9:1571-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu, H., J. P. Cong, and T. Shenk. 1997. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. USA 94:13985-13990. [DOI] [PMC free article] [PubMed] [Google Scholar]