Abstract
Bovine herpesvirus 1 (BHV-1) is an important pathogen of cattle, and infection is usually initiated via the ocular or nasal cavity. Following acute infection, the primary site for BHV-1 latency is the sensory neuron. Reactivation from latency occurs sporadically, resulting in virus shedding and transmission to uninfected cattle. The only abundant viral transcript expressed during latency is the latency-related (LR) RNA, suggesting that it mediates some aspect of latency. An LR mutant was constructed by inserting three stop codons near the beginning of the LR-RNA, suggesting that expression of LR proteins would be altered. The LR mutant grew with wild-type (wt) efficiency in bovine kidney cells (MDBK). When calves were infected with the LR mutant, a dramatic decrease (3 to 4 logs) in ocular, but not nasal, viral shedding occurred during acute infection relative to the wt or the LR-rescued virus (M. Inman, L. Lovato, A. Doster, and C. Jones, J. Virol. 75:8507-8515, 2001). In this study, we examined the latency reactivation cycle in calves infected with the LR mutant and compared these results to those from calves infected with wt BHV-1 or the LR-rescued virus. During acute infection, lower levels of infectious virus were detected in trigeminal ganglion homogenates from calves infected with the LR mutant. As judged by in situ hybridization, BHV-1-positive neurons were detected in trigeminal ganglia of calves infected with the wt but not the LR mutant. Although LR-RNA was detected by reverse transcription-PCR in calves latently infected with the LR mutant, a semiquantitative PCR analysis revealed that lower levels of viral DNA were present in trigeminal ganglia of calves infected with the LR mutant. Dexamethasone treatment of calves latently infected with wt BHV-1 or the LR-rescued virus, but not the LR mutant, consistently induced reactivation from latency, as judged by shedding of infectious virus from the nose or eyes and increases in BHV-1-specific antibodies. In summary, this study demonstrates that wt expression of LR gene products plays an important role in the latency reactivation cycle of BHV-1 in cattle.
Bovine herpesvirus 1 (BHV-1) is an important viral pathogen of cattle that can cause severe respiratory infection, conjunctivitis, abortions, vulvovaginitis, balanopostitis, and systemic infection in neonate calves (52). BHV-1 infection is also an important component of the upper respiratory tract infection referred to as “shipping fever” or bovine respiratory complex (46). BHV-1 is not the sole infectious agent associated with shipping fever, but it initiates the disorder by immunosuppressing infected cattle, which results in secondary bacterial infections and pneumonia. Increased susceptibility to secondary infection correlates with depressed cell-mediated immunity after BHV-1 infection (4, 10-12). CD8+ T-cell recognition of infected cells is impaired by repressing expression of major histocompatibility complex class I and of the transporter associated with antigen presentation (13, 14, 25). CD4+ T-cell function is impaired during acute infection of calves, in part, because BHV-1 infects CD4+ T cells and induces apoptosis (48). BHV-1 infection costs the cattle industry millions of dollars per year in the United States (3; bulletin from the National Agricultural Statistics Service, Agricultural Statistics Board, U.S. Department of Agriculture). Although modified live vaccines are available, they can cause disease in young calves or abortions in cows, and they have the potential to establish latency and reactivate from latency (21).
BHV-1 is a member of the Alphaherpesvirinae subfamily and shares certain biological properties with herpes simplex virus types 1 and 2 (HSV-1 and -2, respectively) (20). Viral gene expression is temporally regulated in three distinct phases: immediate-early (IE), early (E), or late (L). Two IE transcription units exist: IE transcription unit 1 (IEtu1) and IEtu2. IEtu1 encodes functional homologues of two HSV-1 IE proteins, ICP0 and ICP4. IEtu2 encodes a protein that is similar to an essential HSV IE protein, ICP22 (51). bICP0 is very important for productive infection, because it activates all classes of viral promoters and is expressed at high levels throughout infection (9, 50, 51).
BHV-1 establishes lifelong latency in ganglionic neurons of the peripheral nervous system after initial replication in mucosal epithelium. Reactivation from latency and spread to other susceptible animals occur after natural or corticosteroid-induced stress (36, 43). Although the primary site of BHV-1 latency is sensory neurons, there is evidence that long-term persistence and reactivation also occur within germinal centers of pharyngeal tonsil (49). The latency-related RNA (LR-RNA) is the only abundant viral transcript detected in latently infected neurons (22, 36, 37). A fraction of LR-RNA is polyadenylated and alternatively spliced in trigeminal ganglia (TG), suggesting this RNA is translated into more than one LR protein (LRP) (8, 16). LR gene products inhibit S-phase entry, and LRP is associated with cyclin-dependent kinase 2 (cdk2)-cyclin complexes (16, 19). LR gene products also promote cell survival following induction of apoptosis in transiently transfected cells (6). We recently constructed an LR mutant virus that contains three stop codons near the beginning of LR RNA to test whether LR gene products play a role in the ability of BHV-1 to replicate in cattle (17). Calves infected with the LR mutant consistently exhibited diminished clinical symptoms and ocular shedding of the virus compared to calves infected with wild-type (wt) or LR-rescued virus. Conversely, the LR mutant had similar growth properties in productively infected bovine kidney cells (MDBK) and the nasal cavity of calves during acute infection. These results suggested that LR gene products promote virus growth in certain cell types in the eye or optic nerve during acute infection of cattle.
HSV-1 establishes latency in ganglionic sensory neurons, typically TG or sacral dorsal root ganglia (20, 47). In situ hybridization has revealed that a small region, the latency-associated transcript (LAT), within the terminal repeats is abundantly transcribed in latently infected neurons (38, 44). Numerous mutants that do not express detectable levels of LAT have been constructed. Although several studies have suggested that LAT plays no role in a latent infection, for example (2, 15), most have concluded that LAT is important, but not required. LAT enhances establishment of latency in mice (40, 45) and rabbits (34), because certain LAT-null mutants contain lower levels of viral DNA in TG relative to wt virus (7, 24). LAT is also important for in vivo reactivation with two different rabbit eye infection models. The McKrae strain of HSV-1 is frequently shed in the tears of infected rabbits as a result of spontaneous reactivation and LAT is necessary for efficient spontaneous reactivation (28-32).
LAT interferes with apoptosis in transiently transfected cells and in infected mice or rabbits (1, 18, 27). The ability of LAT to interfere with apoptosis correlates with its ability to promote spontaneous reactivation (18), suggesting the antiapoptotic activity of LAT has biological significance with respect to latency. Since the LR gene and LAT interfere with apoptosis, the LR gene was inserted into the LAT locus to test whether it could restore spontaneous reactivation to a LAT-null mutant. The LR gene of BHV-1 is capable of restoring high levels of spontaneous reactivation from latency to a LAT-null mutant (26), adding support to the hypothesis that inhibition of apoptosis plays an important role in the latency reactivation cycle of HSV-1 and BHV-1.
In this study, we examined the effects of the LR mutant on virus production in TG and the latency reactivation cycle. Diminished levels of virus were detected in TG of calves acutely infected with the LR mutant when compared to those infected with wt or LR-rescued virus. Although we consistently detected LR-RNA in TG of calves infected with the LR mutant or the wt by PCR, we were unable to detect viral DNA in neurons of calves infected with the LR mutant by in situ hybridization. PCR analysis confirmed that calves infected with the LR mutant contained lower levels of viral DNA during latency compared to those in calves infected with wt BHV-1. The LR mutant virus was not reactivated from latently infected calves following treatment with dexamethasone (DEX). In contrast, calves infected with wt virus or the LR-rescued virus reactivated efficiently following the same DEX treatment. These results demonstrate that wt expression of LR gene products is crucial for the latency reactivation cycle in cattle.
MATERIALS AND METHODS
Virus and cells.
Bovine kidney cells (MDBK; ATCC CCL-22) were plated at a density of 5 × 105 per 100-mm2 plastic dish in Earle's modified medium supplemented with 5% fetal bovine serum (FBS), penicillin (10 U/ml), and streptomycin (100 μg/ml).
The Cooper strain of BHV-1 (wt virus) was obtained from the National Veterinary Services Laboratory, Animal and Plant Health Inspection Services, Ames, Iowa. A complete description of the LR mutant virus has been previously described (17). In short, the LR mutant virus was developed by replacing wt (Cooper strain) LR gene sequences with an oligonucleotide that contain a unique EcoRI restriction site and three stop codons in each reading frame. In transiently transfected cells, a plasmid with this mutation does not express detectable levels of the LR protein, but does express LR-RNA (6). Viral stocks were prepared by infecting MBDK cells with a multiplicity of infection (MOI) of 0.001 from a plaque-purified virus. Virus was titrated on MDBK cells by using 10-fold dilutions and determining the 50% tissue culture infectious dose (TCID50) or PFU.
Animal experiments.
BHV-1-free crossbred calves (∼250 kg) were randomly assigned and housed in isolation rooms to prevent cross contamination. Calves were anesthetized with Rompun (approximately 1 mg/kg of body weight; Bayer Corp., Shawnee Mission, Kans.). Calves were then inoculated with 1 ml of a solution containing 107 PFU of the indicated virus per ml in each nostril and eye, without scarification, for a total of 4 × 107 PFU per animal as described previously (41, 48, 49). Experiments with animals were performed in accordance with the American Association of Laboratory Animal Care guidelines. At 60 days postinfection (dpi) (latency), calves were injected intravenously with 100 mg of DEX as described previously (49). Additional intramuscular injections (25 mg) of DEX were given at 2 and 4 days after the initial intravenous injection of DEX to ensure that reactivation occurs. We have previously demonstrated that multiple injections of DEX enhance shedding of virus (21). Calves were housed under strict isolation containment and given antibiotics before and after BHV-1 infection to prevent secondary bacterial infection.
Nasal swabs, ocular swabs, and serum samples were taken at the designated times. Nasal and ocular swabs were stored at −80°C in 2 ml of tissue culture medium supplemented with 10 μg of amphotericin B per ml (Fungizone) and 45 μg of gentamicin per ml. Samples were thawed quickly in a 37°C water bath, vortexed, and centrifuged (1,500 × g for 10 min). All titrations were performed with 10-fold serial dilution and plated in quadruplicate.
Virus was isolated from TG by mincing 0.5 g of tissue, suspending the tissue in 9 ml of Dulbecco's modified Eagle's medium (DMEM), and homogenizing the tissue with a tissue grinder (Polytron, Switzerland). One milliliter of fetal bovine serum was added, and the homogenate was subjected to three freeze-thaw cycles with a dry ice-ethanol bath. After the last cycle, the homogenate was centrifuged at 2,000 rpm (Jouan CR412 centrifuge) for 30 min at 4°C. The supernatant from the TG homogenate was subsequently used to infect MDBK cells. The TG supernatant (125 μl) was added to 500 μl of medium (1:5 dilutions), and then 1:5 serial dilutions were made. One hundred microliters of each dilution was added in quadruplicate to a 96-well plate. One hundred microliters of MDBK cells (105 cells) was added to each well. After 4 days of incubation, cells were fixed and then stained with formaldehyde-bromophenol blue. Virus titers were measured with the 50% end point assay as described previously (17).
Nucleic acid tissue extraction.
RNA and DNA extractions from MDBK cells or TG were performed as previously described (21, 41).
RT of RNA.
Reverse transcription (RT) was performed essentially as previously described (21). Briefly, 4 μg of RNA was treated with DNase, and after inactivation, the RNA was reverse transcribed with random hexamers as primers. As a control for DNA contamination in the RNA samples, DNase-treated RNA was mixed with the RT reaction mix lacking reverse transcriptase. Two microliters was used for PCR.
PCR.
PCR was performed on the extracted DNA and synthesized cDNA with the indicated primer pairs p4 (nucleotide [nt] 873, 5′CGTGTATTTGCGACCCCCAGCCT3′) and p5 (nt 596, 5′GCCAGACCAAACCCCCCGCA3′) (17, 41). Actin, L3B, and gC primers have been described previously (16, 17, 21, 41). The gC forward primer is 5′ AAAGCCCCGCCGAAGGAG (bp 550 of the BHV-1 gC gene). The gC reverse primer is 5′ TACGAACAGCAGCACGGGC (bp 756 of BHV-1 gC gene). The forward bovine growth hormone (gH) primer is 5′ GCTTTCGCCCTGCTCTGCC (bp 994 of the bovine growth hormone gene). The reverse gH primer is 5′ TCCTGCCTCCCCACCCCTA (bp 1155 of the bovine growth hormone gene). After a hot start, each cycle consisted of 95°C for 1 min, 60°C for 1 min, and 72°C for 2 min (30 cycles total). To ensure complete elongation of amplified products, the reaction was incubated at 72°C for an additional 10 min. For some studies, the PCR products were digested with EcoRI, electrophoresed on a 2% agarose gel, and the DNA was visualized by staining with ethidium bromide.
Direct FA for the detection of BHV-1.
For the direct fluorescence assay (FA), a 24-well plate of MDBK cells was infected with clarified viral lysate. Twenty-four hours later, cell monolayers were fixed for 5 min with ice-cold methanol-acetone (50:50) and then allowed to dry. A 1:50 dilution of direct conjugate (anti-BHV-1-specific antibody-fluorescein isothiocyanate [FITC] conjugate; American BioResearch, Milton, Tenn.) was added to the fixed cells and incubated at 37°C for 30 min. The monolayers were washed twice with phosphate-buffered saline. The presence of virus was visualized with an FITC filter.
BHV-1-specific neutralizing antibodies.
The Veterinary Diagnostic Service, University of Nebraska, Lincoln, measured neutralizing antibody titers with the Cooper strain as the stock virus.
In situ hybridization.
DNA probes specific for BHV-1 gC, ribonucleotide reductase, bICP0, and the LR gene were used. Labeling of the probes and hybridization steps were performed as previously described (48, 49). After hybridization, slides were washed twice in 4× SSC (1 × SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 5 min at room temperature, once in 2× SSC at 40°C, once in 0.5× SSC at 40°C, twice in 2× SSC at room temperature, twice in 0.5× SSC at room temperature, and once in buffer I (100 mM maleic acid, 150 mM NaCl [pH 7.5]). The conjugate antibody step and reaction development were also performed as previously described (48, 49).
RESULTS
The LR mutant virus does not grow efficiently in TG of acutely infected calves.
We previously constructed a BHV-1 LR mutant virus that contains stop codons near the 5′ terminus of the LR transcript (LR mutant) (17). The LR mutant grew to similar titers relative to wt or LR-rescued virus in the nasal cavity of calves and cultured bovine cells. However, the LR mutant shed approximately 4 logs less infectious virus from eyes of acutely infected calves compared to wt or LR-rescued virus, suggesting the LR gene plays a role in virus growth in certain bovine tissue.
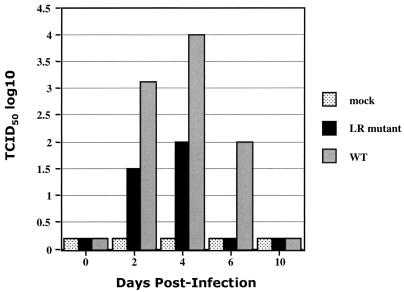
To test whether the LR mutant contained different amounts of infectious virus in TG compared to calves infected with wt BHV-1, calves were infected with the respective viruses, TG were harvested at different times after infection, and infectious virus was measured in TG homogenates. Levels of infectious virus in TG homogenates were measured by incubating the supernatants with MDBK cells. Cultures that produced cytopathic effects were also subjected to FA with BHV-specific antisera as an independent verification that cytopathic effects were due to virus infection and not nonspecific toxic effects of the TG homogenates. At 2 and 4 dpi, calves infected with the LR mutant contained 10- to 100-fold less virus in TG homogenates compared to calves infected with wt virus (Fig. 1). Infectious virus was not detected in TG of calves infected with the LR mutant on 6 dpi. FA-positive samples were detected at 6 dpi in LR mutant-infected calves and 10 dpi in calves infected with the wt (data not shown), but the amount of virus was too low for titration.
FIG. 1.
Isolation and titration of virus present in TG during acute infection. Virus was isolated from TG of mock-, LR mutant-, or wt (WT)-infected calves on the designated days postinfection as described in Materials and Methods. The titer of infectious virus was determined on MDBK cells. The data shown are the averages from each group. A value of 0.2 was used to denote a negative result in order to visualize the bars.
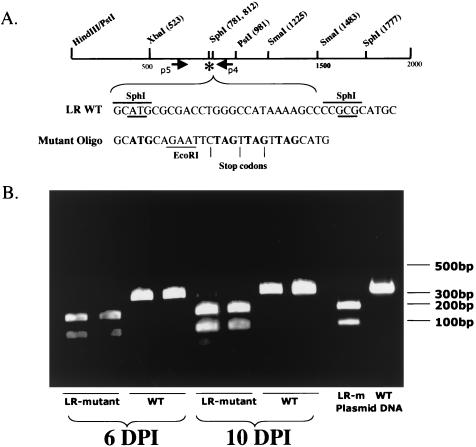
To verify that the LR mutant genome was present in TG of calves infected with the LR mutant, PCR analysis was performed. DNA was prepared from calves infected for 6 or 10 dpi and screened for the presence of the mutant by PCR with the p4 and p5 primers (Fig. 2A). Amplified products were then digested with EcoRI. Calves infected with the LR mutant contained two bands (105 and 193 bp) that were detected following digestion with EcoRI (Fig. 2B, lanes 1, 2, 5, and 6). Wt virus yielded a single band (298 bp) as expected (Fig. 2B, lanes 3, 4, 7, and 8). In summary, this study demonstrated that, during acute infection, lower levels of infectious virus were present in TG of calves infected with the LR mutant.
FIG. 2.
PCR of TG from calves infected with either LR mutant or wt viruses. DNA was prepared from TG as described in Materials and Methods. Primers P4 and P5 (17) (A) were used to amplify a region that contains the mutated sequences, including a unique EcoRI site. The amplified products were then digested with EcoRI (position in LR mutant is denoted by asterisk) and visualized by ethidium bromide staining on 2% agarose gel electrophoresis (B). The presence of wt (WT) virus yields a single band of 298 bp, while LR mutant virus yields two bands migrating at 105 and 193 bp. The LR mutant plasmid (LR-m) and a plasmid with the wt LR gene served as positive controls to show the positions of the expected products. Molecular weight markers (100-bp ladder) are identified. PCR of representative samples from calves infected for 6 or 10 days demonstrates that only the inoculated virus was detected in the TG.
Analysis of BHV-1 DNA in calves infected with the LR mutant or wt DNA.
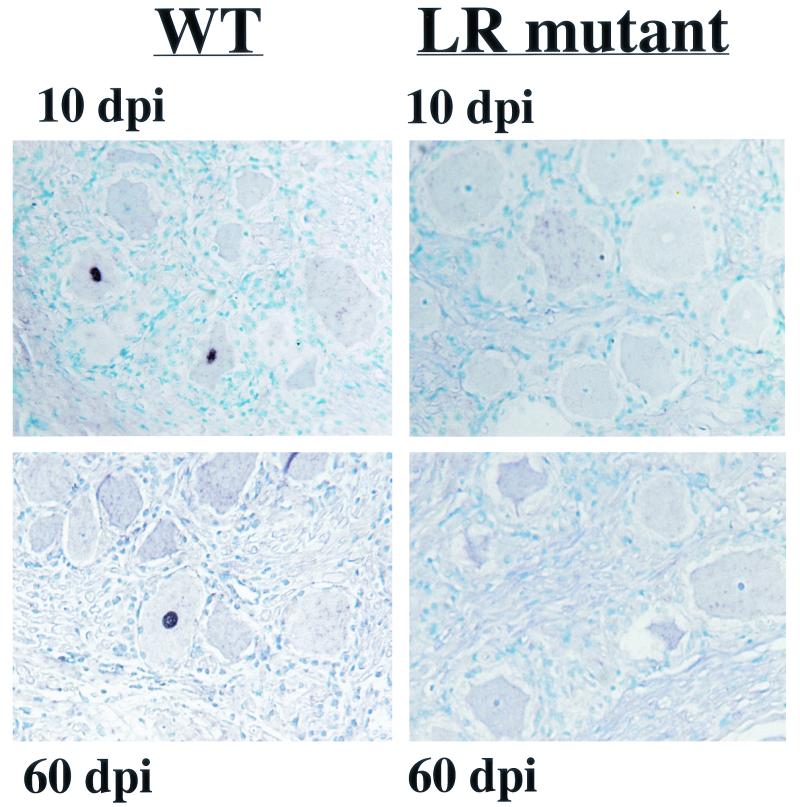
In situ hybridization was performed with probes that specifically bind to four viral genes (48, 49). In situ-positive neurons in TG were consistently detected in thin sections prepared from calves infected with wt virus at 10 and 60 dpi (Fig. 3). We estimated that less than 5% of the total neurons contained viral DNA, as judged by in situ hybridization. In contrast, DNA-positive neurons were not detected by in situ hybridization with TG thin sections prepared from calves infected with the LR mutant for 10 or 60 days after infection. This study suggested that neurons with high copies of viral DNA were not present in TG of calves infected with the LR mutant.
FIG. 3.
In situ hybridization of TG samples after infection. Thin sections were prepared from TG of calves infected with the LR mutant or wt (WT) virus at 10 (end of acute infection or establishment of latency) or 60 (latency) dpi. In situ hybridization was performed as described in Materials and Methods. The results shown are representative of numerous independent sections.
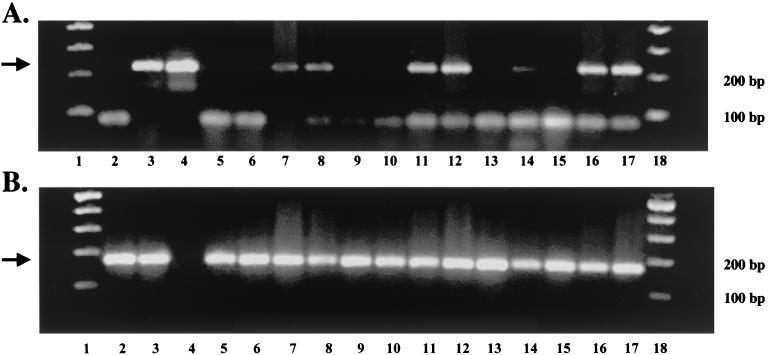
To further compare the levels of viral DNA in TG of calves infected with the wt virus or the LR mutant, a semiquantitative PCR was performed with BHV-1-specific primers. We focused on samples prepared from latently infected calves (60 dpi) or during the early stages of reactivation (24 and 48 h after DEX treatment). As expected, wt viral DNA was readily detected in TG of latently infected calves (Fig. 4, lanes 7 and 8) and at 24 (lanes 11 and 12) or 48 (lanes 16 and 17) h after DEX treatment. In contrast, TG prepared from calves latently infected with the LR mutant did not contain detectable levels of viral DNA (lanes 5 and 6). At 24 h after DEX treatment, neither of two calves latently infected with the LR mutant contained detectable levels of viral DNA (lanes 9 and 10). At 48 h after DEX treatment, one of three calves latently infected with the LR mutant contained detectable levels of viral DNA (lanes 13 to 15). Under the conditions of this PCR assay, we were able to detect approximately 200 to 400 copies of viral DNA per μg of total DNA. In summary, two independent assays (in situ hybridization and PCR) have demonstrated that calves latently infected with the LR mutant contained less viral DNA in TG.
FIG. 4.
PCR of DNA prepared from TG to detect viral DNA during latency and reactivation. DNA was extracted from TG of latently infected calves (60 days after infection) or when latently infected calves were treated with 100 mg of DEX to initiate reactivation (single intravenous injection). PCR was performed as described in Materials and Methods. PCR primers were used to detect BHV-1 gC DNA (A) and bovine growth hormone (gH) (B). PCR products were electrophoresed on 2% agarose gels, and the DNA was stained with ethidium bromide. Lanes 1 and 18 contain the 100-bp ladder (NE Biolabs). Lane 2 contains DNA prepared from TG of a mock-infected calf. Lane 3 contains DNA prepared from MDBK cells infected with BHV-1 (MOI = 1) for 24 h. Lane 4 contains DNA purified from BHV-1 virions. Lanes 5 and 6 contain DNA prepared from TG of calves latently infected with the LR mutant. Lanes 7 and 8 contain DNA prepared from TG of calves latently infected with wt BHV-1. Lanes 9 and 10 contain DNA prepared from TG of calves latently infected with the LR mutant 24 h after DEX treatment. Lanes 11 and 12 contain DNA prepared from TG of calves latently infected with wt BHV-1 24 h after DEX. Lanes 13, 14, and 15 contain DNA prepared from TG of calves latently infected with the LR mutant 48 h after DEX treatment. Lanes 16 and 17 contain DNA prepared from TG of calves latently infected with the wt 48 h after DEX treatment. For each DNA sample, 1 μg of DNA was used for PCR. In the lanes that contained purified viral DNA, 20 ng of DNA was used for PCR. The arrows indicate the position of the expected PCR product.
LR-RNA is expressed in calves latently infected with the LR mutant.
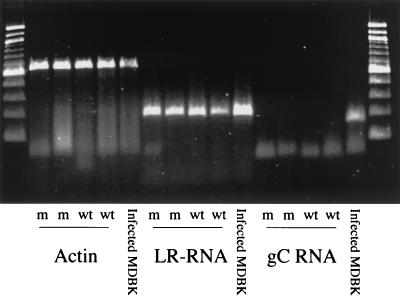
In theory, the LR mutant should express LR-RNA, but not proteins because of the three stop codons (17). In productively infected bovine cells, the LR mutant expressed abundant levels of LR-RNA (Fig. 5, infected MDBK cells). LR-RNA expression was examined in TG of calves infected with the LR mutant at 60 dpi (latency). RT-PCR was performed with primers that specifically amplify LR-RNA (L3B) as described previously (6, 16, 21). LR-RNA was detected in two of two calves infected with the LR mutant and, as expected, in two of two calves infected with the wt at 60 dpi (Fig. 5). gC RNA was not detected by RT-PCR in latently infected calves, demonstrating that high levels of productive infection were not occurring in TG during latency (60 days after infection).
FIG. 5.
RT-PCR of TG from infected calves during latency. RNA was extracted from TG of infected calves or MDBK cells as described in Materials and Methods. cDNA was generated with random primers, and PCR was performed with specific primers. Primers that amplify a segment of the β-actin transcript flank an intron, which allows for detection of contaminating DNA as previously described (17). L3B primers were used to detect the LR transcript. Primers that amplify gC RNA were used to verify that calves were in the latent phase of infection. m, calves infected with the LR mutant virus; wt, calves infected with wt virus. When reverse transcriptase was omitted during cDNA synthesis, no PCR product was detected (data not shown).
Comparison of DEX-induced reactivation of the LR mutant to that of the wt.
Treatment of latently infected rabbits (36) or calves (21, 43) with DEX consistently reactivates BHV-1 from latency. Consequently, virus shedding occurs in ocular or nasal cavities, and virus-specific antibodies increase. A preliminary study was performed with two calves latently infected with the LR mutant, two calves infected with wt virus, and two calves infected with the LR-rescued virus. Following a single intravenous injection of DEX (100 mg), we detected reactivation in calves latently infected with wt or the LR-rescued virus, but not the two calves infected with the LR mutant, suggesting the LR mutant was not capable of reactivating. To confirm this result, a larger study was performed with an initial intravenous injection of DEX (100 mg) followed by two intramuscular injections of DEX (25 mg) to ensure that reactivation occurred efficiently. Our previous studies demonstrated that multiple injections of DEX allowed efficient reactivation of a temperature-sensitive mutant of BHV-1, which is used as a modified live vaccine and is severely attenuated (21).
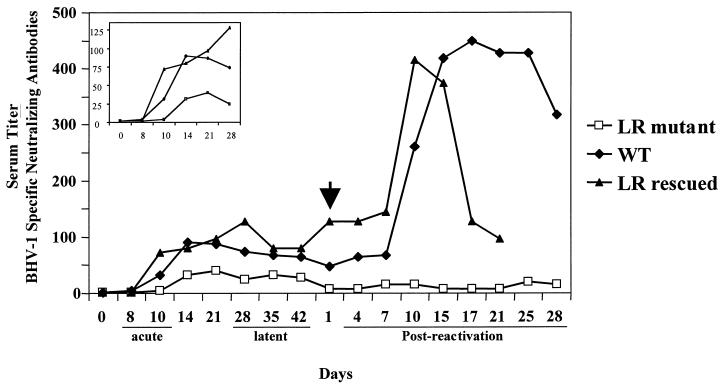
Neutralizing antibody titers were measured after DEX treatment to determine if reactivation occurred. As expected, a dramatic increase in virus-specific antibodies was detected in animals infected with wt or LR-rescued virus after DEX-induced reactivation (Fig. 6), indicating that virus replication and shedding occurred. A five- to sixfold increase in antibody titers was detected, which is consistent with reactivation from latency. In contrast, an increase in virus-specific antibodies was not observed when calves latently infected with the LR mutant were treated with DEX.
FIG. 6.
BHV-1 neutralizing antibody titers. Serum was collected from calves at the indicated times and stored at −20°C until tested. Standard testing was performed with constant amounts of virus (Cooper strain) and twofold serial dilutions of the serum. The Veterinary Diagnostic Services, University of Nebraska, Lincoln, performed the assay. Solid rectangles, calves infected with wt virus; solid triangles, LR-rescued virus; and open rectangles, calves infected with the LR mutant virus. Each time point represents at least four calves. The inset panel is shown to illustrate the differences during acute phase more clearly. The x-axis scale is different. The differences between the time points after 10 days postreactivation were statistically significant (P < 0.05).
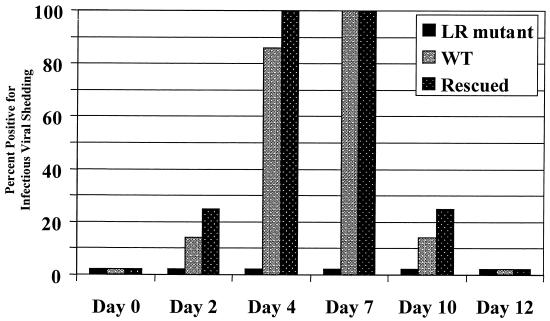
To confirm the neutralizing antibody results, nasal and ocular swabs were collected at different times after DEX treatment, and the presence of infectious virus in swabs was detected by incubation with MDBK cells. As expected, during latency, wt, rescued, or LR mutant virus was not detected in swabs on the day of DEX treatment (day 0). At 4 and 7 days after DEX treatment, infectious virus was detected in swabs obtained from six of seven and seven of seven calves latently infected with wt virus (Fig. 7). At 4 and 7 days after DEX treatment, infectious virus was detected in four of four calves infected with the rescued virus. At these time points, infectious virus was detected in nasal and ocular swabs of calves shedding wt and LR-rescued virus. To simplify the data, the ocular and nasal results were combined. In sharp contrast, infectious virus was not detected in seven of seven calves latently infected with the LR mutant at any time point tested. Infectious virus was not detected in nasal or ocular swabs of any calf after 10 days postreactivation (Fig. 7 and data not shown). These studies demonstrated that the LR mutant did not reactivate from latency following multiple injections of DEX.
FIG. 7.
Viral shedding from ocular and nasal cavities after DEX-induced reactivation. Ocular and nasal swabs were collected from each calf at the designated times after treatment with DEX. Infectious virus in swabs was detected by inoculating MDBK cells with a 1:20 dilution of the swab medium solution. FA was used to confirm the presence of infectious BHV-1 in cultures containing cytopathic effects. Shown are the percentages of calves that shed BHV-1 virus/total number of calves in each group on the designated day after DEX treatment. Four calves were used for the study with the LR-rescued virus, seven were used for the wt (WT) study, and seven were used for the study with the LR mutant.
DISCUSSION
These studies demonstrated that the LR mutant did not reactivate from DEX-induced reactivation, whereas the wt or LR-rescued virus did. The LR mutant contains three stop codons near the beginning of the LR-RNA that are designed to prohibit protein expression from all three reading frames (17). The LR mutant also lacks 25 bp from wt sequence to prevent reversion to the wt. A peptide antibody that is directed against amino acid sequences within the LR open reading frame (ORF2) recognizes a protein of approximately 40 kDa in cells transfected with a wt LR gene construct (6, 16, 19), but not when transfected with a plasmid containing the mutation used to make the LR mutant virus (6). On the surface, it appears that any phenotypic difference between the LR mutant and wt or LR-rescued virus is attributable to lack of protein expression by the LR mutant. Because the LR-RNA is alternatively spliced (8), there is the possibility that more than one protein is expressed. We are using baculovirus expression systems to overexpress the various LR proteins to make polyclonal antibodies directed against these putative proteins. When all of these reagents are available, we will be able to accurately determine which, if any, LR proteins are disrupted by the mutation in the LR mutant virus. At this time, we cannot rule out the possibility that differences in LR-RNA expression between wt BHV-1 and the LR mutant were responsible for the attenuated phenotype. Regardless of whether an LR protein or changes in LR-RNA mediate the altered phenotype of the LR mutant, these studies clearly demonstrated that the latency reactivation cycle was disrupted following infection of calves with the LR mutant.
The TG is divided into three sections (ophthalmic, maxillary, and mandibular), and each section innervates the eye, nose, or mouth, respectively. Following infection of calves with the LR mutant, a 3- to 4-log reduction in virus shedding from ocular swabs was detected compared to the level in calves infected with wt or LR-rescued virus (17). In contrast, shedding of the LR mutant from the nasal cavity during acute infection was not significantly different from that of wt BHV-1 or the LR-rescued virus. These data suggested that “seeding” of the TG by the LR mutant would be fairly normal via the maxillary route following infection, but lower levels of virus would seed the TG via the ocular route. Considering that we were not able to reactivate the LR mutant and lower levels of viral DNA were detected in TG of calves latently infected with the LR mutant, it appears that seeding of viral DNA via the ocular route is more important. It is also possible that regardless of whether the LR mutant seeds the TG via the maxillary or ocular route, efficient amplification of the viral genome or reactivation from latency would not occur. The finding that 10- to 100-fold less infectious virus was present in TG of calves acutely infected with the LR mutant supports the contention that wt expression of LR gene products was important for acute infection of sensory neurons.
Several reports have demonstrated that HSV-1 LAT plays an important role in establishing latency (34, 39, 40), suggesting LR gene products also play a role in establishing latency. Two findings in this study support a role for wt expression of LR gene products in the establishment of latency. First, reduced levels of viral DNA were detected in calves latently infected with the LR mutant. Second, the failure to detect in situ hybridization-positive neurons in calves latently infected with the LR mutant suggested that LR gene products were necessary for latency in neurons containing high copies of viral DNA. Based on these two observations, we suggest that LR gene products promote (i) productive infection in certain neurons, (ii) survival of “permissive” neurons that support productive infection, or (iii) infection of certain types of neurons. Considering that LR gene products interfere with apoptosis in transiently transfected cells (6) and HSV-1 LAT interferes with apoptosis (1, 18, 27), it is tempting to speculate that LR gene products promote neuronal survival in TG of infected calves during acute infection. Since our results do not exclude the possibility that LR gene products play a direct role in reactivation from latency, it is clear that additional experiments are necessary to appreciate the steps in the latency reactivation cycle that LR gene products regulate.
We were unable to detect infectious virus from calves latently infected with the LR mutant following DEX treatment to initiate reactivation (Fig. 6 and 7). One could argue that the efficiency of virus shedding was below the levels of detection in calves latently infected with the LR mutant. However, we were also unable to detect changes in virus-specific antibodies during the course of reactivation. An increase in virus-specific antibodies is a sensitive method to detect reactivation from BHV-1 (21) and HSV-1 (33). Furthermore, we used multiple injections of DEX to ensure that reactivation occurred. By using a similar protocol, we reactivated a modified live vaccine strain of BHV-1 that is severely attenuated (21). It is generally accepted that the amount of viral DNA in TG of HSV-1-infected small animal models plays a role in the efficiency of reactivation (20, 47), implying there is a similar mechanism for BHV-1. As discussed above, calves infected with the wt, but not the LR mutant, have neurons with high levels of viral DNA and reactivate efficiently, suggesting these neurons support reactivation.
In general, HSV-1 LAT has been shown to be important, but not required, for latency reactivation in rabbits and mouse models (20, 47). The results presented in this study indicate that wt expression of LR gene products was required for the latency reactivation cycle in calves when reactivation was initiated by DEX. The simplest explanation for this is that the LR gene is more important than LAT for the latency reactivation. BHV-1 lacks several genes contained in the HSV-1 genome, which mediate pathogenesis and/or latency: the 34.5 gene, for example (42). The 34.5 gene plays a crucial role in neurovirulence by inhibiting antiviral functions of the interferon-inducible double-stranded RNA-dependent protein kinase R (5, 23). 34.5-null mutants have reduced pathogenesis in rabbits and mice, in large part because of poor growth properties in the eyes and TG (35). Since LR gene products play a role in ocular growth of BHV-1 in calves (17) and lower levels of infectious virus were detected in TG of calves acutely infected with the LR mutant (Fig. 1), they have additional properties that have not been described for HSV-1 LAT. The LR gene restores spontaneous reactivation to a McKrae LAT-null mutant (dLAT2903), demonstrating that LAT and LR gene products have common functional properties that are necessary for efficient reactivation from latency (26). In addition, the LR gene enhanced the ability of dLAT2903 to kill mice during acute infection and induce recurrent eye disease in latently infected rabbits, underscoring our hypothesis that LR gene products have expanded roles during the latency reactivation cycle and even pathogenesis when compared to LAT. Although the current studies point to added functions of the LR gene in the life cycle of BHV-1, the importance of HSV-1 LAT in the latency reactivation cycle may be underestimated because its role in the natural host cannot be analyzed.
Acknowledgments
This research was supported by grants from the USDA (9802064 and 2000-02060) and NIH (P20RR15635). L. Lovato was supported by a scholarship from CAPES, Brazil.
We thank B. Clowser for assistance with cattle experiments.
REFERENCES
- 1.Ahmed, M., M. Lock, C. G. Miller, and N. W. Fraser. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J. Virol. 76:717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Block, T. M., J. G. Spivack, I. Steiner, S. Deshmane, M. T. McIntosh, R. P. Lirette, and N. W. Fraser. 1990. A herpes simplex virus type 1 latency-associated transcript mutant reactivates with normal kinetics from latent infection. J. Virol. 64:3417-3426. (Erratum, 64:4603.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowland, S. L., and P. E. Shewen. 2000. Bovine respiratory disease: commercial vaccines currently available in Canada. Can. Vet. J. 41:33-48. [PMC free article] [PubMed] [Google Scholar]
- 4.Carter, J. J., A. D. Weinberg, A. Pollard, R. Reeves, J. A. Magnuson, and N. S. Magnuson. 1989. Inhibition of T-lymphocyte mitogenic responses and effects on cell functions by bovine herpesvirus 1. J. Virol. 63:1525-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou, J., J. J. Chen, M. Gross, and B. Roizman. 1995. Association of a Mr 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5− mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciacci-Zanella, J., M. Stone, G. Henderson, and C. Jones. 1999. The latency-related gene of bovine herpesvirus 1 inhibits programmed cell death. J. Virol. 73:9734-9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devi-Rao, G. B., D. C. Bloom, J. G. Stevens, and E. K. Wagner. 1994. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J. Virol. 68:1271-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devireddy, L. R., and C. Jones. 1998. Alternative splicing of the latency-related transcript of bovine herpesvirus 1 yields RNAs containing unique open reading frames. J. Virol. 72:7294-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraefel, C., J. Zeng, Y. Choffat, M. Engels, M. Schwyzer, and M. Ackermann. 1994. Identification and zinc dependence of the bovine herpesvirus 1 transactivator protein BICP0. J. Virol. 68:3154-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griebel, P. J., H. B. Ohmann, M. J. Lawman, and L. A. Babiuk. 1990. The interaction between bovine herpesvirus type 1 and activated bovine T lymphocytes. J. Gen. Virol. 71:369-377. [DOI] [PubMed] [Google Scholar]
- 11.Griebel, P. J., L. Qualtiere, W. C. Davis, A. Gee, H. Bielefeldt Ohmann, M. J. Lawman, and L. A. Babiuk. 1987. T lymphocyte population dynamics and function following a primary bovine herpesvirus type-1 infection. Viral Immunol. 1:287-304. [DOI] [PubMed] [Google Scholar]
- 12.Griebel, P. J., L. Qualtiere, W. C. Davis, M. J. Lawman, and L. A. Babiuk. 1987. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral Immunol. 1:267-286. [DOI] [PubMed] [Google Scholar]
- 13.Hariharan, M. J., C. Nataraj, and S. Srikumaran. 1993. Down regulation of murine MHC class I expression by bovine herpesvirus 1. Viral Immunol. 6:273-284. [DOI] [PubMed] [Google Scholar]
- 14.Hinkley, S., A. B. Hill, and S. Srikumaran. 1998. Bovine herpesvirus-1 infection affects the peptide transport activity in bovine cells. Virus Res. 53:91-96. [DOI] [PubMed] [Google Scholar]
- 15.Ho, D. Y., and E. S. Mocarski. 1988. Beta-galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology 167:279-283. [DOI] [PubMed] [Google Scholar]
- 16.Hossain, A., L. M. Schang, and C. Jones. 1995. Identification of gene products encoded by the latency-related gene of bovine herpesvirus 1. J. Virol. 69:5345-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inman, M., L. Lovato, A. Doster, and C. Jones. 2001. A mutation in the latency-related gene of bovine herpesvirus 1 leads to impaired ocular shedding in acutely infected calves. J. Virol. 75:8507-8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inman, M., G.-C. Perng, G. Henderson, H. Ghiasi, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2001. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J. Virol. 75:3636-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, Y., A. Hossain, M. T. Winkler, T. Holt, A. Doster, and C. Jones. 1998. A protein encoded by the latency-related gene of bovine herpesvirus 1 is expressed in trigeminal ganglionic neurons of latently infected cattle and interacts with cyclin-dependent kinase 2 during productive infection. J. Virol. 72:8133-8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, C. 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv. Virus Res. 51:81-133. [DOI] [PubMed] [Google Scholar]
- 21.Jones, C., T. J. Newby, T. Holt, A. Doster, M. Stone, J. Ciacci-Zanella, C. J. Webster, and M. W. Jackwood. 2000. Analysis of latency in cattle after inoculation with a temperature sensitive mutant of bovine herpesvirus 1 (RLB106). Vaccine 18:3185-3195. [DOI] [PubMed] [Google Scholar]
- 22.Kutish, G., T. Mainprize, and D. Rock. 1990. Characterization of the latency-related transcriptionally active region of the bovine herpesvirus 1 genome. J. Virol. 64:5730-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggioncalda, J., A. Mehta, Y. H. Su, N. W. Fraser, and T. M. Block. 1996. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology 225:72-81. [DOI] [PubMed] [Google Scholar]
- 25.Nataraj, C., S. Eidmann, M. J. Hariharan, J. H. Sur, G. A. Perry, and S. Srikumaran. 1997. Bovine herpesvirus 1 downregulates the expression of bovine MHC class I molecules. Viral Immunol. 10:21-34. [DOI] [PubMed] [Google Scholar]
- 26.Perng, G.-C., B. Maguen, L. Jin, K. R. Mott, N. Osorio, S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, M. Inman, G. Henderson, C. Jones, and S. L. Wechsler. 2002. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J. Virol. 76:1224-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perng, G.-C., C. Jones, J. Ciacci-Zanella, M. Stone, G. Henderson, A. Yukht, S. M. Slanina, F. M. Hoffman, H. Ghiasi, A. B. Nesburn, and S. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript (LAT). Science 287:1500-1503. [DOI] [PubMed] [Google Scholar]
- 28.Perng, G.-C., K. Chokephaibulkit, R. L. Thompson, N. M. Sawtell, S. M. Slanina, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1996. The region of the herpes simplex virus type 1 LAT gene that is colinear with the ICP34.5 gene is not involved in spontaneous reactivation. J. Virol. 70:282-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perng, G.-C., E. C. Dunkel, P. A. Geary, S. M. Slanina, H. Ghiasi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045-8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perng, G.-C., H. Ghiasi, S. M. Slanina, A. B. Nesburn, and S. L. Wechsler. 1996. The spontaneous reactivation function of the herpes simplex virus type 1 LAT gene resides completely within the first 1.5 kilobases of the 8.3-kilobase primary transcript. J. Virol. 70:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perng, G.-C., S. M. Slanina, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1996. A 371-nucleotide region between the herpes simplex virus type 1 (HSV-1) LAT promoter and the 2-kilobase LAT is not essential for efficient spontaneous reactivation of latent HSV-1. J. Virol. 70:2014-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perng, G.-C., S. M. Slanina, A. Yukht, B. S. Drolet, W. Keleher, Jr., H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1999. A herpes simplex virus type 1 latency-associated transcript mutant with increased virulence and reduced spontaneous reactivation. J. Virol 73:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perng, G.-C., S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1999. Herpes simplex virus type 1 serum neutralizing antibody titers increase during latency in rabbits latently infected with latency-associated transcript (LAT)-positive but not LAT-negative viruses. J. Virol. 73:9669-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perng, G.-C., S. M. Slanina, A. Yukht, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J. Virol. 74:1885-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perng, G.-C., R. L. Thompson, N. M. Sawtell, W. E. Taylor, S. M. Slanina, H. Ghiasi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1995. An avirulent ICP34.5 deletion mutant of herpes simplex virus type 1 is capable of in vivo spontaneous reactivation. J. Virol. 69:3033-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock, D., J. Lokensgard, T. Lewis, and G. Kutish. 1992. Characterization of dexamethasone-induced reactivation of latent bovine herpesvirus 1. J. Virol. 66:2484-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rock, D. L., S. L. Beam, and J. E. Mayfield. 1987. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J. Virol. 61:3827-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rock, D. L., A. B. Nesburn, H. Ghiasi, J. Ong, T. L. Lewis, J. R. Lokensgard, and S. L. Wechsler. 1987. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol. 61:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawtell, N. M. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 71:5423-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawtell, N. M., and R. L. Thompson. 1992. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J. Virol. 66:2157-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schang, L. M., and C. Jones. 1997. Analysis of bovine herpesvirus 1 transcripts during a primary infection of trigeminal ganglia of cattle. J. Virol. 71:6786-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwyzer, M., and M. Ackermann. 1996. Molecular virology of ruminant herpesviruses. Vet. Microbiol. 53:17-29. [DOI] [PubMed] [Google Scholar]
- 43.Sheffy, B. E., and D. H. Davies. 1972. Reactivation of a bovine herpesvirus after corticosteroid treatment. Proc. Soc. Exp. Biol. Med. 140:974-976. [DOI] [PubMed] [Google Scholar]
- 44.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235:1056-1059. [DOI] [PubMed] [Google Scholar]
- 45.Thompson, R. L., and N. M. Sawtell. 1997. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J. Virol. 71:5432-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tikoo, S. K., M. Campos, and L. A. Babiuk. 1995. Bovine herpesvirus 1 (BHV-1): biology, pathogenesis, and control. Adv. Virus Res. 45:191-223. [DOI] [PubMed] [Google Scholar]
- 47.Wagner, E. K., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkler, M. T. C., A. Doster, and C. Jones. 1999. Bovine herpesvirus 1 can infect CD4+ T lymphocytes and induce programmed cell death during acute infection of cattle. J. Virol. 73:8657-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winkler, M. T. C., A. Doster, and C. Jones. 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsil of latently infected calves. J. Virol. 74:5337-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wirth, U. V., C. Fraefel, B. Vogt, C. Vlc̆ek, V. Pac̆es, and M. Schwyzer. 1992. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3′ coterminal and encode a putative zinc finger transactivator protein. J. Virol. 66:2763-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wirth, U. V., B. Vogt, and M. Schwyzer. 1991. The three major immediate-early transcripts of bovine herpesvirus 1 arise from two divergent and spliced transcription units. J. Virol. 65:195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyler, R., M. Engels, and M. Schwyzer. 1989. Infectious bovine rhinotracheitis/vulvovaginitis (BHV-1), p. 1-72. In G. Witman (ed.), Herpesvirus diseases of cattle, horses, and pigs. Kluwer Academic Publishers, Boston, Mass.