Abstract
Herpes simplex virus type 1 (HSV-1) amplicon vectors are promising gene delivery tools, but their utility in gene therapy has been impeded to some extent by their inability to achieve stable transgene expression. In this study, we examined the possibility of improving transduction stability in cultured human cells via site-specific genomic integration mediated by adeno-associated virus (AAV) Rep and inverted terminal repeats (ITRs). A rep− HSV/AAV hybrid amplicon vector was made by inserting a transgene cassette flanked with AAV ITRs into an HSV-1 amplicon backbone, and a rep+ HSV/AAV hybrid amplicon was made by inserting rep68/78 outside the rep− vector 3′ AAV ITR sequence. Both vectors also had a pair of loxP sites flanking the ITRs. The resulting hybrid amplicon vectors were successfully packaged and compared to a standard amplicon vector for stable transduction frequency (STF) in human 293 and Gli36 cell lines and primary myoblasts. The rep+, but not the rep−, hybrid vector improved STF in all three types of cells; 84% of Gli36 and 40% of 293 stable clones transduced by the rep+ hybrid vector integrated the transgene into the AAVS1 site. Due to the difficulty in expanding primary myoblasts, we did not assess site-specific integration in these cells. A strategy to attempt further improvement of STF by “deconcatenating” the hybrid amplicon DNA via Cre-loxP recombination was tested, but it did not increase STF. These data demonstrate that introducing the integrating elements of AAV into HSV-1 amplicon vectors can significantly improve their ability to achieve stable gene transduction by conferring the AAV-like capability of site-specific genomic integration in dividing cells.
The elucidation of the molecular bases of inherited or acquired diseases and the completion of the human genome map have challenged vector development in the field of gene therapy. In many gene therapy paradigms, successful long-term gene transduction has not been achieved due to the loss of transgene expression over time, particularly when nonintegrative vectors, such as those based on adenovirus or herpes simplex virus type 1 (HSV-1), were used. Although the mechanisms involved are not well understood, some evidence suggests that down-regulation of the transgene promoter (46, 50, 54), degradation or extrusion of transgene DNA (50), and rejection of transgene-expressing cells by the immune system (20, 21) may all lead to the instability of transgene expression. The most common approaches to this problem have been to attempt to maintain the transgene vectors as stable episomal elements, e.g., with Epstein-Barr virus or mammalian artificial chromosome sequences, or to promote transgene integration into the cellular genome of the host (for a review, see reference 27). The present study was designed to address the latter approach using elements of adeno-associated virus (AAV) and P1 bacteriophage loxP in the context of a plasmid-based HSV-1 amplicon vector.
HSV-1 amplicon vectors are highly versatile vectors with very attractive aspects in terms of gene therapy. They can very efficiently infect a broad range of dividing and nondividing cells with minimal multiplicities of infection (MOIs) (51, 53) and can deliver up to 153 kb of DNA into mammalian cells (51). A major drawback of all HSV-1 vectors is the transient nature of transgene expression that is most likely related to the nonepisomal and nonintegrated state of amplicon DNA in the host cells. AAV is a human parvovirus with a 4.7-kb single-stranded linear DNA genome, which consists of two open reading frames, encoding four overlapping regulatory proteins (Rep78, -68, -52, and -40) and three capsid proteins, flanked by 145-base inverted terminal repeats (ITRs) (45). This virus has the unique ability during latent infection to undergo site-specific integration of its viral DNA into the AAVS1 region in chromosome 19 (19q13.3-qter) of the human genome (26, 39). The mechanism of the site-specific integration has not been fully elucidated. However, there is consistent evidence from several laboratories suggesting that the ITRs and the two large Rep proteins (Rep68 and Rep78) are responsible for this function. Recombinant AAV (rAAV) vectors which are devoid of all AAV sequences except the two ITRs lose their site-specific integration ability, although they are still capable of random integration (31, 35, 37, 60). However, when Rep68, Rep78, or Rep68 and Rep78 is expressed in cis or trans relative to the ITRs, the AAV ITRs and ITR-flanked DNA can be “rescued” from the plasmid backbone of AAV-derived plasmids and integrated into the AAVS1 site of transduced cells (1, 28, 48, 57). It has not been possible to include rep and transgene together in recombinant AAV vectors because of their relatively small packaging capacity (<5 kb), but in recent years, hybrid vectors have been created which incorporate the AAV ITRs and rep genes in combination with larger transgene capacities. These include the hybrid baculovirus-AAV vector (BAC-AAV), the hybrid adenovirus/AAV ITR vector (Ad/AAV) in combination with an adenovirus/AAV rep68/78 helper vector, and the hybrid HSV-1 amplicon vector (HSV/AAV) (23, 34, 36). With the BAC-AAV and Ad/AAV vectors, both enhanced stable transduction frequencies (STFs) over the nonhybrid parent vectors and site-specific integration were achieved. In the previous versions of the HSV/AAV hybrid vectors, only partial ITR sequences were included inadvertently, and in the later version of the vectors, the green fluorescent protein (GFP)-expressing HSV/AAV hybrid vector, there were no Rep proteins being expressed due to a construction error. Therefore, although improved stability of transgene expression was observed both in cultured cells and in vivo, site-specific integration was not evaluated (6, 10, 23).
The P1 bacteriophage Cre-loxP system has been widely used for in vitro and in vivo genomic manipulation. This system includes the 38-kDa recombinase (Cre) and the 34-bp loxP target sequence (47). When two parallel loxP sites exist in the same DNA molecule, Cre will first bind these two sites together to form a circular protein-DNA complex and then mediate a synaptic union of these two loxP sites. This results in two smaller circular molecules, each of them containing one loxP site (13).
HSV-1 amplicons are plasmid-based vectors containing only two cis-acting HSV-1 elements, a packaging signal and an origin of DNA replication (12, 43, 44). The packaged amplicon vectors have a ∼152-kb DNA genome consisting of multiple head-to-tail copies of the amplicon (amplicon concatemer). Hybrid HSV/AAV amplicons also include multiple copies of the AAV ITR-flanked and rep-containing units. Structurally, this is very different from wild-type (wt) and recombinant AAV in which the ITRs exist at the two ends of the linear genome. Theoretically, Rep should first rescue the ITR-flanked fragment from the vector genome and then target it to the AAVS1 site. Since rescue and integration are two contradictory functions of Rep proteins and each of them might require a different level of Rep expression (2), we predict that HSV/AAV vectors might have a reduced efficiency of integration compared to that of wt AAV. Based on this analysis, we hypothesize that if the ITR-flanked transgene were first excised from the vector concatemer by means of Cre-loxP recombination, Rep expressed at low levels could facilitate site-specific integration.
In this study, we reengineered the HSV/AAV hybrid amplicon to contain the full-length ITRs flanked by loxP sites, with or without rep68/78. This was done to test the hypothesis that inclusion of this AAV “integration machinery” into an HSV-1 hybrid amplicon vector could promote the transgene integration into the host genome and thereby stabilize the transgene expression. The HSV/AAV hybrid vectors were evaluated for STFs as well as site-specific integration events in three different types of human cells. In addition, we tested our hypothesis that deconcatenation of the amplicon DNA via Cre-loxP recombination would further enhance site-specific integration. Our results show that a Rep-expressing HSV/AAV hybrid vector (rep+) enhanced the STF in all cell types tested. The site-specific integration rate was as high as 84% in the stably transduced Gli36 colonies examined. There was no additional improvement in STF after deconcatenation with Cre-loxP recombination, but the vector DNA was appropriately targeted to AAVS1 in the clones transduced by rep+ vector at a slightly higher frequency than without deconcatenation. The ability to achieve site-specific integration of transgene via HSV/AAV hybrid vectors should have a significant impact on stable and reproducible levels of transgene expression in transduced cells that contain the AAVS1 site.
MATERIALS AND METHODS
Cell culture.
Human embryonic kidney 293 cells were obtained from the American Type Culture Collection (Manassas, Va.). 293T/17 cells express the simian virus 40 large T antigen and were selected specifically for high transfection efficiency (provided by David Baltimore, Massachusetts Institute of Technology, Cambridge). Human glioma Gli36 cells were provided by Anthony Campagni (UCLA School of Medicine). African green monkey kidney VERO 2-2 cells were provided by Rozanne Sandri-Goldin (University of California, Irvine). Human primary myoblasts were provided by Emanuela Gussoni (Children's Hospital, Boston, Mass.). The HEK293, 293T/17, Gli36, and VERO 2-2 cells were grown in Dulbecco's modified Eagle medium with 10% fetal bovine serum (Sigma, St. Louis, Mo.) supplemented with 100 U of penicillin/ml and 0.1 mg of streptomycin (Sigma)/ml. VERO 2-2 cell growth medium was further supplemented with 0.4 mg of G418 (Gibco/BRL, Rockville, Md.)/ml. Primary myoblasts were grown in Ham's F10 media supplemented with 20% fetal bovine serum and 5 ng of basic fibroblast growth factor (Promega)/ml. All cells were incubated at 37°C and 5% CO2 with a humidified atmosphere.
A Cre recombinase-expressing HEK293 cell line was generated by infecting wt HEK293 cells with a retroviral vector carrying Cre recombinase and puromycin expression cassettes (kindly provided by Philippe Leboulch, Massachusetts Institute of Technology). Infected cells were selected with 2 μg of puromycin/ml for 1 week. Puromycin-resistant colonies were isolated and expanded. The expression of Cre recombinase in stable cell lines was detected by immunocytochemistry with anti-Cre antibody (Babco, Richmond, Calif.).
Vector constructs.
AAV ITR sequences with unique restriction sites at each end were synthesized manually (Midland Certified Reagent Co.). Plasmid pHSVloxP was created by replacing the polylinker of pHSVprPUC (gift of Howard Federoff, University of Rochester, Rochester, N.Y.) with a new oligonucleotide polylinker. This contained a pair of loxP sites near each end flanked by AscI and PshaI (5′) and EcoRV (3′) and had NotI, PacI, BglII, SalI, and SgfI restriction sites in between loxP sites. Plasmid pHSVLGN was constructed by inserting the BglII-flanked cytomegalovirus promoter-driven enhanced GFP (eGFP) expression cassette and simian virus 40 promoter-driven neomycin (neo)-resistance protein expression cassette derived from plasmid pHyRGN (10) into pHSVloxP (Fig. 1A). Plasmid pHLIGN was constructed by sequentially inserting the 3′ AAV ITR into the SalI and SgfI sites, the eGFP and neo-resistance cassette fragment into the BglII site, and the 5′ AAV ITR into the NotI and PacI sites of pHSVloxP (Fig. 1B). The AAV rep68/78 expression cassette with its p5 promoter (derived from pRep68/78 in which the expression of Rep52 and Rep40 had been aborted with a missense mutation of their starting codon; a gift of Cornel Fraefel, Institute of Virology, University of Zurich, Zurich, Switzerland) was inserted in both orientations either in the PshAI site upstream of the 5′ loxP site or in the EcoRV site downstream of the 3′ loxP site of pHLIGN. Of these four plasmids, the only one used in further experiments was pHLIRGN, in which rep was inserted in the forward orientation after the 3′ loxP site (Fig. 1C) as the other yielded low titers on packaging. The integrities of the ITRs in pHLIGN and pHLIRGN were determined by sequential digestion with either of the enzymes that digest the sites flanking ITRs, followed by digestion with enzymes that digest the sites inside of ITR (MscI and SmalI sites exist inside of the ITRs as palindromes). ITR fragments were resolved by electrophoresis on 5% Nusieve agarose (FMC, Rockland, Maine) gels.
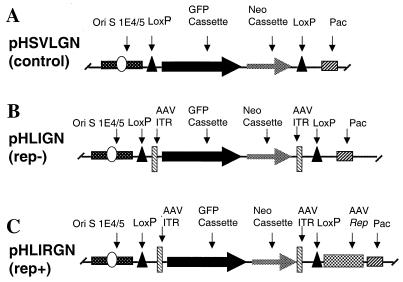
FIG. 1.
Vector constructs. (A) A standard amplicon with a pair of loxP genes flanking GFP and neomycin resistance transgene cassettes. (B) The rep− HSV/AAV hybrid amplicon was made by inserting a pair of AAV ITRs into the standard amplicon flanking the transgene cassettes. (C) The rep+ HSV/AAV hybrid amplicon was made by adding Rep68/78 into the rep− hybrid amplicon downstream of the 3′ loxP site.
Vector packaging.
The helper virus-free packaging system developed by Fraefel et al. (11) was used to package all amplicons. In brief, VERO 2-2 cells were transfected with a mixture of amplicon plasmid, a set of five cosmids, and Lipofectamine (Gibco/BRL). The five cosmids spanned the entire HSV-1 genome, with only the cleavage-packaging signals (“a” sequences containing the pac signals) deleted. Amplicon vectors were harvested 60 h later, freeze thawed three times, sonicated, and centrifuged at 1,000 × g for 10 min. Amplicon vectors were then concentrated and purified by centrifugation at 55,000 × g for 2 h onto a 25% sucrose gradient. Amplicon vector stock titers were determined as transduction units per milliliter (TU/ml) by infecting 3 × 105 293T/17 cells per well in 24-well plates and then counting the GFP-positive cells 18 h postinfection at a 20× magnification.
Western blot analysis.
The expression of Rep proteins was evaluated by Western blot analysis. 293T/17 cells transfected with amplicons pHLIRGN, pHLIGN, pHSRepN1 (from David Jacoby, Massachusetts General Hospital, Boston, Mass.), and pPSSV9 (from Richard J. Samulski, University of North Carolina at Chapel Hill, Chapel Hill, N.C.) were lysed in a buffer containing 1% NP-40, 50 mM Tris (pH 8.0), 150 mM NaCl, and Complete-Mini Cocktail of protease inhibitors (Boehringer Mannheim, Indianapolis, Ind.). Protein concentrations were determined using the Coomassie Plus protein reagent assay (Pierce, Rockford, Ill.) and a bovine serum albumin standard (Bio-Rad, Hercules, Calif.). Equal amounts of cell lysates (300 μg) were denatured and separated by electrophoresis on 10% polyacrylamide gels with sodium dodecyl sulfate. Rainbow Markers (Amersham Life Sciences, Arlington Heights, Ill.) were used as molecular weight markers. Proteins were transferred to nitrocellulose membrane (Bio-Rad Trans-blot Transfer Medium Pure; 0.45-μm pore size) in transfer buffer (25 mM Tris, 192 mM glycine, pH 8.3) by using a Bio-Rad Trans-blot Cell for 3 h at 0.5 mA at 4°C. Membranes were stained with 0.2% Ponceau S (Sigma) to ensure proper transfer and equal loading of samples. After staining, membranes were blocked overnight in 10% nonfat dry milk in TBS-T (150 mM NaCl, 50 mM Tris-HCl [pH 7.9], 0.01% Tween 20). The following day, membranes were washed twice for 15 min and twice for 5 min in TBS-T and then incubated for 1 h at room temperature with primary antibodies in 2% nonfat dry milk in TBS-T. Mouse anti-AAV Rep clone 303.9 (ARP Scientific, Belmont, Mass.) was developed against all four Rep proteins (with molecular masses of 40, 52, 68, and 78 kDa) and used at dilutions of 1:25. The membranes were then washed as before and incubated for 30 min with a 1:10,000 dilution of anti-mouse immunoglobulin G (IgG) horseradish peroxidase-conjugated secondary antibody (Amersham Life Sciences) in TBS-T with 5% nonfat dry milk. After washing, as described above, the blots were developed using enhanced chemiluminescence (ECL) reagents (Amersham Life Sciences). Membranes were then exposed to film for 3 min.
Cloning efficiency and STF.
One day prior to infection, 293 cells, Gli36 cells, or human primary myoblasts were plated at a density of 5 × 105 cells per 60-mm-diameter dish. On the following day, cells were infected at an MOI of 0.1 or 1 TU/cell with either HLIRGN or HLIGN hybrid vectors. In parallel, all cell types were infected with the nonhybrid HSVLGN vector as a control for the presence of AAV ITRs, and 293 cells were also infected with HSVGN as a control for the presence of loxP sites. The day after infection, all vector-containing medium was replaced with fresh growth medium. Forty-eight hours postinfection, cells were harvested and then subjected to fluorescence-activated cell sorter (FACS) analysis for GFP-positive cells, as appropriate. The unsorted cells were replated at specific cloning densities and grown in media with or without 1 mg of G418 (Gibco/BRL)/ml for 293 and Gli36 cells or 0.4 mg/ml for human primary myoblasts for 10 to 20 days. All clones in the plates with or without G418 selection were counted, and the cloning efficiencies and stable transduction frequencies (STFs) were determined, respectively. Cloning efficiency was defined as the number of clones formed without drug selection divided by the number of cells plated. STF was defined as the number of neomycin-resistant clones after G418 selection divided by product of the number of cells plated times the percentage of GFP+ cells in the original sample times the cloning efficiency. In addition, one group of 293 cells was also FACS sorted for GFP-positive cells and plated at cloning density in two groups without G418 selection. One group was removed from their plates at 1 and 2 weeks postsorting and reanalyzed each time by FACS for the percentage of GFP+ cells. The other group had the number of GFP-positive and -negative clones counted at 2 weeks postsorting. The STF of this group of cells without the influence of G418 selection was then determined using the same formula used above.
FACS and analysis.
All cell sorting and dynamic GFP expression analysis was done using a FacsCalibur analyzer (Becton-Dickinson, Franklin Lakes, N.J.).
Hirt DNA extraction and bacteria transformation.
Five million Cre-expressing or wt 293 cells were infected by HLIGN at an MOI of 3. Forty-eight hours after infection, the cells were harvested and the episomal DNA was extracted according to a modified Hirt DNA extraction procedure (18). Five microliters of Hirt DNA was used to transform DH5α-competent bacteria (Invitrogen) according to the manufacturer's instructions. The resulting plasmid DNA was analyzed by digestion with restriction enzymes and followed by gel electrophoresis.
Southern blot analysis.
Isolated GFP+ colonies from Gli36 and 293 cells infected by the each of the three vectors (HLIRGN, HLIGN, or HSVLGN) were expanded, and genomic DNA was extracted according to standard methods. Twenty micrograms of genomic DNA was digested with EcoRI, which doesn't cut the loxP-flanked transgene cassette or the amplicon backbone but does cut the rep gene and also generates a native 8.2-kb AAVS1 fragment from human genomic DNA. After electrophoresis in 0.7% agarose gels, the digested DNA was transferred onto a nylon membrane. The membrane was first hybridized using a 0.7-kb PmeI fragment derived from the eGFP cDNA as probe, and then the blot was stripped off. The blot was exposed to film for 24 h to ensure no radioactive signal was left and then rehybridized using a 1.1-kb BamHI/PvuII fragment derived from the AAVS1 locus (kindly donated by R. M. Kotin, National Institutes of Health) as probe. All probes were labeled with [32P]dCTP using a Radprime DNA labeling system (Invitrogen). The genomic DNA from colonies infected with the rep+ amplicon was also cut with KpnI, which cuts the vector twice, generating 11- and 0.5-kb bands. This blot was hybridized using a 1.1-kb BamHI/XhoI fragment from pRep68/78 as probe to examine the incidence of rep integration. Some of the Hirt DNA from Cre+ or wt 293 cells was digested with NheI, a unique cutter in the transgene region, and hybridized with the eGFP probe. Finally, two sets of genomic DNA from Gli36 and 293 colonies infected with rep+ amplicon were cut with StuI, the enzyme that cuts once inside of the transgene, and were hybridized with the 1.8-kb amplicon backbone probe or 0.7-kb eGFP probe to check the integration of amplicon backbone and the existence of a concatemer of ITR-flanked transgene units.
RESULTS
Construction and packaging of HSV and HSV/AAV hybrid amplicon vectors.
The ITRs of AAV and a transcriptional cassette for the two large AAV Rep proteins were inserted into HSV-1 amplicons. In addition, a pair of loxP sequences was also added into the amplicons flanking the ITRs (in the case of hybrid vector) or into the transgene cassette (in the case of standard amplicon). The resulting HSV/AAV hybrid vectors and a control vector lacking AAV ITRs, all with loxP sites, are shown in Fig. 1.
Since the highly homologous sequences between the two AAV ITRs can cause recombination events leading to mutations of the ITR during plasmid replication and because various deletions were detected in the ITR fragments in plasmids that were made available to us, we synthesized the ITRs (Midland Certified Reagent Co.) flanked by unique restriction sites for insertion into our hybrid amplicons. After the final amplicons were completely constructed, the integrities of the ITRs were verified by digestion with restriction enzymes that flank an individual ITR and then by MscI and SmaI that cut inside the ITRs, and the restriction products were resolved using 5% Nusieve agrose gel electrophoresis. Analysis of the restriction products from all hybrid vectors, including pHLIGN and pHLIRGN, demonstrated the presence of all expected restriction products (data not shown), indicating that the ITRs were intact.
All vectors were packaged using the five-cosmid helper virus-free packaging system (11). The rep− HLIGN vector and both control vectors all had titers of 1 × 106 to 6 × 106 TU/ml in crude stocks and 1 × 108 to 3 × 108 TU/ml after purification. Of the vectors that contained the rep68/78 gene, only when the rep gene was placed downstream of the 3′ ITR at the forward orientation was a comparable amplicon vector titer achieved. This amplicon, pHLIRGN or rep+ hybrid vector (Fig. 1C), was the only one of these four rep-containing vectors to be used for further studies, and its range of titers was 0.4 × 105 to 2 × 105 TU/ml in crude stock and 0.5 × 107 to 1 × 107 TU/ml after purification.
The expression of the Rep68 and Rep78 proteins in cells transduced with the HSV/AAV rep+ amplicon, pHLIRGN, was confirmed by Western blotting. 293T/17 cells were transfected with pHLIRGN, pHLIGN, pHSRepN1, or pSSV9. Plasmids pHSRepN1 and pSSV9 express all four Rep proteins. Mouse anti-AAV Rep clone 303.9 primary antibodies were used to detect the expression of Rep proteins. Four Rep proteins were shown in the lysates of 293T/17 cells transfected by both HSRepN1 and pSSV9. Only Rep68 and -78 were detected in the lysates of pHLIRGN-transfected cells and, as expected, no Rep proteins were expressed in pHLIGN-transfected cells (data not shown).
Evaluation of AAV hybrid function. (i) Stability of transgene expresion.
The aim of this study was to obtain more stable transgene expression through the function of ITRs and Rep proteins of AAV within the context of the HSV-1 amplicon vector. This function was assessed in three cultured human cell types, 293, Gli36 (glioma cells), and primary myoblasts.
First, we examined the dynamics of transgene expression over time after infection without G418 selection. Human primary myoblasts and 293 cells were infected with each of the three vectors in Fig. 1 at an MOI of 1 and were then subjected to FACS sorting for GFP+ cells 48 h postinfection. The initial infection efficiencies were ∼20, ∼90, and ∼40% for 293, Gli36c and primary myoblasts, respectively, at an MOI of 1 for control and rep− vector and a slight decease for rep+. There was no notable cell death observed at this MOI, and no noticeable difference in growth rates was seen among human primary myoblasts infected by the three vectors, but there was a slightly slower growth rate seen in 293 cells infected by the rep+ vector. The cell populations were analyzed sequentially by FACS sorting for the percentage of GFP+ cells at 1 and 2 weeks postinfection. For both cell types, there were more GFP+ cells at 1 and 2 weeks postinfection in the rep+ group than in the control group (2.7- and 14.4-fold more at 1 week postinfection and 13.2- and 5-fold more at 2 weeks postinfection for 293 cells and primary myoblasts, respectively, compared to the cells infected by the standard amplicon, HSVLGN) (Table 1). However, there were 6.2- and 7.5-fold decreases in the number of GFP+ 293 cells and human primary myoblasts, respectively, at 2 weeks postinfection compared to the number at 1 week postinfection. Although the rep− vector had a slight advantage over the control vector at 1 week postinfection (1.5- and 3-fold, respectively), this advantage was lost at 2 weeks postinfection (P > 0.05).
TABLE 1.
Dynamics of transgene expression
| Cell | Vector | % of GFP+ cells ata:
|
|
|---|---|---|---|
| 1 week | 2 weeks | ||
| 293 | HLIRGN | 11.7 ± 0.4 | 1.9 ± 0.4 |
| HLIGN | 6.4 ± 0.2 | 0.1 ± 0.04 | |
| HSVLGN | 4.4 ± 0.3 | 0.2 ± 0.1 | |
| HPMb | HLIRGN | 15.3 ± 2.3 | 2.0 ± 0.4 |
| HLIGN | 3.2 ± 0.6 | 0.6 ± 0.2 | |
| HSVLGN | 1.0 ± 0.3 | 0.4 ± 0.1 | |
FACS analysis data. n = 6 for all of the groups.
HPM, human primary myoblast.
Second, the STF with G418 selection was determined for vectors HLIRGN, HLIGN, or HSVLGN. The results from all three cell types show that the rep+ HSV/AAV hybrid vector gave rise to significantly higher STFs (P < 0.01) than the standard amplicon. The rep− hybrid vector yielded the same to slightly decreased STFs in 293 cells as the standard amplicon, and no stable colonies were seen in the Gli36 cells and primary myoblasts (Table 2). These observations indicate that the AAV ITRs alone do not enhance the integration of amplicon vector DNA into the host genome. However, the large Rep proteins together with ITRs were able to supply this AAV function to the HSV/AAV hybrid vector. In all the three cell types tested, HLIRGN at both MOIs yielded the highest STF.
TABLE 2.
STFs
| Cell | MOI | Vector | STF (%)a |
|---|---|---|---|
| 293 | 0.1 | HLIRGN | 9.4 ± 2.9 |
| HLIGN | 3.1 ± 0.9 | ||
| HSVLGN | 5.7 ± 1.1 | ||
| 1 | HLIRGN | 7.4 ± 1.1 | |
| HLIGN | 0.02 ± 0.04 | ||
| HSVLGN | 3.1 ± 0.2 | ||
| Gli36 | 0.1 | HLIRGN | 10.3 ± 1.29 |
| HLIGN | 0.5 ± 0.1 | ||
| HSVLGN | 0.8 ± 0.1 | ||
| 1 | HLIRGN | 8.1 ± 0.7 | |
| HLIGN | 1.1 ± 0.3 | ||
| HSVLGN | 2.8 ± 0.4 | ||
| HPM | 1 | HLIRGN | 2.2 ± 0.5 |
| HLIGN | 0 | ||
| HSVLGN | 0.1 ± 0.1 |
STF = Number of stable transduced colonies/number of cells infected × efficiency of colony formation × 100%. n = 8 for all of the groups.
(ii) Site-specific integration.
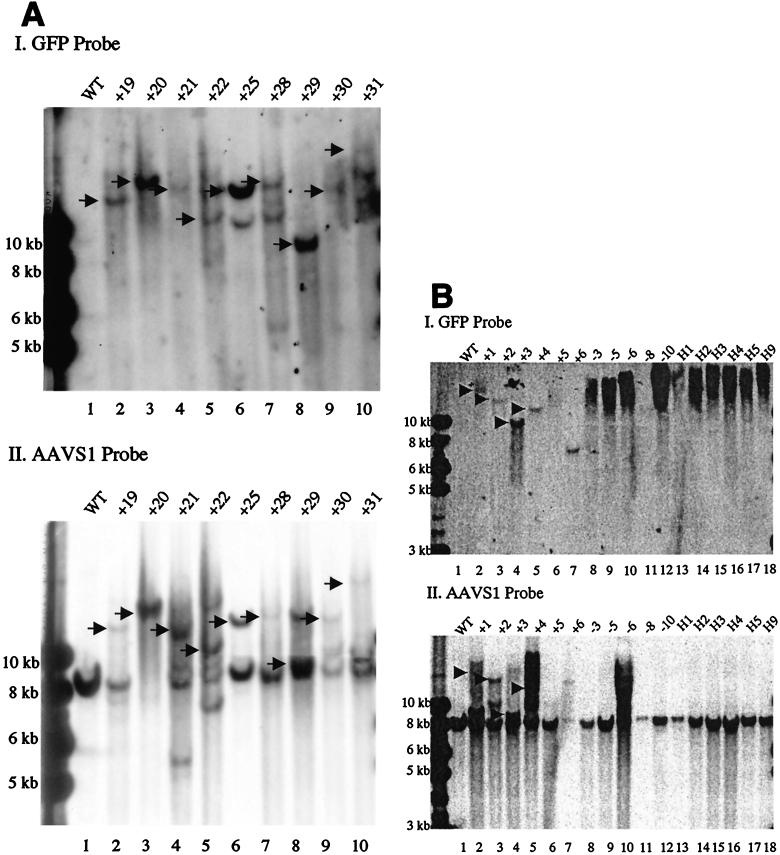
A variety of vectors with AAV ITRs and rep sequences included have been demonstrated to be able to mediate transgene integration into the AAVS1 site in the transduced human cells (1, 28, 34, 40, 48). To determine the efficiency of site specific integration mediated by the HSV/AAV hybrid amplicon, stable GFP+ clones from Gli36 cells infected with HLIRGN, HLIGN, or HSVLGN were expanded, and genomic DNA was extracted and then subjected to Southern blot analysis. Twenty-seven clones infected with the rep+ vector, 14 clones infected with the rep− vector, and 18 clones infected with a standard amplicon were examined. Integration at AAVS1 was assessed by hybridizing EcoRI-digested genomic DNA from these cells with a GFP probe followed by an AAVS1 probe. Shifted AAVS1 bands were detected in 26 out of 27 clones infected with the rep+ vector, and 22 of them also hybridized to the GFP probe in the same location. In contrast, there was no shift of AAVS1 bands seen in any of the clones derived from rep− or control vectors (Fig. 2).
FIG. 2.
Southern blot analysis of site-specific integration in Gli36 cells. Cellular genomic DNA was cut with EcoRI, an enzyme that does not cut the two control vectors, HSVLGN and HLIGN, but cuts inside the rep gene of HLIRGN and generates a native 8.2-kb AAVS1 band in the human genome. The blots were probed with GFP sequences first and then with AAVS1 sequences. (A) Colonies from Gli36 cells infected with HLIRGN. (I) The genomic DNA was hybridized with a GFP probe. Lane 1, noninfected cells; lanes 2 to 10, cells infected with HLIRGN. The arrows point out the bands that are coordinated with an AAVS1-positive band in the panel below. (II) The same blot was probed with AAVS1 sequences. The arrows point out the bands that are coordinated with GFP-positive bands in the panel above. (B) Additional samples from Gli36 cells infected with HLIRGN and samples from cells infected with HLIGN and HSVLGN. (I) Hybridization to the GFP probe. Lane 1, noninfected Gli36 cells; lanes 2 to 7, samples infected with HLIRGN; lanes 8 to 12, samples infected with HLIGN; lanes 13 to 18, samples infected with HSVLGN. The arrows point out the GFP+ bands that are coordinated with AAVS1 bands in the panel below. In lanes 8 to 18, there were no specific GFP+ bands observed, but a blurred GFP+ signal was seen on the top of the gel. (II) The same blot was probed with AAVS1 sequences. Lanes 2 to 7, shifted AAVS1 bands were detected in every colony. The arrows point out the shifted AAVS1 bands that are coordinated with GFP+ bands in I. In lanes 8 to 18 (except for lane 10), no shifted AAVS1 band was detected in samples from HLIGN or HSVLGN-infected cells. Lane 10, clone −6, the genomic DNA was only partially digested because of overloaded DNA; the presence of an 8-kb AAVS1 band was proven in two other blots with a reduced amount of DNA.
(iii) Integration of rep gene, amplicon backbone, and AAV-like concatemer.
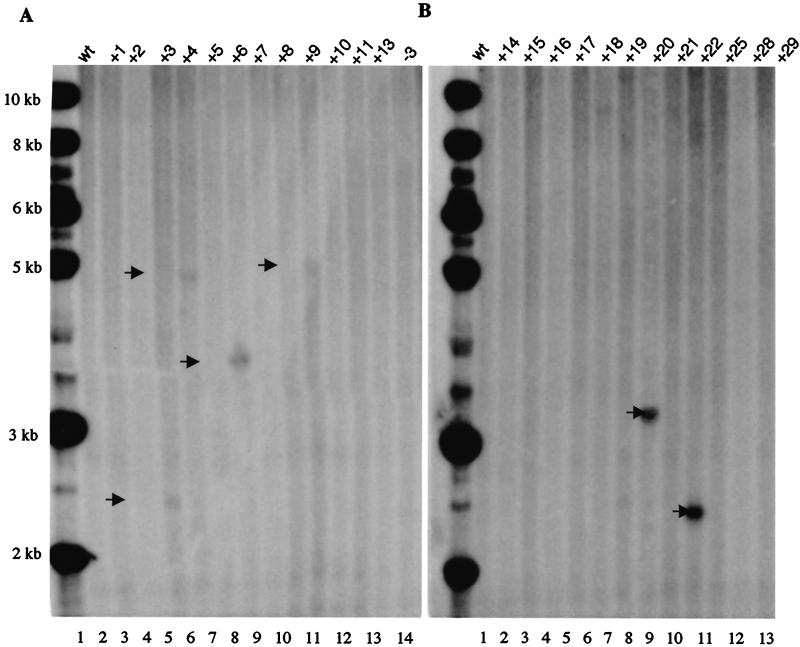
In the rep+ HSV/AAV construct, the rep gene is placed outside of the ITRs. Theoretically, transgene integration mediated by Rep proteins should occur primarily at the ITRs, thus excluding the rep gene and other amplicon sequences from the integration so that the expression and the potential toxicity of Rep proteins would only be temporary. To test this hypothesis, the genomic DNA of Gli36 cells from the clones infected with the rep+ vector was cut with KpnI and analyzed by Southern blotting with a cDNA probe coding for the rep sequence. Six of 27 clones showed a rep+ band in various sizes. This indicates that rep integration is not completely avoided by this vector design but occurs only in a small fraction of the integration events (Fig. 3). The integration of amplicon backbone was also examined. Interestingly, the backbone sequence was detected in 3 of 27 clones but only 1 of those clones was included an integrated rep sequence (data not shown). In the latent infection of wt AAV and rAAV, integration could occur as concatemers. To examine if this was the case in the integration mediated by the rep+ hybrid vector, the genomic DNA was also cut with StuI, which cuts once inside of transgene, and hybridized with eGFP probe. Putatively, it should generate an extra band with a 4-, 6-, or 0.3-kb size in the case of head-to-tail, head-to-head, or tail-to-tail concatemers, respectively. There was no matched image found (data not shown(.
FIG. 3.
Southern blot analysis of integration of rep gene in Gli36 cells. The cellular genomic DNA of colonies infected by HLIRGN was cut with KpnI, the enzyme which cuts only inside of the rep region and yields ∼11- and ∼0.5-kb bands. The blot was probed with rep sequence. (A) Lane 1, DNA from noninfected Gli36 cells; lanes 2 to 13, DNA from cells infected with HLIRGN. In lanes 4, 5, 7, and 10, rep+ bands are seen. Lane 14, DNA from cells infected with HLIGN. (B) Lane 1, DNA from noninfected Gli36 cells; lanes 2 to 13, DNA from Gli36 cells infected with HLIRGN. In lanes 8 and 10, the rep+ band is shown with arrow. Lane 14, DNA from cells infected with HLIGN.
Effects of deconcatenation. (i) Confirmation of Cre/LoxP function.
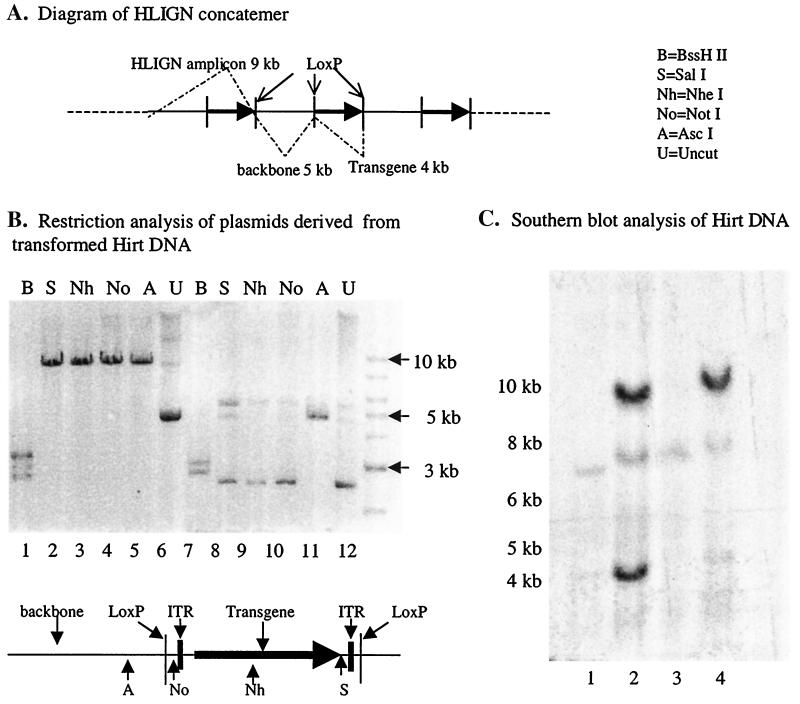
The efficiency of Cre-loxP recombination for deconcatenation was determined by analyzing the Hirt DNA extracted from both Cre-expressing 293 cells (293 Cre+ cells) and wt 293 cells infected by the HLIGN vector. Two approaches were utilized. First, Hirt DNA from Cre-expressing 293 Cre+ cells or wt 293 cells was used to transform bacteria. Twenty-eight colonies were obtained in the plate transformed by the Hirt DNA from 293 Cre+ cells and one colony from the wt 293 plate. Ten colonies from the 293 Cre+ plate and the single colony from the wt 293 plate were analyzed by restriction digestion and agarose gel electrophoresis. Nine out of 10 293 Cre+ colonies were found to contain only the amplicon backbone (5 kb) as demonstrated by their size and the fact that all of the enzymes that cut in between the loxP sites (NheI, NotI, and SalI) failed to digest the plasmid, but AscI, which cuts outside of the 5′ loxP, did linearize the plasmid (Fig. 4B). The remaining Cre+ colony as well as the colony from wt 293 contained the intact HLIGN (9-kb) plasmid (Fig. 4B). Southern blot analysis performed directly on the Hirt DNA digested with NheI that cuts once inside the ITRs of the rep− amplicon within the transgene should generate a single 9-kb band in monomers and multimers of rep− vector DNA. On the other hand, NheI should generate a 4-kb band in transgene-containing minicircles yielded by Cre-loxP recombination. Identical amounts of uncut and NheI-cut Hirt DNA from 293 Cre+ and wt 293 cells were probed with a 0.7-kb PmeI fragment containing the transgene sequence (Fig. 4B). The resulst showed that both of the 4- and 9-kb bands could be found in the lane loaded with 293 Cre+ Hirt DNA, and both bands showed similar signal intensity. This indicates that about half of the transgene had been dissociated from the amplicon backbone (Fig. 4C). There was no 4-kb band observed in wt 293 Hirt DNA. In the lanes loaded with uncut Hirt DNA from both Cre+ and wt 293 cells, no positive signal was observed. This is probably a result of inefficient transfer of circularized DNA. Both of these experiments support excision of the ITR-flanked transgene from the amplicon genome in the 293 Cre+ cells but not in the wt 293 cells.
FIG. 4.
Confirmation of deconcatenation of amplicon vectors via Cre-loxP recombination. (A). Diagram of an HLIGN concatemer. HLIGN is 9 kb in length and is composed of a 5-kb plasmid backbone and a 4-kb loxP-flanked transgene cassette. Note that when packaged head to tail in the amplicon concatemer, the plasmid backbone, in turn, is also flanked by the loxP sequences. (B) Restriction endonuclease analysis of DNA derived from bacteria transformed with Hirt DNA from HLIGN-infected cells. The 5-kb plasmid derived from 293 Cre+ cells displayed the pattern of the amplicon backbone after being digested with BssHII (lane 7). This plasmid no longer contained the unique restriction sites that are located in between loxP sequences (lane 8, NotI; lane 9, SalI; lane 10, NheI) but retained the restriction sites (e.g., in lane 11, AscI) that are located outside of the loxP sequences. The 9-kb plasmid derived from a single 293 Cre+ clone displayed the restriction map of complete pHLIGN amplicon (lanes 1 to 5). (C) Southern blot analysis of Hirt DNA from 293 Cre+ and wt 293 cells infected with HLIGN. Lanes 1 and 3, uncut Hirt DNA from 293 Cre+ and wt 293 cells; lane 2, Hirt DNA from 293 Cre+ cut by NheI. GFP+ bands were at 4- and 9-kb positions. Lane 4, the Hirt DNA from wt 293 cells cut by NheI. Only the 9-kb GFP+ band was observed.
(ii) Stability of transgene expression after deconcatenation.
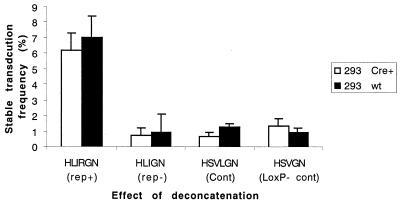
Next, we examined the effects of deconcatenation by Cre/LoxP recombination on STF in 293 Cre+ and wt 293 cells following infection by HLIRGN, HLIGN, HSVLGN, and HSVGN at an MOI of 1. Vector HSVGN is identical to HSVLGN except that it lacks the loxP signals, and it was added as a control to rule out nonspecific effects of Cre recombinase. GFP-positive cells were FACS sorted 48 h postinfection and plated at cloning density. The sorted cells were grown without G418 selection for 2 weeks, and the GFP+ colonies were counted. The STF for each vector was calculated using the formula described in the previous section (Fig. 5). In 293 Cre+ cells, among the four vectors, HLIRGN generated the highest STF. However, when compared to wt 293 cells, deconcatenation did not improve STF for any of the vectors.
FIG. 5.
Effect of deconcatenation. The STFs yielded from 293 Cre+ and wt 293 cells infected with HLIRGN, HLIGN, HSVLGN, and HSVGN were compared. HSVGN was identical to HSVLGN except that the former lacks loxP sites. There was no significant difference between 293 Cre+ cells infected with loxP-containing vectors and the STFs yielded in wt 293 cells.
(iii) Site-specific vector integration and rep and amplicon backbone integration after deconcatenation.
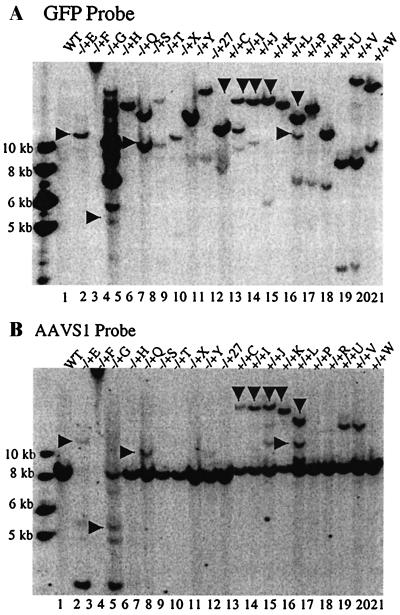
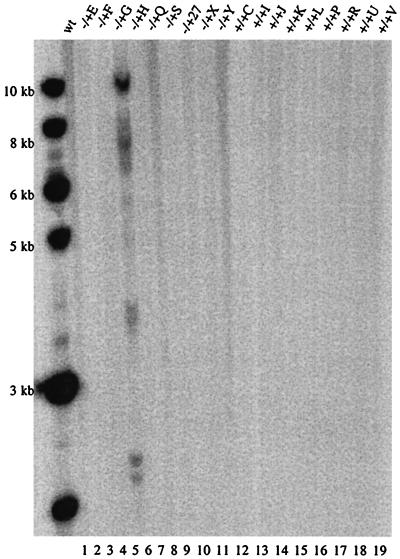
Site-specific integration after deconcatenation was also examined. A total of 40 clones, 10 each of GFP+ clones from 293 Cre+ and wt 293 cells infected by HLIRGN or HLIGN, were expanded and their EcoRI-digested genomic DNA was analyzed by Southern blotting. Site-specific integration was found in 40% of wt 293 and 50% of 293 Cre+ colonies infected by HLIRGN (Fig. 6). As expected, no AAVS1 band shifts indicative of site-specific integration were detected in any of the colonies infected by HLIGN in either wt 293 or 293 Cre+ cells. The effect of deconcatenation on integration of the rep gene was also examined in the clones infected by HLIRGN. One of 10 clones from wt 293 cells showed integration of the rep gene, and this particular clone integrated into the host genome at multiple sites as shown in the Southern blot probed with rep sequence (Fig. 7). None of clones from 293 Cre+ cells showed integration of the rep gene, which suggests that physically separating the rep gene from the ITR-flanked transgene fragments may help to solve the potential problem of rep gene integration (Fig. 7). The integration of amplicon backbone was also checked. None of the clones from either cells showed positive band of amplicon backbone sequence (data not shown).
FIG. 6.
Southern blot analysis of Cre+ and wt 293 cells to detect site-specific integration into AAVS1 site. Genomic DNA was digested with EcoRI. The blot was first probed with a GFP probe and then reprobed with an AAVS1 probe. (A) Blot was probed with GFP sequences. Lane 1, DNA from wt 293; lanes 2 to 11, DNA from wt 293 cells; lanes 12 to 21, DNA from 293 Cre+ cells infected by HLIRGN. The GFP+ bands coordinated with shifted AAVS1 bands were indicated by arrows. In lane 3, clone −/+F was not digested because no enzyme was added in this experiment, but it was proved to have site-specific integration in another experiment. (B) The same blot was probed with GFP probe. In lanes 2, 4, 6, 10, 12 to 16, 20, and 21, shifted AAVS1 bands are shown. The shifted AAVS1 bands that coordinate with GFP bands are indicated with arrows.
FIG. 7.
Southern blot analysis for rep integration in Cre+ and wt 293 cells. Lane 1, DNA from noninfected wt 293; lanes 2 to 11, DNA from wt 293 cells infected by HLIRGN. In lane 4, the clone −/+G shows multiple rep+ bands; the same clone showed multiple copies of integration. Lanes 12 to 21, DNA from 293 Cre+ cells infected by HLIRGN. There was no rep+ band observed in any of these clones.
DISCUSSION
One obstacle that has impeded the application of HSV-1 amplicon vectors in gene therapy is the instability of transgene expression (6, 9, 10, 23, 50). Although some studies observed prolonged physiological changes in experimental animals after gene transfer mediated by HSV-1 amplicon vectors, the number of cells retaining transgene expression was small (8, 33, 42). It has been believed that the primary cause of this failure in nondividing cells was promoter shut down, and strategies to replace viral promoters by tissue-specific promoters have improved the stability of transgene expression to a certain degree (11, 22, 54, 61). However, despite these changes, obtaining a significant fraction of infected cells to sustain expression of transgenes for a considerable therapeutic period has been difficult. Although the factors related to the instability of transgene expression mediated by this type of vector remain to be defined, a recent study by Tsai et al. (50) suggested that peripheralization of amplicon vector DNA in the nuclei of transduced neurons was correlated with the loss of transgene expression and that at 60 days postinfection the majority of the initially transduced vector DNA (80%) disappeared from transduced nuclei (50). This provides direct evidence that the extrachromosomal state of HSV-1 amplicon DNA is, at least in a large part, responsible for the instability of transgene expression. This aspect can't be overcome by changing promoters or adding introns and suggests that changing the state of vector DNA in the nuclei of transduced cells could be a solution. Previously, we have constructed an HSV/AAV vector by inserting an ITR and rep gene of AAV into an HSV-1 amplicon vector containing a LacZ reporter gene under control of a human cytomegalovirus immediate-early 1 (IE1) promoter. This vector was able to sustain transgene expression in cultured glioma cells for 2 weeks, whereas the control amplicon lost the transgene after 10 days postinfection (23). The HSV/AAV hybrid vector was modified subsequently by changing the LacZ gene into GFP and neomycin resistance genes to facilitate further evaluation of the vector. The GFP-neomycin version of HSV/AAV hybrid vector was demonstrated to prolong transgene expression in brain and liver (6, 10) but other hybrid characteristics, such as site-specific integration, couldn't be proven. Consequentially, sequence data demonstrated that there was a construction error in the GFP-neomycin version of hybrid vector so that the rep sequence was interrupted by a transgene cassette. Furthermore, the ITRs were incomplete, containing only the D element important in replication of AAV genome (52) but not the trs and RBS sequences needed for site-specific integration (3). This incomplete ITR was the result of a preexisting deletion in the original material that we used to construct the amplicon vector. Nevertheless, these observations suggested that the AAV integration elements, even if they were not intact, could help stabilize transgene expression from HSV-1 amplicon vector, and we presumed that it should be further improved when their full function was added. In this study, we reconstructed HSV/AAV hybrid vectors by using manually synthesized ITRs and modified the design by using a rep cassette expressing only Rep68/78 instead of a rep cassette expressing all four Rep proteins and by adding loxP signals in flanking the ITRs. We examined the new hybrid vectors for their ability to confer stable transgene expression and genomic integration in three cell lines that were undergoing active growth. Our data indicate that the addition of the AAV ITRs alone, in rep− hybrid vector HLIGN, did not improve the stability of transgene expression when compared to a standard amplicon vector HSVLGN, in all three types of human origin cells tested. However, the addition of the AAV ITRs and rep68/78, in rep+ hybrid vector HLIRGN, substantially increased the stability of transgene expression in all cell types studied. Southern blot analysis identified that this increase in STF was conferred primarily by site-specific genomic integration of transgene into the AAVS1 site, which is known to require Rep protein mediation.
Like adenovirus, HSV-1 is one of the natural helper viruses of AAV (14, 55). To date, attempts to produce an adeno/AAV hybrid vector that includes both the rep genes and the ITRs of AAV in the same vector have been unsuccessful. It has only been possible to make an adenovirus expressing Rep when the rep genes are driven by some promoter other than the natural p5 promoter (36, 56). It has also been reported that Rep proteins suppress DNA replication mediated by HSV-1 (16). Thus, it was reasonable to be concerned about achieving adequate vector titers with an HSV/AAV rep+ hybrid system. As expected, when rep68/78 was expressed by amplicon DNA, there was a significant decrease in the production of amplicon vector virions. However, the extent of this reduction could be modulated by the position of the rep68/78 cassette relative to the position of the OriS/IE 4/5 promoter component of the HSV-1 amplicon vector. VP16, a viral tegument protein that is abundant during viral replication and carried by the virion, activates the I/E45 promoter present upstream of the 5′ ITR and/or transgene cassette in all amplicons. When the rep68 and rep78 genes were placed immediately adjacent to this promoter, viral production decreased by 100- to 1,000-fold. However, when the rep68/78 cassette was placed some distance from OriS/IE 4/5 promoter, but in the same orientation, a reasonable titer of rep+ hybrid amplicon vector (107 TU/ml after concentration) could be achieved. We hypothesize that to recover the full production of amplicon vector, the expression of Rep proteins will have to be suppressed during the packaging process. This decrease in titer caused by rep68/78 has been confirmed in the companion paper by Heister et al. (17).
There are two distinct stages in the life cycle of wt AAV infection. In the absence of a helper virus, AAV normally enters a latent stage. In this stage, there is a limited expression of AAV Rep proteins, which prevents further expression of the viral genes and thereby inhibits virus production. However, even the limited expression of Rep proteins is effective in facilitating the genomic integration of the viral genome at a specific site (AAVS1) (5, 15, 24-26, 39). In the presence of a helper virus (e.g., adenol or herpes virus) or cellular stress reagents (e.g., UV irradiation, chemical carcinogens), AAV enters a lytic stage in which infectious AAV particles are produced (4, 19, 58, 59). The first step in the lytic stage is to rescue the integrated AAV DNA from the host genome and then replicate the AAV DNA (29, 38). In the context of the HSV/AAV amplicon vector, in order to achieve the site-specific integration of the ITR-flanked transgene, the machinery used in both stages of wt AAV infection must have been involved but in a reverse order. First, the Rep proteins (Rep68 and -78) needed to rescue the ITR-flanked fragment from amplicon concatemers. Next, the Rep proteins had to replicate the rescued ITR-flanked DNA and, finally, target them to the AAVS1 site of the host genome. Our data on the dynamic expression of transgene expression at 1 and 2 weeks postinfection (Fig. 3A) revealed that there were much larger percentages of HLIRGN-transduced cells that retained transgene expression at 1 week postinfection than at 2 weeks postinfection, when almost all the nonintegrative expression had vanished. This indicates that additional copies of the transgene produced in cells infected by rep+ hybrid vector, either by transgene release from the amplicon backbone via the rescue mechanism and/or by ITR-flanked transgene replication mediated by Rep proteins. This was more obvious in primary myoblasts in which at 1 week postinfection only 1% of cells infected by a control vector retained transgene expression compared to 15% of cells infected by rep+ hybrid vector.
The result of Southern blot analysis suggests that the integration that occurred in the cells transduced by the rep+ hybrid amplicon vector underwent a different mechanism of integration than standard or rep− hybrid amplicon vectors. In the latter vectors, the whole ∼150-kb concatemer of the amplicon vector genome was integrated randomly into the cell genome (data not shown). However, in the case of the rep+ hybrid amplicon vector, in most cases only a single copy of the 4-kb transgene cassette appeared to be integrated into the AAVS1 site. In the Gli36 cells, the frequency of integration was 10% of cells infected by rep+ hybrid vector, and as many as 84% of these integration events were at the AAVS1 site. This gives a positive indication that active and specific Rep functions were involved in the integration events. In 293 cells, the frequency of site-specific integrations was 40% among the stable colonies transduced by rep+ hybrid vector. This frequency of site-specific integration was somehow lower than the observation with infection by wt AAV (60 to 70%) in the same type of cells (26, 39). This could reflect either the effect of drug selection in their studies and the absence in drug selection in ours or an inherent difference in the efficiency of site-specific integration between the two vectors.
In the HSV/AAV rep+ hybrid vector construct, the rep gene was placed outside of the ITRs. Theoretically, such a design should exclude the rep gene from integration. Our Southern blot analysis results showed that this happened in most but not all of the cases. In 23% of Gli36 and 10% of 293 stably transduced clones, rep was detected in the cellular genome. Interestingly, in these clones, with only one exception, there was no amplicon backbone detected, which raised a question of whether the rep gene we detected was intact and functional. It is not clear how the rep gene was included in the integrated sequence without the backbone sequence. The general concern regarding the integration of the rep gene is Rep-induced toxicity to the transduced cells and the potential excision of the integrated transgene from cellular genome when the cell is exposed to conditions that render the cell permissive for AAV replication. In addition to that, there are also some concerns about the potential problems that would accompany coinfection with other viruses, such as HSV, adenovirus, and wt AAV. In the cases of coinfection with HSV or adenovirus, it's unlikely that the presence of the rep gene will worsen the infections of HSV or adenovirus, since the role of Rep proteins is to suppress rather than enhance the DNA replication of these two viruses. In the case of coinfection with a latent wt AAV, although it is possible that Rep from the transduced vector could rescue the latent wt AAV genome and elevate the expression of viral genes, it is unlikely that Rep would produce infectious AAV particles since neither Rep proteins nor amplicon vector can provide helper functions. Nevertheless, the integration of the rep gene is an undesirable consequence and needs to be avoided. We sought to minimize this possibility by using the strategy of deconcatenation through Cre-loxP combination. When Cre recombinase combines two adjacent loxP sequences to form an ITR-containing minicircle, the rep gene as well as the amplicon backbone are physically excluded from the preintegration unit. It appears that this approach was successful because when it was used, there was no rep integration detected in any colonies derived from 293 Cre+ cells in this study.
In both 293 and Gli36 cells, HLIRGN was tested at MOIs of both 0.1 and 1. An MOI of 0.1 yielded a slightly higher STF than was seen at the higher MOI, suggesting that the toxicity of Rep proteins and higher level of GFP expression might actually artificially decrease the STF. We also noticed that there were fewer GFP+ cells seen at 2 weeks by FACS than would be suggested by STF seen using cloning. This might indicate that the stable transduction didn't always happen in the first generation of infected cells and/or that cytotoxicity of GFP caused cell death in some GFP-expressing cells, since GFP has been shown to be toxic to a number of cell types (30). In fact, GFP+ colonies were generally smaller than GFP− colonies in the non-G418-selected group. It was unlikely to be caused by the Rep protein or related to drug selection because a similar phenomenon was observed in the control GFP+ groups and in the rep− GFP+ group and in the group in which drug selection was not used.
To draw a comprehensive conclusion about the rep+ hybrid vector integration mechanism and the factors governing STF, more information, e.g., the sequences of integration junctions and the evidence of rescued ITR-flanked fragments, needs to be analyzed. However, based on the recent work of Fraefel et al. using rep+ hybrid amplicon vectors with similar design, such conclusions appear to be justified (17).
In the design of the HSV/AAV hybrid vectors, we had some concerns regarding the efficacy of these vectors based on studies of AAV. In the natural life cycle of AAV, the rescue and replication of the AAV genome depend on increased expression of Rep proteins, which is stimulated by the helper virus or the cellular stress agents (2). Could the HSV/AAV vector express enough Rep proteins to support the rescue and/or replication of the ITR-flanked transgene from the amplicon backbone? Could Rep proteins rescue and/or replicate genes larger than the parent virus, i.e., larger than 4.7 kb? To address these issues, loxP sequences were inserted on either side of the ITR-flanked transgene cassette. Our hypothesis was that in the presence of Cre recombinase, the ITR-flanked transgene cassette could be excised from the amplicon background via Cre-loxP recombination, and thus, rescue and replication could be avoided. In the present study, we evaluated the STFs yielded in Cre-expressing 293 cells (293 Cre+) infected with the three vectors in comparison with those yielded in non-Cre-expressing 293 cells. The Cre-loxP recombination events were validated by the existence of a circularized 5-kb amplicon backbone and 4-kb ITR-flanked transgene in the vector-infected 293 Cre+ cells. Although that there was no improvement on the efficiency of stable integration after the deconcatenation, this experiment does show that it is possible for Rep68/78 to process preformed circularized ITR-containing DNA for genomic integration. This could prove useful for integrating transgene cassettes larger than wt AAV. Our Southern blot analysis results indicated that 50% of the integrations in 293 Cre+ cells were targeted to the AAVS1 site, and the integration of preformed circles appeared to be less disturbing to the genome of AAVS1 region than integration in wt 293 cells. Three out of five stable colonies had a shift in AAVS1 band comparable to the sum of the AAVS1 band (8.2-kb) plus the 4.3-kb ITR-flanked transgene cassette. In contrast, in the colonies from wt 293 cells, the sizes of shifted AAVS1 bands were much more diverse (Fig. 7).
We anticipated that the deconcatenation of rep− hybrid amplicon vector would produce circularized AAV ITR-containing transgene units which resemble the double-stranded circular form of recombinant AAV vectors (rAAV) (7, 32). Presumably, this would promote random integration similar to the mechanism used by rAAV, thereby stabilizing transgene expression. The fact that it did not (Fig. 7) suggests that simply adding AAV ITRs to the amplicon vector will not promote genomic integration either as concatemer or as a monomer but that the presence of Rep proteins is a necessary addition, at least in dividing cells, for both random and site-specific integrations. It remains to be determined whether stable transgene expression can be achieved in nondividing cells or in vivo by the deconcatenated or even nondeconcatenated rep− hybrid amplicon vectors. Studies of gene transfer by rAAV in fully differentiated muscle and liver of mice have all suggested that extrachromosomal double-stranded circular monomers and concatemers of rAAV genomes are important structures for long-term gene expression (7, 32, 60). In a recent investigation of gene transfer by rAAV in liver, ∼90% of long-term transgene expression was determined to associate with extrachromosmal forms of AAV vector DNA (32). Structurally, the products of deconcatenated HSV/AAV rep− vector should be similar to those of rAAV genomes. Therefore, it is possible that this vector could be used to confer stable transgene expression in vivo, potentially with a transgene larger than 4.7 kb.
A pseudo-loxP site has been shown to exist in the human genome (49), and based on this, Cre-loxP recombination has been proposed as a possible alternative way to promote integration of transgenes into the human genome (41). However, the milieu in the present study could not provide insight into this approach. In this study, the effect of Cre-loxP recombination was evaluated in 293 Cre+ cell lines where Cre is constantly being expressed. Because the predominant function of Cre recombinase is excision rather than insertion, the final result would not be insertion even if an insertion occurred sometime during the process. To direct a successful loxP-containing transgene insertion into the pseudo-loxP site, transient expression of Cre recombinase would be desirable and its efficacy in promoting integration would be affected by the level of Cre recombinase expressed.
In summary, inserting the ITRs and rep68/78 of AAV into an HSV-1 amplicon vector improved the frequency of stable transgene expression in immortalized human cell lines (293 cells and Gli36 cells) as well as in human primary myoblasts, compared to standard and rep− hybrid amplicon vectors by means of rescue-replication and genomic integration. For rep+ hybrid amplicon, the genomic integration was frequently targeted to the AAVS1 site in 84% of stable Gli36 clones and 40% of stable 293 clones. In addition, deconcatenation of HSV/AAV rep+ amplicon concatemers using Cre-loxP recombination did not enhance STF but did improve the percentage of cells that integrated a single copy of the transgene into the AAVS1 site without associated rep integration. The HSV/AAV rep+ amplicon vector described here provides an advanced gene delivery tool for ex vivo genetic manipulation of dividing cells through their high infection efficiency, large gene capacity, and high frequency of site-specific integration. The HSV/AAV rep+ amplicon vector also holds promise for solving the problem of transient transgene expression in vivo through both site-specific integration and the rAAV-like mechanism of episomal retention.
Acknowledgments
We thank D. Baltimore, A. Compagni, H. Federoff, C. Fraefel, E. Gussoni, D. Jacoby, P. M. Kotin, P. Leboulch, R. J. Samulski, and R. Sandri-Goldin for their gifts of reagents used in this study. We thank S. Muhkerjee, R. Hirsch, W. Liu, and A. F. Flint for their technical assistance.
The work was supported by grants from MDA to P.D.A. and Y.W., NIAMS grant K01AR02148 to Y.W., and NINDS grants NS-24279 and NCI CA-69246 to X.O.B.
REFERENCES
- 1.Balague, C., M. Kalla, and W. W. Zhang. 1997. Adeno-associated virus Rep78 protein and terminal repeats enhance integration of DNA sequences into the cellular genome. J. Virol. 71:3299-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berns, K. I., and C. Giraud. 1996. Biology of adeno-associated virus. Curr. Top. Microbiol. Immunol. 218:1-23. [DOI] [PubMed] [Google Scholar]
- 3.Brister, J. R., and N. Muzyczka. 2000. Mechanism of Rep-mediated adeno-associated virus origin nicking. J. Virol. 74:7762-7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buller, R. M., J. E. Janik, E. D. Sebring, and J. A. Rose. 1981. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J. Virol. 40:241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung, A. K., M. D. Hoggan, W. W. Hauswirth, and K. I. Berns. 1980. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J. Virol. 33:739-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costantini, L. C., D. R. Jacoby, S. Wang, C. Fraefel, X. O. Breakefield, and O. Isacson. 1999. Gene transfer to the nigrostriatal system by hybrid herpes simplex virus/adeno-associated virus amplicon vectors. Hum. Gene Ther. 10:2481-2494. [DOI] [PubMed] [Google Scholar]
- 7.Duan, D., P. Sharma, J. Yang, Y. Yue, L. Dudus, Y. Zhang, K. J. Fisher, and J. F. Engelhardt. 1998. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J. Virol. 72:8568-8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.During, M. J., J. R. Naegele, K. L. O'Malley, and A. I. Geller. 1994. Long-term behavioral recovery in parkinsonian rats by an HSV vector expressing tyrosine hydroxylase. Science 266:1399-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federoff, H. J., A. Brooks, B. Muhkerjee, and T. Corden. 1997. Somatic gene transfer approaches to manipulate neural networks. J. Neurosci. Methods 71:133-142. [DOI] [PubMed] [Google Scholar]
- 10.Fraefel, C., D. R. Jacoby, C. Lage, H. Hilderbrand, J. Y. Chou, F. W. Alt, X. O. Breakefield, and J. A. Majzoub. 1997. Gene transfer into hepatocytes mediated by helper virus-free HSV/AAV hybrid vectors. Mol. Med. 3:813-825. [PMC free article] [PubMed] [Google Scholar]
- 11.Fraefel, C., S. Song, F. Lim, P. Lang, L. Yu, Y. Wang, P. Wild, and A. I. Geller. 1996. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J. Virol. 70:7190-7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geller, A. I., and X. O. Breakefield. 1988. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science 241:1667-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton, D. L., and K. Abremski. 1984. Site-specific recombination by the bacteriophage P1 lox-Cre system. Cre-mediated synapsis of two lox sites. J. Mol. Biol. 178:481-486. [DOI] [PubMed] [Google Scholar]
- 14.Handa, H., and B. J. Carter. 1979. Adeno-associated virus DNA replication complexes in herpes simplex virus or adenovirus-infected cells. J. Biol. Chem. 254:6603-6610. [PubMed] [Google Scholar]
- 15.Handa, H., K. Shiroki, and H. Shimojo. 1977. Establishment and characterization of KB cell lines latently infected with adeno-associated virus type 1. Virology 82:84-92. [DOI] [PubMed] [Google Scholar]
- 16.Heilbronn, R., A. Burkle, S. Stephan, and H. zur Hausen. 1990. The adeno-associated virus rep gene suppresses herpes simplex virus-induced DNA amplification. J. Virol. 64:3012-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heister, T., I. Heid, M. Ackermann, and C. Fraefel. 2002. Herpes simplex virus type 1/adeno-associated virus hybrid vectors mediate site-specific integration at the adeno-associated virus preintegration site, AAVS1, on human chromosome 19. J. Virol. 76:7163-7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heller, R. A., K. Song, D. Villaret, R. Margolskee, J. Dunne, H. Hayakawa, and G. M. Ringold. 1990. Amplified expression of tumor necrosis factor receptor in cells transfected with Epstein-Barr virus shuttle vector cDNA libraries. J. Biol. Chem. 265:5708-5717. [PubMed] [Google Scholar]
- 19.Hoggan, M. D., N. R. Blacklow, and W. P. Rowe. 1966. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc. Natl. Acad. Sci. USA 55:1467-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huard, J., G. Acsadi, A. Jani, B. Massie, and G. Karpati. 1994. Gene transfer into skeletal muscles by isogenic myoblasts. Hum. Gene Ther. 5:949-958. [DOI] [PubMed] [Google Scholar]
- 21.Huard, J., G. Akkaraju, S. C. Watkins, M. Pike-Cavalcoli, and J. C. Glorioso. 1997. LacZ gene transfer to skeletal muscle using a replication-defective herpes simplex virus type 1 mutant vector. Hum. Gene Ther. 8:439-452. [DOI] [PubMed] [Google Scholar]
- 22.Jin, B. K., M. Belloni, B. Conti, H. J. Federoff, R. Starr, J. H. Son, H. Baker, and T. H. Joh. 1996. Prolonged in vivo gene expression driven by a tyrosine hydroxylase promoter in a defective herpes simplex virus amplicon vector. Hum. Gene Ther. 7:2015-2024. [DOI] [PubMed] [Google Scholar]
- 23.Johnston, K. M., D. Jacoby, P. A. Pechan, C. Fraefel, P. Borghesani, D. Schuback, R. J. Dunn, F. I. Smith, and X. O. Breakefield. 1997. HSV/AAV hybrid amplicon vectors extend transgene expression in human glioma cells. Hum. Gene Ther. 8:359-370. [DOI] [PubMed] [Google Scholar]
- 24.Kotin, R. M., R. M. Linden, and K. I. Berns. 1992. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 11:5071-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotin, R. M., J. C. Menninger, D. C. Ward, and K. I. Berns. 1991. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics 10:831-834. [DOI] [PubMed] [Google Scholar]
- 26.Kotin, R. M., M. Siniscalco, R. J. Samulski, X. D. Zhu, L. Hunter, C. A. Laughlin, S. McLaughlin, N. Muzyczka, M. Rocchi, and K. I. Berns. 1990. Site-specific integration by adeno-associated virus. Proc. Natl. Acad. Sci. USA 87:2211-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam, P. Y., and X. O. Breakefield. 2000. Hybrid vector designs to control the delivery, fate and expression of transgenes. J. Gene Med. 2:395-408. [DOI] [PubMed] [Google Scholar]
- 28.Lamartina, S., G. Roscilli, D. Rinaudo, P. Delmastro, and C. Toniatti. 1998. Lipofection of purified adeno-associated virus Rep68 protein: toward a chromosome-targeting nonviral particle. J. Virol. 72:7653-7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laughlin, C. A., J. D. Tratschin, H. Coon, and B. J. Carter. 1983. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene 23:65-73. [DOI] [PubMed] [Google Scholar]
- 30.Liu, H. S., M. S. Jan, C. K. Chou, P. H. Chen, and N. J. Ke. 1999. Is green fluorescent protein toxic to the living cells? Biochem. Biophys. Res. Commun. 260:712-717. [DOI] [PubMed] [Google Scholar]
- 31.Miao, C. H., R. O. Snyder, D. B. Schowalter, G. A. Patijn, B. Donahue, B. Winther, and M. A. Kay. 1998. The kinetics of rAAV integration in the liver. Nat. Genet. 19:13-15. [DOI] [PubMed] [Google Scholar]
- 32.Nakai, H., S. R. Yant, T. A. Storm, S. Fuess, L. Meuse, and M. A. Kay. 2001. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol. 75:6969-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neill, J. C., M. R. Sarkisian, Y. Wang, Z. Liu, L. Yu, P. Tandon, G. Zhang, G. L. Holmes, and A. I. Geller. 2001. Enhanced auditory reversal learning by genetic activation of protein kinase C in small groups of rat hippocampal neurons. Brain Res. Mol. Brain Res. 93:127-136. [DOI] [PubMed] [Google Scholar]
- 34.Palombo, F., A. Monciotti, A. Recchia, R. Cortese, G. Ciliberto, and N. La Monica. 1998. Site-specific integration in mammalian cells mediated by a new hybrid baculovirus-adeno-associated virus vector. J. Virol. 72:5025-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponnazhagan, S., D. Erikson, W. G. Kearns, S. Z. Zhou, P. Nahreini, X. S. Wang, and A. Srivastava. 1997. Lack of site-specific integration of the recombinant adeno-associated virus 2 genomes in human cells. Hum. Gene Ther. 8:275-284. [DOI] [PubMed] [Google Scholar]
- 36.Recchia, A., R. J. Parks, S. Lamartina, C. Toniatti, L. Pieroni, F. Palombo, G. Ciliberto, F. L. Graham, R. Cortese, N. La Monica, and S. Colloca. 1999. Site-specific integration mediated by a hybrid adenovirus/adeno- associated virus vector. Proc. Natl. Acad. Sci. USA 96:2615-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutledge, E. A., and D. W. Russell. 1997. Adeno-associated virus vector integration junctions. J. Virol. 71:8429-8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samulski, R. J., K. I. Berns, M. Tan, and N. Muzyczka. 1982. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc. Natl. Acad. Sci. USA 79:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samulski, R. J., X. Zhu, X. Xiao, J. D. Brook, D. E. Housman, N. Epstein, and L. A. Hunter. 1991. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 10:3941-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satoh, W., Y. Hirai, K. Tamayose, and T. Shimada. 2000. Site-specific integration of an adeno-associated virus vector plasmid mediated by regulated expression of rep based on Cre-loxP recombination. J. Virol. 74:10631-10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sena-Esteves, M., Y. Saeki, C. Fraefel, and X. O. Breakefield. 2000. HSV-1 amplicon vectors—simplicity and versatility. Mol. Ther. 2:9-15. [DOI] [PubMed] [Google Scholar]
- 42.Song, S., Y. Wang, S. Y. Bak, M. J. During, J. Bryan, O. Ashe, D. B. Ullrey, L. E. Trask, F. D. Grant, K. L. O'Malley, H. Riedel, D. S. Goldstein, K. A. Neve, G. J. LaHoste, J. F. Marshall, J. W. Haycock, R. L. Neve, and A. I. Geller. 1998. Modulation of rat rotational behavior by direct gene transfer of constitutively active protein kinase C into nigrostriatal neurons. J. Neurosci. 18:4119-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spaete, R. R., and N. Frenkel. 1982. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell 30:295-304. [DOI] [PubMed] [Google Scholar]
- 44.Spaete, R. R., and N. Frenkel. 1985. The herpes simplex virus amplicon: analyses of cis-acting replication functions. Proc. Natl. Acad. Sci. USA 82:694-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Srivastava, A., E. W. Lusby, and K. I. Berns. 1983. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J. Virol. 45:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starr, P. A., F. Lim, F. D. Grant, L. Trask, P. Lang, L. Yu, and A. I. Geller. 1996. Long-term persistence of defective HSV-1 vectors in the rat brain is demonstrated by reactivation of vector gene expression. Gene Ther. 3:615-623. [PubMed] [Google Scholar]
- 47.Sternberg, N., and D. Hamilton. 1981. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J. Mol. Biol. 150:467-486. [DOI] [PubMed] [Google Scholar]
- 48.Surosky, R. T., M. Urabe, S. G. Godwin, S. A. McQuiston, G. J. Kurtzman, K. Ozawa, and G. Natsoulis. 1997. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J. Virol. 71:7951-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thyagarajan, B., M. J. Guimaraes, A. C. Groth, and M. P. Calos. 2000. Mammalian genomes contain active recombinase recognition sites. Gene 244:47-54. [DOI] [PubMed] [Google Scholar]
- 50.Tsai, D. J., J. J. Ho, C. R. Ozawa, and R. M. Sapolsky. 2000. Long-term expression driven by herpes simplex virus type-1 amplicons may fail due to eventual degradation or extrusion of introduced transgenes. Exp. Neurol. 165:58-65. [DOI] [PubMed] [Google Scholar]
- 51.Wade-Martins, R., E. R. Smith, E. Tyminski, E. A. Chiocca, and Y. Saeki. 2001. An infectious transfer and expression system for genomic DNA loci in human and mouse cells. Nat. Biotechnol. 19:1067-1070. [DOI] [PubMed] [Google Scholar]
- 52.Wang, X. S., S. Ponnazhagan, and A. Srivastava. 1996. Rescue and replication of adeno-associated virus type 2 as well as vector DNA sequences from recombinant plasmids containing deletions in the viral inverted terminal repeats: selective encapsidation of viral genomes in progeny virions. J. Virol. 70:1668-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, Y., C. Fraefel, F. Protasi, R. A. Moore, J. D. Fessenden, I. N. Pessah, A. DiFrancesco, X. Breakefield, and P. D. Allen. 2000. HSV-1 amplicon vectors are a highly efficient gene delivery system for skeletal muscle myoblasts and myotubes. Am. J. Physiol. Cell. Physiol. 278:C619-C626. [DOI] [PubMed] [Google Scholar]
- 54.Wang, Y., L. Yu, and A. I. Geller. 1999. Diverse stabilities of expression in the rat brain from different cellular promoters in a helper virus-free herpes simplex virus type 1 vector system. Hum. Gene Ther. 10:1763-1771. [DOI] [PubMed] [Google Scholar]
- 55.Weindler, F. W., and R. Heilbronn. 1991. A subset of herpes simplex virus replication genes provides helper functions for productive adeno-associated virus replication. J. Virol. 65:2476-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weitzman, M. D., K. J. Fisher, and J. M. Wilson. 1996. Recruitment of wild-type and recombinant adeno-associated virus into adenovirus replication centers. J. Virol. 70:1845-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weitzman, M. D., S. R. Kyostio, R. M. Kotin, and R. A. Owens. 1994. Adeno-associated virus (AAV) Rep proteins mediate complex formation between AAV DNA and its integration site in human DNA. Proc. Natl. Acad. Sci. USA 91:5808-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yakinoglu, A. O., R. Heilbronn, A. Burkle, J. R. Schlehofer, and H. zur Hausen. 1988. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 48:3123-3129. [PubMed] [Google Scholar]
- 59.Yakobson, B., T. A. Hrynko, M. J. Peak, and E. Winocour. 1989. Replication of adeno-associated virus in cells irradiated with UV light at 254 nm. J. Virol. 63:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang, C. C., X. Xiao, X. Zhu, D. C. Ansardi, N. D. Epstein, M. R. Frey, A. G. Matera, and R. J. Samulski. 1997. Cellular recombination pathways and viral terminal repeat hairpin structures are sufficient for adeno-associated virus integration in vivo and in vitro. J. Virol. 71:9231-9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, G. R., X. Wang, T. Yang, M. Sun, W. Zhang, Y. Wang, and A. I. Geller. 2000. A tyrosine hydroxylase-neurofilament chimeric promoter enhances long-term expression in rat forebrain neurons from helper virus-free HSV-1 vectors. Brain Res. Mol. Brain Res. 84:17-31. [DOI] [PubMed] [Google Scholar]