Abstract
Despite active immune responses, gammaherpesviruses establish latency. In a related process, these viruses also persistently replicate by using a mechanism that requires different viral genes than acute-phase replication. Many questions remain about the role of immunity in chronic gammaherpesvirus infection, including whether the immune system controls latency by regulating latent cell numbers and/or other properties and what specific immune mediators control latency and persistent replication. We show here that CD8+ T cells regulate both latency and persistent replication and demonstrate for the first time that CD8+ T cells regulate both the number of latently infected cells and the efficiency with which infected cells reactivate from latency. Furthermore, we show that gamma interferon (IFN-γ) and perforin, which play no significant role during acute infection, are essential for immune control of latency and persistent replication. Surprisingly, the effects of perforin and IFN-γ are site specific, with IFN-γ being important in peritoneal cells while perforin is important in the spleen. Studies of the mechanisms of action of IFN-γ and perforin revealed that perforin acts primarily by controlling the number of latently infected cells while IFN-γ acts primarily by controlling reactivation efficiency. The immune system therefore controls chronic gammaherpesvirus infection by site-specific mechanisms that regulate both the number and reactivation phenotype of latently infected cells.
Despite clearance of acute infection by the immune response, herpesviruses establish chronic infections via latency and persistent replication. Latency and persistent replication are critically important parts of the gammaherpesvirus life cycle, contributing to spread within the population and diseases such as cancer. The human gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus establish latent infection in lymphoid cells and persistently replicate at sites such as the oropharynx and the genital tract (17, 18, 23, 43). These viruses are particularly problematic in immunocompromised hosts, in whom persistent replication and expansion of latently infected cell populations likely contribute to disease. Despite the importance of these pathogens, immunity during chronic infection is poorly understood. In particular, the role of the immune system in controlling latency and persistent replication is incompletely defined.
Murine gammaherpesvirus 68 (γHV68) is genetically related to EBV and Kaposi's sarcoma-associated herpesvirus and infects laboratory mice, providing a small-animal model for mechanistic studies. Although the physiology of gammaherpesvirus infection is not completely understood, through the use of the γHV68 system, it has become apparent that there are at least four experimentally distinguishable components of infection: (i) acute infection, (ii) latent infection, (iii) reactivation from latency, and (iv) persistent replication. These components of infection, and how they relate to each other, are not yet fully characterized.
In this report, we will use the following definitions. Acute replication refers to production of infectious virus prior to day 15 of infection. Following inoculation with γHV68, acute replication occurs in multiple organs but is cleared 9 to 15 days postinfection (4, 28, 39). By the time acute replication has cleared, γHV68 establishes a latent infection in macrophages and B cells in the peritoneum (42) and in B cells, macrophages, and dendritic cells in the spleen (9, 29). Latency is stable cellular carriage of the nonreplicating viral genome in a form that can undergo reactivation (production of infectious virus from a latently infected cell). It appears that latency has multiple forms in vivo, with early forms transitioning to stable long-term latency (2, 13, 34, 35). For γHV68, the reactivation phenotype of latently infected cells reflects this transition: cells during the early form of latency (e.g., day 16 after infection) spontaneously reactivate more efficiently ex vivo than cells during long-term latency (41).
Persistent replication refers to the production of infectious virus after 15 days, during the chronic phase of infection. Persistent replication and acute replication are distinct processes requiring different viral genes. For example, γHV68-encoded v-cyclin and v-bcl-2 play a critical role in persistent virus replication in IFN-γ−/− mice but play no role in replication during acute infection (10). In contrast to acute replication, persistent replication may be secondary to reactivation from latency since both γHV68 v-cyclin and v-bcl-2 play an important role in both persistent virus replication and reactivation from latency (10). After the establishment of latency, persistent replication is not detectable in immunocompetent mice (41). However, the finding that activated CD8+ T cells specific for epitopes in lytic-cycle proteins are detectable well after the clearance of acute infection (15, 25, 26) supports the hypothesis that persistent replication occurs at a low level throughout latency.
While it is clear that immunity plays an important role in controlling gammaherpesvirus latency (1), the mechanisms responsible are not known. In particular, it is not known what mechanisms T cells use to control latency, whether the immune system determines the number of latently infected cells or whether immunity can control other properties of latent cells such as their capacity to reactivate from latency. Similarly, immune control of persistent replication is poorly understood. Since acute replication and persistent replication are distinct processes, it is possible that certain immune mechanisms can control both latency and persistent replication but not acute infection.
CD8+ T cells play an important role in limiting acute γHV68 infection, but IFN-γ and perforin apparently do not (7, 8,24, 27, 32). CD8+ T cells are also important for controlling some aspects of chronic infection (8, 27, 34); however, it is not known whether they regulate the number of latently infected cells or some other aspect of latency. Furthermore, the molecular mechanisms responsible for CD8+ T cell actions during chronic infection have not been identified. The roles of IFN-γ and perforin, two major mediators of CD8+ T-cell action, have previously been evaluated by using infectious-center assays, with the conclusion that these proteins are not important for control of long-term latency (7, 24, 32). The role of perforin and IFN-γ in regulating persistent γHV68 replication has not been evaluated, but it is notable that murine cytomegalovirus (MCMV) persistently replicates in IFN-γ-depleted or IFN-γR−/− mice (16, 21).
In work presented here, we confirm that CD8+ T cells are important for controlling γHV68 latency and show for the first time that both IFN-γ and perforin play important roles in regulating long-term latent infection despite their lack of a role during acute infection. Importantly, we demonstrate that CD8+ T cells and perforin, but not IFN-γ, control the frequency of latently infected cells. In addition, we show for the first time that specific immune mechanisms (CD8+ T cells, IFN-γ, and perforin) also alter the reactivation phenotype of latently infected cells, as manifested by changes in the efficiency of ex vivo reactivation from latency. Furthermore, we demonstrate that CD8+ T cells and IFN-γ, but not perforin, control persistent replication. Surprisingly, the roles of perforin and IFN-γ in controlling chronic γHV68 infection are site specific, with IFN-γ predominating over perforin in peritoneal cells and perforin regulating splenic latency without any apparent involvement of IFN-γ.
MATERIALS AND METHODS
Virus and mouse infections.
γHV68 clone WUMS (ATCC VR1465) was passaged, and titers were determined by plaque assay on NIH 3T12 cells (39). Mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) and housed and bred at the Washington University School of Medicine in accordance with all federal and university guidelines. C57BL/6J (B6) or B6 mice deficient in the CD8 α chain (CD8−/−; Jackson no. 002665), in IFN-γ (IFN-γ−/−; Jackson no. 002287), or in perforin (Pfp−/−; Jackson no. 002407) were used. Mice were infected intraperitoneally (i.p.) between 8 and 12 weeks of age with 2 × 106 PFU of γHV68 in 0.5 ml of Dulbecco modified Eagle medium containing 10% fetal calf serum. Mice were sacrificed, and spleens and peritoneal exudate cells (PEC) from five mice per group were harvested and pooled as previously described (12, 41). As we have previously reported (42), prior to infection, peritoneal cells were 40 to 50% macrophages, 35 to 40% B cells, and a small number of T cells; after γHV68 infection, the number of peritoneal cells increased significantly but macrophages still predominated.
Ex vivo assays for latency and persistent replication by LD reactivation analysis.
Reactivation from latency was assayed as previously described (39) by plating limiting dilutions (LDs) of cells onto permissive mouse embryonic fibroblast monolayers and scoring a cytopathic effect as a result of emerging virus. Serial twofold dilutions of cells (24 wells/dilution starting at 105 cells/well for splenocytes and 4 × 104 cells/well for PEC) were plated onto an indicator monolayer of mouse embryonic fibroblasts in 96-well tissue culture plates. To detect the presence of infectious virus in cell samples, replicate cell aliquots were mechanically disrupted in 1/3× Dulbecco modified Eagle medium in the presence of 0.5-mm silica beads. This procedure kills >99% of the cells but has, at most, a twofold effect on the viral titer (41), thus allowing experimental distinction between reactivation from latency (which requires live cells) and persistent replication.
Determination of the frequency of latently infected cells by LD PCR analysis.
To determine the frequency of cells carrying the γHV68 genome, single-copy sensitivity nested PCR assays for γHV68 gene 50 were performed on serial dilutions of cells by using a previously published method with slight modifications (36, 41). Briefly, test cells were thawed, washed, resuspended in an isotonic solution, and counted. Starting at 104 cells/reaction, cells were serially diluted threefold in a background of uninfected NIH 3T12 cells such that a total of 104 cells were present in each well (10-μl total volume) and plated in a 96-well PCR plate at 12 wells/sample per experiment. Single copies of a plasmid containing γHV68 gene 50 in a background of 3T12 cells were included as positive controls to verify sensitivity. 3T12 samples with no plasmid were included as negative controls. After overnight lysis of cells with proteinase K, a round one PCR was performed in 20 μl/reaction. A nested PCR (30 cycles) was performed following addition of 10 μl of round two reaction buffer to the same well, and nested products were visualized on a 1.5% agarose gel. False positives were detected in 0.01% of all reaction mixtures. Positive control reaction mixtures containing 10 copies, 1 copy, or 0.1 copy of plasmid DNA were positive at 96, 35, and 6%, respectively.
Statistical analysis.
All data points represent the mean ± the standard error of the mean for all experiments (cells from five mice were pooled for each experiment). To quantify the number of cells from which the virus reactivated or that carry the latent viral genome, data were subjected to nonlinear regression (sigmoidal dose curve with a nonvariable slope) by using GraphPad Prism (GraphPad, San Diego, Calif.). Frequencies of reactivation events or genome-positive cells were determined (on the basis of the Poisson distribution) by calculating the cell density at which 63.2% of the wells scored positive for reactivation or the viral genome, respectively. To calculate significance, frequencies of reactivation events were statistically analyzed by paired t test over all 12 cell dilutions. For frequencies of genome-positive cells, samples were statistically analyzed by unpaired t test over the range of dilutions.
RESULTS
CD8+ T cells contribute to long-term control of γHV68 latency.
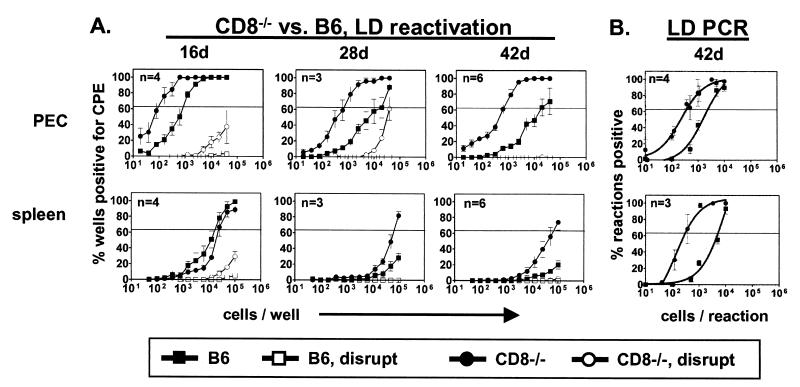
To evaluate the role of CD8+ T cells during chronic γHV68 infection, we compared the abilities of B6 and CD8−/− mice to control γHV68 latency (Fig. 1). PEC and spleens were harvested 16, 28, and 42 days after infection, and the frequency of cells reactivating from latency ex vivo was determined (Fig. 1A). This is a well-established assay (6, 36, 39, 42) that relies on observation of cytopathic effects of harvested cells on fibroblast monolayers over 21 days. Unless preformed infectious virus is present at the time of harvest, a cytopathic effect can only result from reactivation from latency. To control for preformed virus, replicate samples were mechanically disrupted and plated alongside nondisrupted samples. Mechanical disruption destroys the cells, and therefore any possibility of reactivation from latency, but does not inactivate preformed virus, allowing us to simultaneously quantify the frequency of cells reactivation from latency and detect persistently replicating virus. We can estimate the maximum number of lytically infected cells on the basis of the sensitivity of the assay (39, 41) and a very conservative estimate of 1 PFU OF γHV68 per lytically infected cell (see below). In the absence of a significant contribution from preformed virus, increased reactivation indicates either an increase in the frequency of cells that harbor the viral genome or a change in the latency phenotype of the infected cells as measured by reactivation efficiency.
FIG. 1.
CD8+ T cells control the frequency of cells that reactivate or carry the viral genome. Data points reflect the mean of all experiments ± the standard error (n = number of experiments; five mice per experiment). The horizontal line indicates 63%, which was used to calculate the frequency of cells reactivating virus or containing viral DNA by the Poisson distribution. (A) Ex vivo reactivation. The frequencies of cells reactivating in serial dilutions of PEC or splenocytes from B6 and CD8−/− mice at various days (d) postinfection with γHV68 are shown. To detect preformed infectious virus, duplicate samples were mechanically disrupted and plated (disrupt). On the y axis is the percentage of wells positive for a cytopathic effect (CPE; 24 wells per dilution). For PEC, the statistical significances of differences between CD8−/− and B6 mice were P = 0.004, 0.0001, and 0.0001 at 16, 28, and 42 days, respectively. For the spleen, the statistical significances of differences between CD8−/− and B6 mice were P = 0.06 and 0.03 at 28 and 42 days, respectively. At 28 days, the number of PEC reactivating from latency was 1 in 600 and the maximum number of persistently infected cells was 1 in 150,000 (see Fig. 2 results for calculations). (B) Frequency of cells containing viral DNA. PCR was used to detect the γHV68 genome in several dilutions of PEC or splenocytes harvested at 42 days postinfection. On the y axis is the percentage of reaction mixtures positive for viral DNA at each cell dilution (12 wells per dilution per experiment). For both PEC and the spleen, the statistical significance of the difference between CD8−/− and B6 mice was P < 0.0001.
At 42 days postinfection, the frequency of PEC reactivation from latency in CD8−/− mice was increased 30-fold compared to that in B6 mice (Fig. 1A). The frequency of reactivation was also increased in spleens of CD8−/− mice compared to that in spleens of B6 mice. As expected, both the PEC and spleens of B6 mice were clear of persistently replicating virus (<1 PFU/200,000 PEC, <1 PFU/500,000 splenocytes) by 16 days postinfection (41). In contrast, preformed virus was present in the PEC of CD8−/− mice as late as 28 days after infection, demonstrating that CD8+ T cells are required for efficient clearance of persistently replicating virus. Importantly, this small amount of persistently replicating virus (Fig. 1, legend) is not sufficient to impact our finding that CD8+ T cells control the frequency of cells reactivating from latency.
We next tested the hypothesis that CD8+ T cells control latency by regulating the frequency of cells carrying the viral genome. We previously demonstrated that the frequency of γHV68 genome-positive cells in normal mice is maintained at a constant level during the latent stage of γHV68 infection (41). At 42 days postinfection, the frequency of cells harboring the γHV68 genome was sixfold higher in CD8−/− mice than in B6 mice (Fig. 1B). Notably, this increase does not fully account for the increase in reactivation, demonstrating that CD8+ T cells control both the number of latently infected cells and their reactivation phenotype.
IFN-γ controls γHV68 latency and persistent replication.
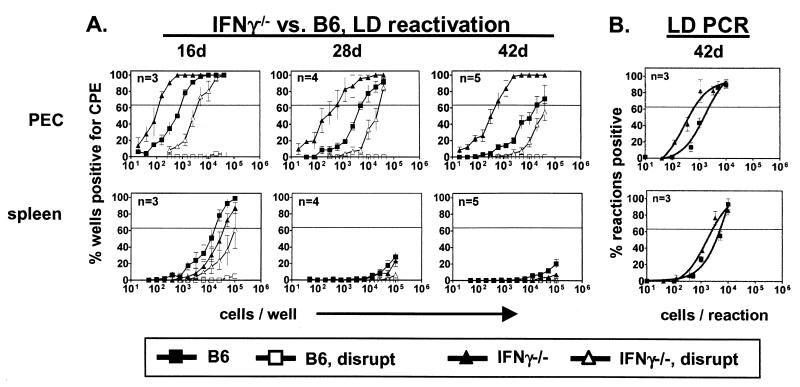
We next defined the role of IFN-γ in latency and persistent replication (Fig. 2A). At all time points, the frequency of PEC reactivation from latency was significantly increased in IFN-γ−/− mice (30-fold) compared to that in B6 mice. In striking contrast, reactivation from splenocytes was slightly decreased in IFN-γ−/− mice compared to that in B6 mice. Thus, IFN-γ is involved in regulating latent γHV68 infection in PEC but not in the spleen. Notably, persistently replicating virus was detected as late as 42 days postinfection in the PEC but not in the spleens of IFN-γ−/− mice, demonstrating that IFN-γ controls persistent replication in the PEC but an IFN-γ-independent mechanism controls persistent replication in the spleen.
FIG. 2.
IFN-γ controls γHV68 latency in PEC but not in the spleen. Experiments with IFN-γ−/− mice are identical to those described in the legend to Fig. 1. B6 data (from Fig. 1) are included for comparison. (A) Ex vivo reactivation. On the y axis is the percentage of wells positive for a cytopathic effect (CPE). For PEC, statistical significances of differences between IFN-γ−/− and B6 mice were P = 0.007, 0.0001, and 0.001 at 16, 28, and 42 days (d), respectively. (B) Frequency of cells containing viral DNA at 42 days postinfection. On the y axis is the percentage of reactions positive for viral DNA at each cell dilution. For both PEC and spleens, the statistical significance of the difference between IFN-γ−/− and B6 mice was P < 0.0001.
Interestingly, the frequency of cells carrying viral DNA was only modestly increased in the PEC (3.5-fold) and spleens (1.9-fold) of IFN-γ−/− mice compared to those of B6 controls (Fig. 2B). Notably, the presence of lytically infected cells that contain high levels of the γHV68 genome do not affect analysis of the frequency of viral genome-positive cells since the cells are lysed and subjected to PCR only after limiting dilution. Thus, cells containing many copies of the γHV68 genome will score the same as cells containing one copy. Persistent replication in IFN-γ−/− mice also cannot explain the observed increase in reactivation from latency. Assuming that every lytically infected cell produces only 1 PFU of γHV68 (a very conservative estimate) and utilizing the fact that the reactivation assay detects approximately 0.2 PFU (39, 41), there is, at most, 1 cell in 200,000 (0.2 PFU at 4 × 104 cells = 1 PFU/200,000 cells) productively infected 42 days after infection. Thus, the net change in the frequency of reactivating cells potentially due to productively infected cells is negligible (1 reactivating cell/660 cells compared to 1 PFU/200,000 cells). Importantly, the 30-fold increase in reactivation must therefore be due to a change in the reactivation phenotype. Thus, IFN-γ contributes to the control of latent γHV68 infection in PEC but not in the spleen, primarily controlling efficiency of reactivation, and is required for limiting persistent replication.
Perforin controls γHV68 latency but not persistent replication.
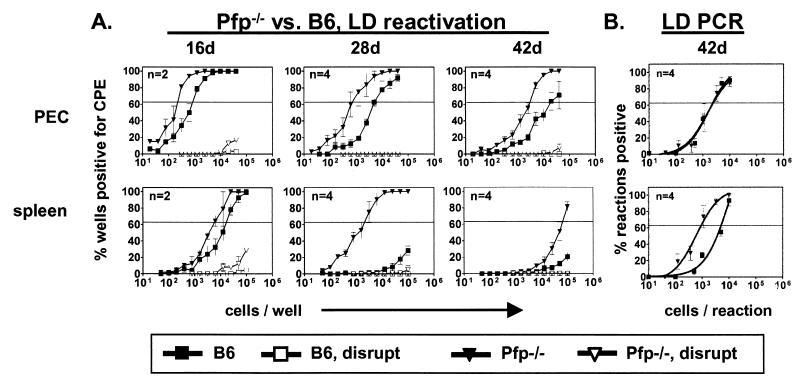
We next defined the role of perforin in the control of γHV68 latency (Fig. 3A). On day 42, the frequency of reactivation from latency was increased eightfold in PEC of Pfp−/− mice compared to those of B6 animals. The frequency of reactivation from splenocytes was also markedly increased in Pfp−/− mice on day 42, demonstrating a key role for perforin in regulating splenic latency. In contrast to IFN-γ, perforin was not required for control of persistent replication, since no preformed infectious virus could be detected at either site. Absence of perforin did not alter the frequency of cells carrying the viral genome in PEC but resulted in a fivefold increase in the frequency of viral genome-positive cells in the spleen compared to that in B6 mice (Fig. 3B). These data demonstrate that perforin regulates latency in the spleen and PEC via different mechanisms, controlling both the number of latently infected cells and the latency phenotype in the spleen while controlling only the reactivation phenotype in PEC.
FIG. 3.
Perforin controls γHV68 latency in the spleen. Experiments with Pfp−/− mice are identical to those described in the legend to Fig. 1. B6 data (from Fig. 1) are included for comparison. (A) Ex vivo reactivation. On the y axis is the percentage of wells positive for a cytopathic effect (CPE). For PEC, statistical significances of differences between Pfp−/− and B6 mice were P = 0.008, 0.0005, and 0.001 at 16, 28, and 42 days (d), respectively. For the spleen, statistical significances of differences between Pfp−/− and B6 mice were P = 0.005, 0.0001, and 0.06 at 16, 28, and 42 days, respectively. (B) Frequency of cells containing viral DNA at 42 days postinfection. On the y axis is the percentage of reaction mixtures positive for viral DNA at each cell dilution. For the spleen, the statistical significance of the difference between Pfp−/− and B6 mice was P < 0.0001.
Comparison of the roles of CD8+ T cells, perforin, and IFN-γ at different sites during chronic γHV68 infection.
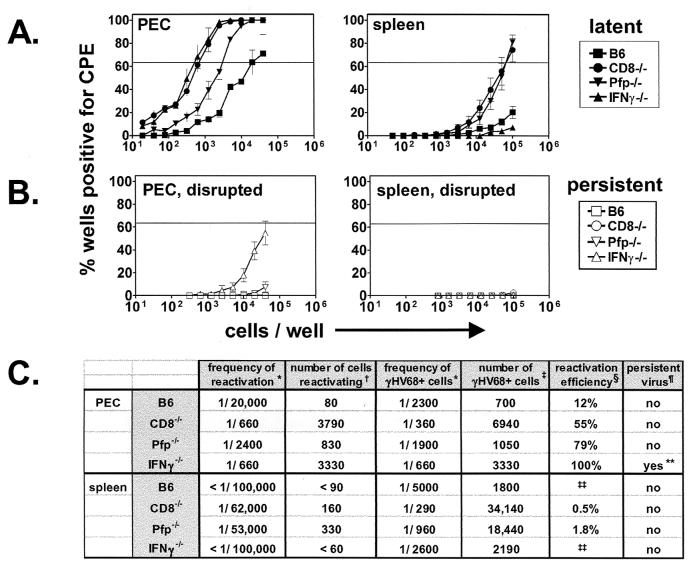
The results of reactivation assays at 42 days after infection of B6, IFN-γ−/−, CD8−/−, and Pfp−/− mice were compiled and are compared in Fig. 4A and B. CD8+ T cells and IFN-γ make important and approximately equivalent contributions to the control of γHV68 latency in PEC. In contrast, CD8+ T cells and perforin make equivalent contributions to the control of γHV68 latency in the spleen but IFN-γ deficiency has little impact. Frequencies of cells reactivating from latency and cells harboring γHV68 DNA are presented in Fig. 4C. We also calculated both the numbers of cells at each site that were viral genome positive and the percentage of those cells that reactivated from latency (reactivation efficiency). These calculations revealed a striking role for IFN-γ in the control of γHV68 reactivation. In IFN-γ−/− mice, 100% of latently infected cells reactivated from latency, demonstrating that IFN-γ alters the latency phenotype and therefore suppresses reactivation. CD8 and perforin also contribute to control of reactivation efficiency, but to a lesser degree than IFN-γ. Together, these data demonstrate that different mechanisms of immunity are required to control chronic γHV68 infection at distinct sites and that immune effectors are capable of controlling both the number of latently infected cells and the latency phenotype.
FIG. 4.
Summary of γHV68 infection in B6, CD8−/−, Pfp−/−, and IFN-γ−/− mice at 42 days postinfection. (A) Latent infection. LD reactivation data from nondisrupted samples from Fig. 1 to 3 are summarized. (B) Persistent infection. LD reactivation data from disrupted samples from Fig. 1 to 3 are summarized. (C) Frequencies of reactivation and frequencies of cells harboring γHV68 DNA. Symbols: ∗, frequencies calculated on the basis of the Poisson distribution of results from Fig. 1 to 3; †, number of cells reactivating = total number of cells recovered × frequency of cells reactivating; ‡, number of γHV68+ cells = total number of cells × frequency of cells γHV68+;. §, reactivation efficiency = (frequency of reactivation/frequency of cells γHV68+) × 100; ¶, presence of persistent virus as detected by LD reactivation of disrupted samples; ∗∗, quantity of persistent virus is 1 PFU/200,000 cells; ‡‡, reactivation efficiency could not be calculated because LD reactivation curve did not reach 63.2%. CPE, cytopathic effect.
DISCUSSION
Chronic gammaherpesvirus infection is distinct from acute infection and is characterized by two key components—latency (stable carriage of the viral genome in cells without production of infectious virus) and persistent replication (production of infectious virus during the chronic phase of infection). While it has long been thought that immunity controls chronic gammaherpesvirus infection, the mechanisms responsible for control have not been identified. In this report, we demonstrate that CD8+ T cells regulate both γHV68 latency and persistent replication. Furthermore, we demonstrate that the lymphocyte effector molecules IFN-γ and perforin regulate gammaherpesvirus latency and persistent replication and make the surprising observation that the actions of these proteins are specific to certain anatomic sites. Importantly, we directly demonstrate for the first time that the immune system controls the number of cells that carry the viral genome and that the immune system, IFN-γ in particular, controls the reactivation phenotype of latently infected cells.
Immune control of the number of latently infected cells.
The data presented here directly demonstrate that CD8+ T cells and perforin regulate the frequency of cells that carry the viral genome during latency. This is consistent with a role for these immune components in killing or limiting the proliferation of latently infected cells. These conclusions are supported by a previous demonstration that the number of cells latently infected by EBV is increased in immunosuppressed patients (1). Our work confirms splenic latency data from experiments using in vivo depletion of CD8+ T cells (27) and demonstrates the generality of these findings since PEC latency is also regulated by CD8+ T cells. Other groups have not observed significant alterations in γHV68 latency in the spleens of Pfp−/− mice following intranasal (i.n.) infection (32), but this may reflect the route of infection or the increased sensitivity of the LD reactivation assay compared to that of infectious-center assays (37, 39). It is also possible that the LD reactivation assay detects a form of latency different from that detected by infectious-center assays, since the LD reactivation assay measures reactivation over 21 days, compared to plaque formation reading at 5 to 6 days (7, 24).
Immune control of the reactivation phenotype.
Importantly, we demonstrate here that the immune system controls the reactivation phenotype of latently infected cells. In fact, the primary action of IFN-γ on PEC is on the efficiency of reactivation from latency although both CD8+ T cells and perforin also alter the reactivation phenotype. Mechanisms underlying alterations in ex vivo reactivation efficiency are unknown, but these findings challenge the commonly held view that herpesvirus latency is primarily defined by interactions between the virus and the host cell, rather than the immunological environment of the latently infected cell. One interesting and testable hypothesis is that immune factors directly alter the expression of viral or host genes in latently infected cells, resulting in alterations in reactivation efficiency. This could be via alterations in the activity of specific viral promoters (e.g., via IFN-γ response elements) or by inducing (or selecting cells harboring) global changes in viral genome methylation or transcriptional machinery (19). For example, if a viral gene essential for latency contained an IFN-γ-responsive promoter, this could prevent reactivation and thereby alter the latency phenotype of the infected cell.
Immune control of persistent replication.
In addition to their roles in controlling latency, we also demonstrate that CD8+ T cells and IFN-γ control persistent replication. CD8−/− mice contained preformed infectious virus up to 28 days after infection, and IFN-γ−/− mice contained preformed virus as late as 42 days after infection—well beyond the time that lytic infection is detectable in immunocompetent mice (4, 28, 39). It is noteworthy that we and other groups have demonstrated that IFN-γ plays a minimal role during acute infection (24, 40), although these experiments have not been verified by depletion of IFN-γ from wild-type mice, as has been done for CD8+ T cells (8, 27). One explanation for our findings that preformed infectious virus is present in IFN-γ−/− mice after clearance of acute infection is that IFN-γ may be essential for control of persistent replication but not that of acute replication. This is possible if persistent replication and acute replication are distinct process. We have recently demonstrated that persistent replication is genetically distinct from acute infection. Two γHV68 mutant viruses lacking either a functional M11 gene (encoding a Bcl-2 homolog) or gene 72 (encoding a cyclin D homolog) replicate normally during acute infection but, in contrast to wild-type γHV68, produce little or no detectable persistent virus in IFN-γ−/− mice (10). Data presented here show that IFN-γ is key to control of the persistent form of infection, despite the absence of an important role for IFN-γ during acute infection (7, 24, 40). These data, together with the demonstration that CD8+ T cells and IFN-γ control the reactivation efficiency of infected cells, are consistent with the view that reactivation from latency and persistent replication are interrelated (10). Our working hypothesis is that these findings are explained by persistent virus originating via reactivation from latency. Notably, other work has implicated IFN-γ as a key factor in controlling persistent MCMV infection of the salivary glands, spleen, and PEC (16, 21) and lymphocytic choriomeningitis virus infection of the central nervous system (31, 38).
Several lines of evidence demonstrate that our results are not related to the route of infection or the dose used. The virus dose makes very little difference to the level of latency, regardless of the route of infection. We have noted that i.p. infection of B6 mice with 0.1 PFU of γHV68 is as effective at establishing latency in splenocytes and PEC as is infection with 106 PFU (data not shown). Similar results were obtained by i.n. infection with 40 to 4 × 105 PFU (data not shown). Furthermore, the activation of the immune system to latent antigens is similar. Infection with 400 PFU of γHV68 i.n. or 106 PFU i.p. primes an anti-M2 CD8+ T-cell response of a similar magnitude (34). Finally, the fundamental conclusions regarding the ability of specific immune components to control aspects of latent infection and persistent replication are similar, regardless of the route or dose used. To verify the role of IFN-γ in the control of γHV68 latency, we infected 129 and 129/IFN-γR−/− mice either i.p. with 106 PFU or i.n. with 100 PFU. Infection by either regimen resulted in loss of control of reactivation in PEC and high levels of persistent replication (data not shown), confirming an essential role for IFN-γ in control of latency. We have also previously noted that B cells are important for controlling latent infection in peritoneal cells, regardless of whether the virus is inoculated i.p. or i.n. (41). Thus, our fundamental conclusions regarding the roles of CD8+ T cells, IFN-γ, and perforin in the control of γHV68 latency and persistent replication are not related to the route of infection or the dose used.
Source of perforin and IFN-γ.
While it is likely that during chronic γHV68 infection, both perforin and IFN-γ are provided by CD8+ T cells, our results do not rule out the possibility that CD4+ T cells or NK cells also control latency via these molecules. Notably, several reports suggest that CD4+ T cells are important during both the acute and chronic stages of γHV68 infection (4, 5,8, 33, 40) and that the CD4+ response is mediated via IFN-γ (5). CD8+- and CD4+-derived IFN-γ is also involved in the control of persistent MCMV infection (20). Consistent with these observations, we demonstrated higher levels and longer durations of persistent virus in IFN-γ−/− mice than in CD8−/− mice, arguing that γHV68 persistent replication is also regulated by IFN-γ derived from CD4+ T cells or NK cells.
Site-specific immune control of latency.
Surprisingly, our data demonstrate that immune control of gammaherpesvirus latency is site specific. Perforin was critical for regulation of latency in the spleen but had a lesser role in PEC. In contrast, in IFN-γ−/− mice, we observed a loss of control of latent infection in PEC but not in the spleen. The lack of a role for IFN-γ in the spleen is consistent with other studies showing that quantities of latent virus in the spleens of IFN-γ−/− and IFN-γR−/− mice are not different from those in the spleens of B6 mice (with the exception of one report of a difference at 17 days after infection) (7, 24). Other groups have not demonstrated a role for IFN-γ in controlling latency.
Possible explanations for the site specificity of IFN-γ and perforin are intriguing. Since the primary cell type carrying latent virus in PEC is the macrophage (42), while in the spleen, B cells and dendritic cells are the primary carriers (9, 29), it is possible that site-specific regulation of latency is due to selective actions of IFN-γ and perforin on macrophages versus B cells or dendritic cells. Alternatively, it is possible that this is the result of different latent genes being expressed in macrophages versus B cells or the presence of specific subsets of immune mediators at different sites.
Other groups have reported site-specific or cell-type-specific control of infection for other pathogens. Induction of cytokines results in clearance of persistent lymphocytic choriomeningitis virus infection from hepatocytes but not from nonparenchymal cells or splenocytes (11). IFN-γ controls MCMV infection in bone marrow macrophages but not as well in embryonic fibroblasts (22). It has been reported that NK cell control of acute MCMV infection is mediated by perforin in the spleen and by IFN-γ in the liver (30), although we have not been able to reproduce these results (A. O'Guinn and H. W. Virgin IV, unpublished data). In addition, perforin is important for controlling Listeria monocytogenes infection in the spleen but not in the liver (14). It should also be noted that, in addition to a direct contribution of perforin and IFN-γ to control of chronic γHV68 infection, it is also conceivable that absence of either perforin or IFN-γ results in an alteration of CD8+ T-cell homeostasis (3).
Acknowledgments
H.W.V. was supported by NIH grants AI39616, CA74730, and HL60090, and S.H.S. was supported by NIH grants CA43143, CA52004, CA58524, and CA74730. S.A.T. was supported by NIH grant 5 T32 CA09547-14 and is a Leukemia and Lymphoma Society Fellow (5609-01). L.V.D. was supported by NIH grant 5 T32 AI07163.
We thank members of the Speck and Virgin laboratories, as well as members of the laboratories of D. Leib, P. MacDonald, L. Morrison, and P. Olivo for helpful discussions. We thank Darren Kreamalmeyer for expert assistance with mouse strains and Gil Akos for technical assistance.
REFERENCES
- 1.Babcock, G. J., L. L. Decker, R. B. Freeman, and D. A. Thorley-Lawson. 1999. Epstein-Barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J. Exp. Med. 190:567-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babcock, G. J., D. Hochberg, and D. A. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. [DOI] [PubMed] [Google Scholar]
- 3.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 290:1354-1358. [DOI] [PubMed] [Google Scholar]
- 4.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, J. P., R. D. Cardin, K. C. Branum, and P. C. Doherty. 1999. CD4+ T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc. Natl. Acad. Sci. 96:5135-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clambey, E. T., H. W. Virgin IV, and S. H. Speck. 2000. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J. Virol. 74:1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutia, B. M., C. J. Clarke, D. J. Allen, and A. A. Nash. 1997. Pathological changes in the spleens of gamma interferon receptor-deficient mice infected with murine gammaherpesvirus: a role for CD8 T cells. J. Virol. 71:4278-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehtisham, S., N. P. Sunil-Chandra, and A. A. Nash. 1993. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J. Virol. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 10.Gangappa, S., L. F. Van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 12.Heise, M. T., and H. W. Virgin IV. 1995. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J. Virol. 69:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacoby, M. A., H. W. Virgin IV, and S. H. Speck. 2002. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J. Virol. 76:1790-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagi, D., B. Ledermann, K. Burki, H. Hengartner, and R. M. Zinkernagel. 1994. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur. J. Immunol. 24:3068-3072. [DOI] [PubMed] [Google Scholar]
- 15.Liu, L., E. Flano, E. J. Usherwood, S. Surman, M. A. Blackman, and D. L. Woodland. 1999. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J. Immunol. 163:868-874. [PubMed] [Google Scholar]
- 16.Lučin, P., I. Pavić, B. Polić, S. Jonjić, and U. H. Koszinowski. 1992. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 66:1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monini, P., L. De Lellis, M. Fabris, F. Rigolin, and E. Cassai. 1996. Kaposi's sarcoma-associated herpesvirus DNA sequences in prostate tissue and human semen. N. Engl. J. Med. 334:1168-1172. [DOI] [PubMed] [Google Scholar]
- 18.Pauk, J., M. L. Huang, S. J. Brodie, A. Wald, D. M. Koelle, T. Schacker, C. Celum, S. Selke, and L. Corey. 2000. Mucosal shedding of human herpesvirus 8 in men. N. Engl. J. Med. 343:1369-1377. [DOI] [PubMed] [Google Scholar]
- 19.Paulson, E. J., and S. H. Speck. 1999. Differential methylation of Epstein-Barr virus latency promoters facilitates viral persistence in healthy seropositive individuals. J. Virol. 73:9959-9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Lucin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Presti, R. M., J. L. Pollock, A. J. Dal Canto, A. K. O'Guin, and H. W. Virgin. 1998. Interferon-gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J. Exp. Med. 188:577-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Presti, R. M., D. L. Popkin, M. Connick, S. Paetzold, and H. W. Virgin. 2002. Novel cell type specific anti-viral mechanism of IFN-gamma action in macrophages. J. Exp. Med. 193:483-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology. Lippincott-Raven, Philadelphia, Pa.
- 24.Sarawar, S. R., R. D. Cardin, J. W. Brooks, M. Mehrpooya, A.-M. Hamilton-Easton, X. Y. Mo, and P. C. Doherty. 1997. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J. Virol. 71:3916-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1998. Virus-specific CD8+ T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc. Natl. Acad. Sci. USA 95:15565-15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1999. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur. J. Immunol. 29:1059-1067. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson, P. G., R. D. Cardin, J. P. Christensen, and P. C. Doherty. 1999. Immunological control of a murine gammaherpesvirus independent of CD8+ T cells. J. Gen. Virol. 80(Pt. 2):477-483. [DOI] [PubMed] [Google Scholar]
- 28.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gammaherpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 29.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 30.Tay, C. H., and R. M. Welsh. 1997. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 71:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tishon, A., H. Lewicki, G. Rall, M. Von Herath, and M. B. A. Oldstone. 1995. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology 212:244-250. [DOI] [PubMed] [Google Scholar]
- 32.Usherwood, E. J., J. W. Brooks, S. R. Sarawar, R. D. Cardin, W. D. Young, D. J. Allen, P. C. Doherty, and A. A. Nash. 1997. Immunological control of murine gammaherpesvirus infection is independent of perforin. J. Gen. Virol. 78:2025-2030. [DOI] [PubMed] [Google Scholar]
- 33.Usherwood, E. J., A. J. Ross, D. J. Allen, and A. A. Nash. 1996. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J. Gen. Virol. 77:627-630. [DOI] [PubMed] [Google Scholar]
- 34.Usherwood, E. J., D. J. Roy, K. Ward, S. L. Surman, B. M. Dutia, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2000. Control of gammaherpesvirus latency by latent antigen-specific CD8+ T cells. J. Exp. Med. 192:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usherwood, E. J., K. A. Ward, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2001. Latent antigen vaccination in a model gammaherpesvirus infection. J. Virol. 75:8283-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virgin, H. W., and S. H. Speck. 1999. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr. Opin. Immunol. 11:371-379. [DOI] [PubMed] [Google Scholar]
- 38.von Herrath, M. G., B. Coon, and M. B. Oldstone. 1997. Low-affinity cytotoxic T-lymphocytes require IFN-gamma to clear an acute viral infection. Virology 229:349-359. [DOI] [PubMed] [Google Scholar]
- 39.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. W. Virgin IV. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weck, K. E., A. J. Dal Canto, J. D. Gould, A. K. O'Guin, K. A. Roth, J. E. Saffitz, S. H. Speck, and H. W. Virgin. 1997. Murine gammaherpesvirus 68 causes large vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus induced vascular disease. Nat. Med. 3:1346-1353. [DOI] [PubMed] [Google Scholar]
- 41.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitby, D., N. A. Smith, S. Matthews, S. O'Shea, C. A. Sabin, R. Kulasegaram, C. Boshoff, R. A. Weiss, A. de Ruiter, and J. M. Best. 1999. Human herpesvirus 8: seroepidemiology among women and detection in the genital tract of seropositive women. J. Infect. Dis. 179:234-236. [DOI] [PubMed] [Google Scholar]