Abstract
Human T-cell leukemia virus type 1 (HTLV-1) causes adult T-cell leukemia (ATL) in infected individuals after a long incubation period. Despite the apparent transforming ability of HTLV-1 under experimental conditions, most HTLV-1 carriers are asymptomatic. These facts suggest that HTLV-1 is controlled by host immunity in most carriers. To understand the interplay between host immunity and HTLV-1-infected cells, in this study, we isolated several HTLV-1 Tax-specific cytotoxic T-lymphocyte (CTL) lines from rats inoculated with Tax-coding DNA and investigated the long-term effects of the CTL on syngeneic HTLV-1-infected T cells. Our results demonstrated that long-term mixed culture of these CTL and the virus-infected T cells led to the emergence of CTL-resistant HTLV-1-infected cells. Although the Tax expression level in these resistant cells was equivalent to that in the parental cells, expression of surface major histocompatibility complex class I (MHC-I) was significantly downregulated in the resistant cells. Downregulation of MHC-I was more apparent in RT1.Al, which presents a Tax epitope recognized by the CTL established in this study. Moreover, peptide pulsing resulted in killing of the resistant cells by CTL, indicating that resistance was caused by a decreased epitope density on the infected cell surface. This may be one of the mechanisms for persistence of HTLV-1-infected cells that evade CTL lysis and potentially develop ATL.
Human T-cell leukemia virus type 1 (HTLV-1) is etiologically linked to adult T-cell leukemia (ATL) (15, 42), a chronic progressive neurological disorder termed HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (11, 38), and various other human diseases (12, 28, 32, 35). Since examination of the viral nucleotide sequences among different disease groups has not revealed any specific determinants that distinguish a particular HTLV-1-associated disease, it is speculated that a primary determinant of HTLV-1-associated disease is host related (5, 25, 54).
ATL is a malignant lymphoproliferative disease affecting a subgroup of middle-aged HTLV-1 carriers characterized by the presence of a mature T-cell phenotype (51). HTLV-1 has been shown to activate and immortalize human T cells in vitro, resulting in polyclonal proliferation of infected cells and subsequent oligoclonal or monoclonal growth (10, 53). The HTLV-1 genome contains a unique 3′ region, designated pX, that encodes the viral transactivator protein Tax (45). Tax transactivates not only the viral long terminal repeat (7, 46, 48) but also the promoters of cellular genes such as interleukin-2 (IL-2) (47), IL-2 receptor (17), myc (6), and fos (8). Thus, it is speculated that Tax plays a central role in HTLV-1-associated immortalization and transformation of T cells, which may lead to the development of ATL.
Despite the apparent transforming ability of Tax in HTLV-1 infection under experimental conditions, most HTLV-1 carriers are asymptomatic. One explanation for this is that HTLV-1 is controlled by host immunity in most carriers, as is the case with many other viruses. In this regard, Tax is known as a major target protein recognized by cytotoxic T lymphocytes (CTL) of HTLV-1 carriers (18). It has been reported that the levels of HTLV-1-specific CTL are quite diverse among HTLV-1 carriers and that ATL patients have impaired levels of HTLV-1-specific CTL, in contrast to the high CTL response levels of HTLV-1 carriers with HAM/TSP (21, 22, 40). Since HTLV-1 Tax-specific CTL can recognize and lyse ATL cells in vitro (20), it is reasonable to assume that the low CTL activity in ATL patients is disadvantageous as it may allow uncontrolled proliferation and evolution of HTLV-1-infected cells in vivo.
On the other hand, it is also known that Tax expression is rarely detected in fresh peripheral ATL cells and that the level of Tax mRNA expression in ATL is much lower than that in those with HAM/TSP or in asymptomatic carriers (20, 24). This observation raised the possibility that HTLV-1-infected cells that do not require Tax expression are selected in the course of ATL development and that the appearance of these cells may lead to the reduction of HTLV-1-specific CTL activities. Thus, to understand the mechanism of ATL development, it is very important to dissect the interplay between HTLV-1-infected T cells and virus-specific CTL.
We have previously established a rat model of ATL-like disease that allows examination of the growth and spread of HTLV-1-infected cells, as well assessment of the effects of immune T cells on the development of the disease (14, 36). By using this model system, we recently reported the therapeutic effect of Tax-coding DNA or peptide against the disease (13, 37). In this study, we isolated several Tax-specific CTL lines from rats inoculated with Tax-coding DNA and examined the long-term effects of the CTL on syngeneic HTLV-1-infected T cells. Our results demonstrated that long-term mixed culture of these CTL and infected T cells leads to the emergence of CTL-resistant infected cells. Although the Tax expression level in these resistant cells was equivalent to that in the parental cells, they expressed a lower level of surface major histocompatibility complex class I (MHC-I). Downregulation of MHC-I was more apparent when we examined the expression of surface RT1.Al, which presents a Tax epitope recognized by the CTL used in the mixed culture. These results suggested the involvement of MHC-I downregulation in the mechanism for evading immune response in the course of ATL development.
MATERIALS AND METHODS
Animals.
Female F344/N Jcl-rnu/rnu (nu/nu) and F344/N Jcl-rnu/+ (nu/+) rats were purchased from Clea Japan, Inc. (Tokyo, Japan). All rats were maintained at the experimental animal facilities of the Tokyo Medical and Dental University. The experimental protocol was approved by the Animal Ethics Review Committee of our university.
Cell lines.
An HTLV-1-immortalized cell line, FPM1, was established in our laboratory by cocultivating thymocytes of a nu/+ rat with HTLV-1-producing human cell line MT-2, which was treated with mitomycin C (50 μg/ml) for 30 min at 37°C (27). The cells were maintained in RPMI 1640 medium with 10% heat-inactivated fetal calf serum (FCS; Whittaker, Walkersville, Md.), penicillin, and streptomycin. Ten units of IL-2 (Shionogi, Osaka, Japan) per milliliter was added at the beginning of mixed culture. Cells were eventually freed from exogenous IL-2. FPM1-V1AX is a subclone of FPM1 cells that possesses in vivo growth ability in nu/nu rats. To establish FPM1V.EFGFP cells, we made an EGFP expression vector by inserting the human elongation factor promoter between the HindIII and SalI sites of pEGFP-1 (Clontech, Palo Alto, Calif.) and designated it pEFGFP. FPM1-V1AX cells were electrically transfected with pEFGFP by using Gene Pulser II systems (Bio-Rad, Hercules, Calif.), and stable transfectants were selected with 400 μg of Geneticin (Gibco-BRL, Rockville, Md.) per ml. Enhanced green fluorescent protein (EGFP) expression in the resulting FPM1V.EFGFP cells was confirmed by flow cytometric analysis. Another HTLV-1-immortalized rat T-cell line derived from a WKA rat, TARS-1 (50), was kindly provided by Takashi Yoshiki (Hokkaido University School of Medicine, Sapporo, Japan). TARS-1.EFGFP cells were established from TARS-1 cells transfected with pEFGFP.
An IL-2-dependent, HTLV-1-negative rat cell line, G14, was also established in our laboratory from a nu/+ rat (37). Briefly, nylon wool column-purified splenic T cells of an HTLV-1-infected nu/+ rat were stimulated in vitro with formalin-fixed FPM1 cells twice in a month in the presence of IL-2. These T cells maintained FPM1-specific cytotoxic activities for the first 3 months of mixed culture and then started to lose the specific activities. After 4 months, these cells became capable of continuously growing in a medium containing 10 U of IL-2 per ml in the absence of FPM1 for stimulation. We designated these cells the G14 cell line. This cell line was positive for rat CD5, CD8, CD25, MHC-I, and MHC-II (Kato et al., unpublished data). To establish G14-Tax cells, G14 cells were electrically transfected with Tax-expressing plasmids by using Gene Pulser II systems (Bio-Rad) and selecting stable transfectants with 400 μg of Geneticin (Gibco-BRL) per ml. Tax expression in the resulting G14-Tax cells was confirmed by Western blotting with human serum containing Tax antibody. G14-Tax cells were maintained in RPMI 1640 medium with 10% FCS and 10 U of IL-2 per ml.
Induction of Tax-specific CTL lines.
Wild-type Tax cloned into the pHbPr.1-neo expression vector (pbMT-2Tax) was kindly provided by Kayoko Matsumoto (33). Fifty milligrams of Au particles (radius, 1.6 μm; Bio-Rad) were coated with 100 μg of the expression vectors. The DNA-coated Au particles were introduced into Tefzel tubing (Bio-Rad), placed on a Tubing Prep Station, and dried by rotation of the tubing under nitrogen flow (0.3 to 0.4 ml/min) for 15 min. The tubing was then cut into 12-mm-long cartridges, which were used in the Helios Gene Gun (Bio-Rad). nu/+ rats were anesthetized with ketamine, and their fur was completely removed by a commercial depilatory agent. DNA-coated Au particles in a cartridge were accelerated by pressurized helium gas for penetration through cell membranes and multiple layers of cells in the epidermis. The concentrations of DNA and Au were 1 μg/shot and 0.5 mg/shot, respectively. Immunization was performed twice with a 1-week interval and 10 shots per immunization.
For induction of Tax-specific CTL in long-term cultivation, splenic T cells (2.5 × 106/well) from immunized rats were cocultured with the same number of formalin-fixed FPM1-V1AX cells in 24-well flat-bottom culture plates in RPMI 1640 medium with 10% FCS and 20 U of IL-2 per ml and stimulated with formalin-fixed FPM1-V1AX cells every 2 weeks. T cells that maintained HTLV-1-specific CTL activities for more than 3 months were judged to be the CTL lines and were used in the experiments.
Analysis of cell surface markers.
Expression of cell surface markers was examined by flow cytometry. Briefly, 106 cells were stained with various mouse monoclonal antibodies for 30 min on ice, washed three times with 1% FCS in phosphate-buffered saline, and then stained with phycoerythrin (PE)-conjugated goat anti-mouse immunoglobulin G (IgG; heavy and light chains; Biomeda Co., Foster City, Calif.). After being washed, the cells were fixed with 1% formalin in phosphate-buffered saline prior to analysis on a FACScalibur (Becton Dickinson, San Jose, Calif.). For some experiments, fluorescein isothiocyanate-conjugated anti-rat CD4 (clone OX-38; BD PharMingen Co., San Diego, Calif.) and PE-conjugated anti-rat CD8 (clone OX-8; BD PharMingen Co.) were used. Anti-rat MHC-I (RT1.A) and MHC-II (RT1.B) were purchased from Cedarlane Laboratories (Hornby, Ontario, Canada), an anti-rat CD25 antibody was from Chemicon International Inc. (Temecula, Calif.), and an antibody against rat RT1.Al was from BD PharMingen Co. Antibodies against rat CD80 and CD86 were generously provided by Ko Okumura and Hideo Yagita (Juntendo University, Tokyo, Japan) (30).
Protein analysis.
Cells were resuspended in ice-cold extraction buffer (20 mmol of HEPES [pH 7.9] per liter, 10 mmol of KCl per liter, 1 mmol of MgCl2 per liter, 150 mmol of NaCl per liter, 1% Triton X-100, 0.5 mmol of dithiothreitol per liter, 0.5 mmol of phenylmethylsulfonyl fluoride per liter, 1 μg of aprotinin per ml, and 1 μg of leupeptin per ml) and gently rocked for 30 min. After centrifugation at 14,000 × g for 20 min at 4°C, the supernatant was collected as a whole-cell extract. The protein concentration of each sample was determined with a protein assay kit (Bio-Rad). Fifty micrograms of whole-cell extract was separated by sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis and transferred to a nitrocellulose filter. After incubation with blocking buffer (2% bovine serum albumin in 10 mmol of Tris-HCl [pH 7.5] per liter and 100 mmol of NaCl per liter), the filter was incubated with 1:1,000-diluted sera containing anti-Tax antibody and then with an anti-human Ig antibody conjugated to horseradish peroxidase (Amersham, Arlington Heights, Ill.). Antibodies bound to the filter were detected by the enhanced-chemiluminescence method (Amersham).
RT-PCR.
Total RNA was extracted from cells with Isogen (Nippon Gene Co., Toyama, Japan) and then treated with DNase I (Gibco-BRL). One microgram of total RNA was subjected to reverse transcription (RT)-PCR with an RT-PCR High kit (Toyobo Co., Osaka, Japan). To amplify Tax mRNA, we used primers RPX3 (5′-ATCCCGTGGAGACTCCTCAA-3′) and RPX4 (5′-AACACGTAGACTGGGTATCC-3′) as described previously (24). As an internal control, rat glyceraldehyde-3-phosphate dehydrogenase (G3PDH) mRNA was also examined by using primers 5′-CATTGACCTCAACTACATGG-3′ and 5′-AGTGATGGCATGGACTGTGG-3′, which amplify 435-bp cDNA fragments of G3PDH mRNA spliced over five intron regions. For a two-step RT-PCR, RT at 42°C for 20 min was terminated by heating at 99°C for 5 min; the PCR mixture was then cooled at 4°C for 5 min and subjected to 35 cycles of a three-temperature PCR (94°C for 30 s, 60°C for 30 s, and 72°C for 30 s). A thermal cycler (Touch Down; Hybaid, Middlesex, United Kingdom) was used for all PCR amplification.
51Cr release cytotoxicity assay.
CTL activity against Tax-expressing or HTLV-1-infected cells was measured by a 6-h 51Cr release assay at various effector-to-target (E/T) cell ratios. T-cell lines derived from DNA-immunized rats were used as effector cells. 51Cr-labeled FPM1-V1AX, TARS-1, G14, or G14-Tax cells were used as target cells. The 51Cr-labeled target cells (104/well) were cocultured with various numbers of effector cells in 96-well round-bottom culture plates at 37°C for 6 h, and then the 51Cr activity released into the supernatants was measured. Specific cytotoxicity was calculated as follows: specific cytotoxicity = ([experimental 51Cr release − spontaneous 51Cr release]/[maximum 51Cr release − spontaneous 51Cr release]) × 100%.
Flow cytometric CTL killing assay.
EGFP-expressing target cells (5 × 104 cells/well) were cocultured with 5 × 105 or 1 × 106 CTL. These mixed cultures were immediately subjected to flow-cytometric analysis or incubated for 48 h and then subjected to flow-cytometric analysis. Cytofluorimetry was done on a FACScalibur and analyzed with Cell Quest software. Target cells were clearly gated away from CTL by light scatter properties and EGFP expression. In some experiments, cells were treated with anti-rat CD8 antibody (clone R1-10B5; Seikagaku Co., Tokyo, Japan), followed by staining with PE-conjugated anti-mouse IgG (heavy and light chains; Biomeda Co.) and examination of the CD8 expression of cells exhibiting high forward scatter and a low EGFP profile.
Peptide pulsing.
Target cells were incubated with [3H]thymidine ([3H]TdR) at 3.7 MBq/106 cells for 12 h at 37°C and then washed three times. These target cells were placed into a round-bottom 96-well plate at a concentration of 5 × 103/well in RPMI 1640 medium with 10% FCS and 10 μM oligopeptide corresponding to Tax 180-188 (GAFLTNVPY) or 11-19 (LLFGYPVYV). Following 1 h of incubation at 37°C, 5 × 104 CTL were added per well for a cytotoxicity assay. Oligopeptides were carried over in the cytotoxicity assay medium. After an additional 6-h incubation at 37°C, cells were harvested with a Micro 96 Harvester (Skatron, Lier, Norway) and the [3H]TdR remaining in the target cells was measured in a microplate beta counter (Micro Beta Plus; Wallac, Turku, Finland). Specific cytotoxicity (percent lysis) was calculated as follows: percent lysis = [(counts per minute without effector − counts per minute with effector)/counts per minute without effector] × 100. Oligopeptides were purchased from Hokudo Co. (Hokkaido, Japan).
Epitope sequences.
An HTLV-I Tax epitope presented by rat RT1.Al, Tax 180-188, was examined for variability in FPM1V.EFGFP and FPM1V.EFGFP/8R cells. Total RNA was extracted from these cell lines with Isogen (Nippon Gene Co.) and then treated with DNase I (Gibco-BRL). One microgram of total RNA was subjected to RT-PCR with an RT-PCR High kit (Toyobo Co.). The primers used for the RT reaction and the first PCR were RPX3 and PX7 (5′-GGCCCGGAAATCATAGGCGTGC-3′). Three microliters of the first PCR product was then subjected to a nested PCR with primers PX5 (5′-TATGTTCGGCCCGCCTACATCGTCACGCCC-3′) and PX9 (5′-CTGGAGTGGTGAGGGTTGAGTGG-3′). The RT reaction and PCR conditions were the same as those described above. The product was cloned into vector pCR2.1 by using a TA Cloning Kit (Invitrogen Co., Carlsbad, Calif.) and subjected to sequence analysis. Thirteen clones from FPM1V.EFGFP and 22 from FPM1V.EFGFP/8R were sequenced by using the BigDye terminator and an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, Calif.) in accordance with the manufacturer's instructions.
RESULTS
Isolation of Tax-specific CTL lines from rats inoculated with Tax-coding DNA.
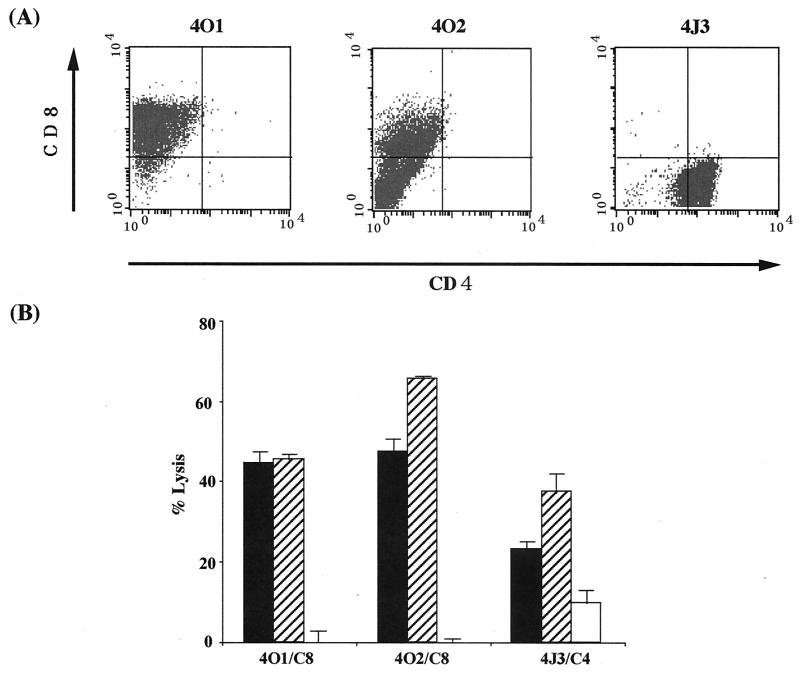
Our previous examination revealed that inoculation of Tax-coding DNA induced specific CTL activity against Tax in nu/+ rats (37). In this study, we attempted to establish Tax-specific CTL lines from rats inoculated with Tax-coding DNA. Spleen T cells were isolated from the DNA-inoculated rats and were stimulated in vitro with an HTLV-1-infected cell line, FPM1-V1AX, every 2 weeks in a 24-well plate. After 3 months of stimulation, we obtained three cell lines from independent wells and examined the expression of CD4 and CD8 by flow cytometry. As shown in Fig. 1A, two of them were positive for CD8 whereas the other one expressed CD4. We designated these CD4+ or CD8+ lines 4J3/C4 or 4O1/C8 and 4O2/C8, respectively, and examined their CTL activity by 51Cr release assay. As shown in Fig. 1B, all three of the lines were able to kill syngeneic HTLV-1-infected cells. These CTL activities were specific to Tax, since all of the lines specifically recognized and killed Tax-expressing G14-Tax cells but not parental G14 cells. These results indicated that Tax-coding DNA was able to induce Tax-specific CTL that express either CD4 or CD8 on their surface.
FIG. 1.
Tax-coding DNA induced CD4+ or CD8+ CTL lines specific to Tax. Splenic T cells were isolated from nu/+ rats inoculated with a Tax expression vector and periodically stimulated with formalin-fixed FPM1-V1AX cells. After 3 months of stimulation, three CTL lines (4O1, 4O2, and 4J3) were established. (A) These cells were labeled with fluorescein isothiocyanate-conjugated anti-rat CD4 and PE-conjugated anti-rat CD8 for 30 min. After being washed, these cells were subjected to flow-cytometric analysis. (B) FPM1-V1AX (▪), G14-Tax (▨), or G14 (□) cells were labeled with 51Cr for 1 h and used as target cells. CTL lines were used as effectors at an E/T cell ratio of 10. Results are expressed as mean percent lysis ± the standard deviation of triplicate wells. Similar results were obtained in four independent experiments.
Emergence of CTL-resistant HTLV-1-infected T cells after mixed culture with 4O1/C8 cells.
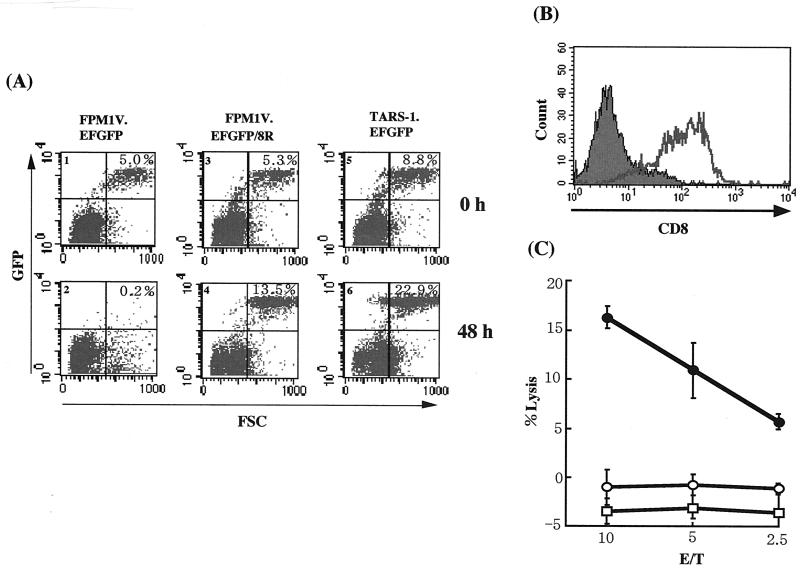
To investigate the interplay between Tax-specific CTL and HTLV-1-infected T cells, we attempted to establish a culture system in which we can distinguish the CTL from infected cells in a mixed culture. At first, we stably introduced an EGFP expression vector into FPM1-V1AX cells and established the FPM1V.EFGFP cell line, which expresses a high level of EGFP. We next examined whether we could distinguish FPM1V.EFGFP cells from 4O1/C8 CTL in mixed culture by flow-cytometric analysis. On the basis of their forward light-scattering profile and EGFP expression, FPM1V.EFGFP cells were accumulated in the upper right column whereas 4O1/C8 cells were detected in the lower left column (Fig. 2A, part 1). After 48 h of mixed culture, we observed a dramatic reduction in the number of FPM1V.EFGFP cells (Fig. 2A, part 2). These results indicated that EGFP-expressing HTLV-1-infected cells were distinguished from 4O1/C8 CTL and that we could evaluate the susceptibility of the virus-infected cells to CTL killing by flow-cytometric analysis. CTL killing by 4O1/C8 was restricted to the MHC, since allogeneic HTLV-1-infected TARS-1.EFGFP T cells were not killed by the CTL (Fig. 2A, parts 5 and 6).
FIG. 2.
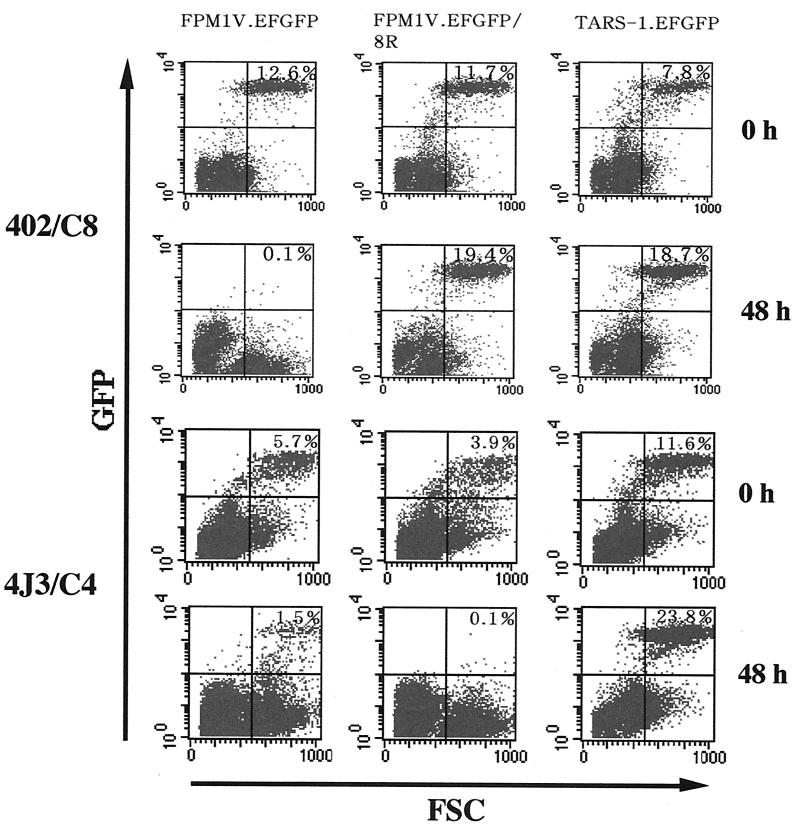
Emergence of CTL-resistant HTLV-1-infected cells after long-term mixed culture with 4O1/C8 CTL. FPM1V.EFGFP and 4O1/C8 cells were mixed in a 24-well plate and continuously cultivated with periodic addition of virus-infected cells. After 3 months of mixed culture, surviving cells with EGFP expression were isolated and designated FPM1V.EFGFP/8R. (A) FPM1V.EFGFP (parts 1 and 2), FPM1V.EFGFP/8R (parts 3 and 4), or TARS-1.EFGFP (parts 5 and 6) cells (5 × 104/well) were mixed with 4O1/C8 cells (106/well) and subjected to flow-cytometric analysis for the expression of EGFP at the indicated times. The percentage of EGFP-positive cells is indicated in each part. (B) Cells from part 2 of panel A were left untreated (solid histogram) or treated with anti-rat CD8 antibody (open histogram) and stained with PE-conjugated anti-mouse IgG (heavy and light chains). Cells with a high level of forward scatter (FSC) and a low EGFP profile were gated from the lower right column of part 2 and examined for CD8 expression. (C) FPM1V.EFGFP (•), FPM1V.EFGFP/8R (○), or TARS1.EFGFP (□) cells (5 × 104/well) were labeled with 51Cr for 1 h and used as target cells. 4O1/C8 cells were used as effectors at the indicated E/T cell ratios. Results are expressed as the mean percent lysis ± the standard deviation of triplicate wells. Similar results were obtained in three independent experiments.
To investigate the long-term effects of Tax-specific CTL on HTLV-1-infected cells, we continuously cultivated FPM1V.EFGFP cells together with 4O1/C8 CTL by periodically adding virus-infected cells to the mixed culture. After 3 months of mixed culture, we isolated EGFP-expressing cells that could survive in the presence of 4O1/C8 CTL. In contrast to the dramatic decrease in the population of FPM1V.EFGFP cells (Fig. 2A, parts 1 and 2), the proportion of these surviving cells markedly increased after 48 h of mixed culture (Fig. 2A, parts 3 and 4). These results indicated that CTL-resistant FPM1V.EFGFP cells were generated after long-term mixed culture with Tax-specific CTL. We also observed the appearance of a new cell population in the lower right column after 48 h of mixed culture (Fig. 2A, part 2). The cells in this population expressed CD8 on their surface, indicating that they were 4O1/C8 cells (Fig. 2B). Since this population appeared only when EGFP-expressing target cells were killed, it is suggested that the CTL increased their volume after being activated by target cells. We further performed a 51Cr release CTL assay to verify the fluorescence-activated cell sorter analysis results. As shown in Fig. 2C, 4O1/C8 cells did not lyse CTL-resistant cells or allogeneic TARS-1.EFGFP cells and killed only parental FPM1V.EFGFP cells. This result was consistent with that obtained by fluorescence-activated cell sorter analysis and confirmed that CTL-resistant FPM1V.EFGFP cells were able to evade CTL lysis. We designated the resistant cell line FPM1V.EFGFP/8R and used it for further study.
MHC-I, but not Tax, expression was downregulated in the FPM1V.EFGFP/8R line.
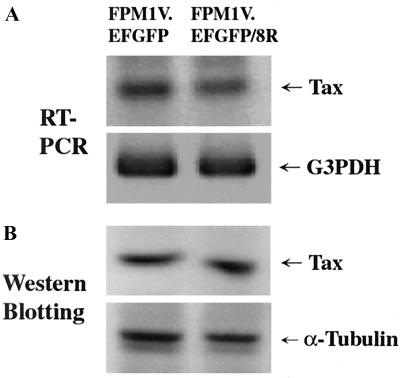
In HTLV-1 infection, it has been suggested that scarcity of viral antigens in vivo is one of the strategies by which the virus can escape host immune surveillance (20, 21). Thus, it was speculated that FPM1V.EFGFP/8R can escape from Tax-specific CTL because of the lack of Tax protein. To examine this possibility, we compared Tax expression in FPM1V.EFGFP cells with that in FPM1V.EFGFP/8R cells. As shown in Fig. 3, the CTL-resistant cells expressed levels of Tax mRNA and protein equivalent to those expressed by the parental cells, suggesting that downregulation of Tax is not the cause of evasion of Tax-specific CTL.
FIG. 3.
Expression of Tax mRNA and protein in FPM1V.EFGFP/8R cells was analyzed by RT-PCR and Western blotting. (A) Total RNAs were isolated from FPM1V.EFGFP or FPM1V.EFGFP/8R cells, and 1 μg of each RNA was subjected to RT-PCR analysis for the expression of Tax and G3PDH mRNAs. (B) Whole-cell extracts were isolated from FPM1V.EFGFP or FPM1V.EFGFP/8R cells, and 50 μg of each protein was subjected to Western blotting analysis for the expression of the Tax and α-tubulin proteins.
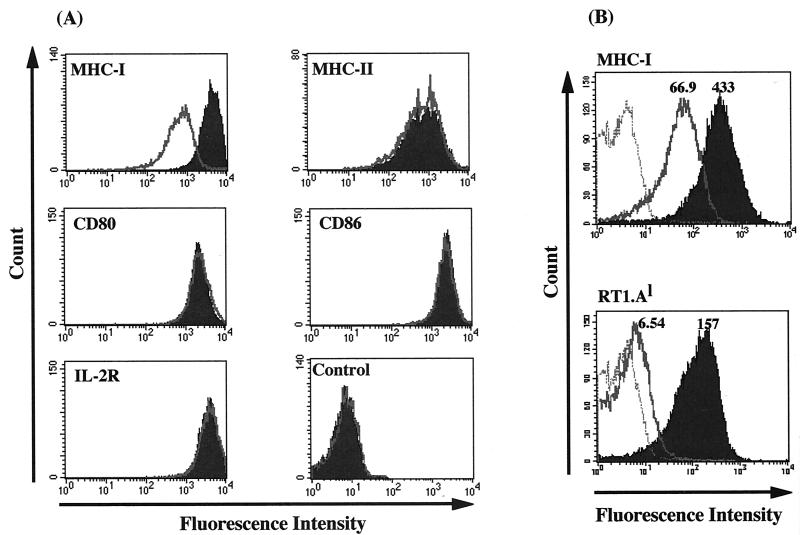
We next examined the expression of cell surface molecules, including MHC-I, MHC-II, CD25, CD80, and CD86, which are related to T-cell activation. As shown in Fig. 4A, MHC-I expression was markedly downregulated in FPM1V.EFGFP/8R cells whereas all of the other molecules were equivalently expressed in both cell lines. Since we recently revealed that 4O1/C8 cells recognize RT1.Al-restricted Tax 180-188 peptide (Hanabuchi et al., unpublished data), we further measured the level of RT1.Al expression on FPM1V.EFGFP/8R cells. As shown in Fig. 4B, RT1.Al expression was decreased dramatically (95.8%), in comparison with an 84.5% reduction in total MHC-I expression. These results suggested that FPM1V.EFGFP/8R cells could evade CTL by downregulating MHC-I, which presents a target epitope for CTL.
FIG. 4.
MHC-I expression is downregulated in FPM1V.EFGFP/8R cells. (A) FPM1V.EFGFP (solid histograms) or FPM1V.EFGFP/8R (open histograms) cells were left untreated (control) or stained with anti-rat MHC-I, MHC-II, CD80, CD86, or IL-2R antibodies for 30 min and then labeled with PE-conjugated anti-mouse IgG antibody. After being washed, labeled cells were subjected to flow-cytometric analysis. (B) FPM1V.EFGFP (solid histograms) or FPM1V.EFGFP/8R (open histograms) cells were stained with anti-rat MHC-I or RT1.Al for 30 min and then labeled with PE-conjugated anti-mouse IgG. After being washed, labeled cells were subjected to flow-cytometric analysis. Histograms of FPM1V.EFGFP/8R cells stained only with PE-conjugated second antibody are represented by the dotted line. The values shown indicate the mean fluorescence intensity in each histogram.
CD4+ CTL line can effectively kill FPM1V.EFGFP/8R cells.
Since MHC-I, but not MHC-II, was downregulated in FPM1V.EFGFP/8R cells, we next examined whether these cells are susceptible to killing by CD4+ CTL. As shown in Fig. 5, another CD8+ Tax-specific CTL line, 4O2/C8, was not able to kill FPM1V.EFGFP/8R cells. In contrast, a CD4+ CTL line, 4J3/C4, effectively killed EGFP-expressing FPM1V.EFGFP/8R cells in 48 h of mixed culture. Killing of FPM1V.EFGFP cells was also observed, whereas 4J3/C4 cells did not lyse an allogeneic cell line, TARS-1.EFGFP. This result indicated that FPM1V.EFGFP/8R cells were still susceptible to MHC-II-restricted CTL activity, further suggesting the importance of the MHC-I expression level for target recognition by Tax-specific CD8+ CTL. We also observed the appearance of a cell population in the lower right column when EGFP-expressing target cells were killed after 48 h of mixed culture, suggesting that it consists of CTL activated by HTLV-I-infected target cells as described in Fig. 2.
FIG. 5.
CD4+, but not CD8+, CTL kill FPM1V.EFGFP/8R cells. FPM1V.EFGFP, FPM1V.EFGFP/8R, or TARS-1.EFGFP cells (105/well) were mixed with 4O2/C8 or 4J3/C4 cells (106/well) and subjected to flow-cytometric analysis for EGFP expression at the indicated times. The percentage of EGFP-positive cells is indicated in each panel. FSC, forward scatter.
Resistance of FPM1V.EFGFP/8R cells to CTL killing was overcome by adding an excess of the relevant Tax epitope.
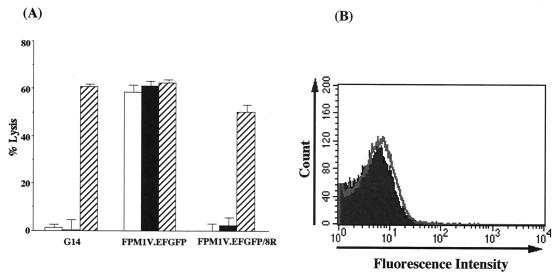
Resistance of HTLV-1-infected cells to CTL killing correlated with downregulation of MHC-I and therefore seemed to result from decreased epitope density on the surface of infected cells. To confirm that this was the mechanism, FPM1V.EFGFP/8R cells were sensitized to CTL by incubation with the relevant RT1.Al-restricted Tax epitope (Tax 180-188). As shown in Fig. 6A, addition of the relevant epitope peptide, but not that of the irrelevant Tax 11-19 peptide, resulted in killing of FPM1V.EFGFP/8R cells by 4O1/C8 CTL. This was not due to upregulation of MHC-I expression by the peptide, because peptide pulsing did not alter the expression of RT1.Al (Fig. 6B). These results demonstrated that a low level of RT1.Al present on the resistant cells was available to exogenous soluble peptide and that addition of the epitope peptide did not affect the stability of RT1.Al on the cell surface. Once they were recognized, HTLV-I-infected cells could be killed, indicating that evasion of CTL lysis occurred at the level of epitope presentation.
FIG. 6.
Killing of peptide-pulsed FPM1V.EFGFP/8R cells by CD8+ CTL. (A) [3H]TdR-labeled G14, FPM1V.EFGFP, or FPM1V.EFGFP/8R cells (5 × 103/well) were mixed with 4O1/C8 cells (5 × 103/well) in 96-well round-bottom plates in the absence (□) or presence of 10 μM peptide Tax 11-19 (▪) or 180-188 (▨). After a 6-h incubation at 37°C, cells were harvested with a Micro 96 Harvester and the [3H]TdR remaining in the target cells was measured in a microplate beta counter. Results are expressed as the mean percent lysis ± the standard deviation of triplicate wells. Similar results were obtained in three independent experiments. (B) FPM1V.EFGFP/8R cells left untreated (solid histograms) or treated with 10 μM peptide Tax 180-188 (open histograms) were stained with anti-rat RT1.Al for 30 min and then labeled with PE-conjugated anti-mouse IgG. After being washed, labeled cells were subjected to flow-cytometric analysis.
An RT1.Al-restricted epitope, Tax 180-188, was conserved in CTL-resistant FPM1V.EFGFP/8R cells.
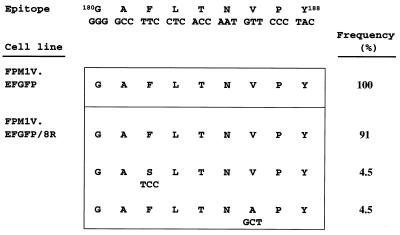
It has been reported that naturally occurring mutations in CTL epitopes in the Tax protein lead to escape from CTL recognition (34). Thus, we examined the nucleotide sequence encoding Tax 180-188 in FPM1V.EFGFP/8R cells to determine the involvement of epitope mutations as an alternative mechanism of CTL resistance. Our results demonstrated that more than 90% of the Tax 180-188 epitopes expressed in CTL-resistant FPM1V.EFGFP/8R cells were identical to wild-type Tax, although 2 of 22 clones had mutations in the region (Fig. 7). All of the clones isolated from CTL-sensitive FPM1V.EFGFP cells had the conserved epitope sequence. These results indicated that generation of epitope variants in FPM1V.EFGFP/8R cells is not the cause of evasion of CTL recognition.
FIG. 7.
Variation in an RT1.Al-restricted epitope of HTLV-1 Tax. Tax-coding cDNA was amplified from FPM1V.EFGFP and FPM1V.EFGFP/8R cells and cloned into vector pCR2.1. Thirteen clones from FPM1V.EFGFP and 22 from FPM1V.EFGFP/8R were sequenced. The sequences of consensus and variant peptides are shown with the corresponding nucleotide sequences and the frequency.
DISCUSSION
In this study, we have demonstrated that HTLV-1-infected T cells obtained the ability to escape CTL recognition by altering the expression of MHC-I on the cell surface. This happened after long-term mixed culture of the infected cells with Tax-specific CTL. Since a similar interaction should exist in HTLV-1-infected individuals in vivo, it is possible that HTLV-1-infected cells evolve the mechanism of MHC-I downregulation to escape host immune systems. In agreement with this idea, It was reported that peripheral mononuclear lymphocytes isolated from ATL patients had altered HLA expression on the cell surface (52). Moreover, Sonoda et al. demonstrated the possibility of loss of HLA antigens in a population of asymptomatic HTLV-1 carriers and a gain in their cell surface after the development of ATL (49). Thus, the rat model system presented here will be a useful tool with which to further dissect the mechanisms by which HTLV-1-infected cells escape the host immune system.
Several viruses that induce chronic infections have been reported to have mechanisms by which to escape immune recognition by altering MHC-I expression on the cell surface (41). In human immunodeficiency virus type 1 infection, Vpu, Tat, and Nef appear to be responsible for MHC-I downregulation (16, 23, 44). Nef has been shown to have a profound effect on MHC-I expression in primary peripheral lymphocytes and to be able to protect infected cells from anti-human immunodeficiency virus type 1 CTL recognition (4). Nef-mediated MHC-I downregulation has been shown to be associated with its interaction with the intracellular sorting machinery of the cell (3, 31). It has also been shown that Nef misroutes MHC-I complexes to clathrin-coated vesicles (29). As for the mechanisms by which HTLV-1 affects MHC-I expression, it has been reported that Tax increases MHC-I expression in glial cells (43). However, our present observation of MHC-I downregulation was not related to repression of Tax expression, since both CTL-sensitive and -resistant lines showed equivalent levels of Tax mRNA and protein expression. As for Tax-independent mechanisms, it was recently reported that an HTLV-1-encoded protein, p12(I), physically binds to the free human MHC-I heavy chains and that this interaction leads to a significant decrease in MHC-I levels on the human T cell surface (19). Thus, further study is required to determine whether p12 is involved in modulation of MHC-I expression in our rat model.
It is well documented that viral protein expression is repressed in most cases of HTLV-1-infected individuals (20, 21). This may be one of the strategies by which the virus can escape the host immune system. The mechanisms of downregulation in vivo are not clear. One of the candidates may be a selection force in the presence of HTLV-1-specific CTL (39). In the present study, we tested this possibility in vitro. The results indicate that although the presence of CTL induced HTLV-1-infected cell resistance to CTL, its mechanism was downregulation of MHC-I but not Tax. Therefore, with regard to Tax expression, the present study did not reproduce the situation in vivo. However, this study elucidated another aspect of escape mechanisms of HTLV-1-infected cells. In addition, controversy has existed as to whether such a low level of HTLV-1 expression in vivo is sufficient, for immortalizing infected cells, to cause infection of other cells in order to establish a variable repertoire of infected clones and for the activation of host immune responses. We show here that by MHC-I downregulation, HTLV-1-infected T cells can escape from CTL without reducing Tax expression. This observation may explain how HTLV-1 evades immune surveillance without losing its ability to propagate itself and to immortalize infected cells. However, we should also point out that our present findings are based on the use of a single HTLV-1-infected cell line and a rat model system. Cell lines from other sources will be useful tools with which to generalize the present findings. Future studies utilizing CTL and HTLV-1-infected cells from HTLV-1 carriers will also help us to understand the clinical significance of the observations presented here.
In addition to MHC-I downregulation, there are other mechanisms related to evasion of CTL lysis, such as the generation of sequence variants in HTLV-1 infection (9, 34) and resistance to Fas-mediated killing (1, 26). Our present examination of the Tax-encoding gene revealed that CTL-resistant FPM1V.EFGFP/8R cells predominantly expressed Tax protein with the wild-type Tax 180-188 epitope, indicating that epitope mutation was not likely the cause of CTL resistance in this study. Intriguingly, we also detected Tax mutants with altered epitope sequences only in FPM1V.EFGFP/8R cells, although the incidence was low. This observation suggested the possibility that Tax-specific CTL could not only induce MHC-I downregulation but also lead to the CTL epitope alteration during long-term mixed culture in this experimental system. Since host CTL responses to Tax have been suggested to play a decisive part in the outcome of HTLV-1 infection (2), this model will be more valuable if it can be applied to studies on CTL epitope generation. Detailed studies on Tax mutants in this model are under way. Moreover, we demonstrated, in this study, that a CD4+ CTL line was able to kill FPM1V.EFGFP/8R cells and that peptide pulsing with an RT1.Al-restricted Tax epitope rendered these resistant cells sensitive to CD8+ CTL. These data indicated that the resistant cells held intact mechanisms required for cell lysis induced by CTL. The peptide-pulsing results also suggested that a low level of RT1.Al present on the cell surface was available to exogenous soluble peptide and that the resistant cells could be killed once the epitope density was increased enough to be recognized by CTL. Thus, we could conclude that resistance to CTL killing occurred mainly at the level of epitope presentation associated with MHC-I downregulation.
In this study, we obtained Tax-specific CTL from rats immunized with Tax-coding DNA. We recently established similar CTL recognizing the same epitope from rats inoculated with HTLV-1-infected cells (13). Since CTL of both origins exhibited similar levels of CTL activity against HTLV-1-infected cells, it is anticipated that CTL from HTLV-1-infected rats could affect infected cells in a similar manner. It will also be interesting to determine whether CTL recognizing other HTLV-1 proteins can induce similar changes in MHC-I expression. Since Env-specific CTL were also induced in the rats used in this study (13), this model system will be useful in the clarification of this question.
In conclusion, we have demonstrated that long-term mixed culture of HTLV-1-infected T cells with Tax-specific CTL led to evasion of CTL lysis by HTLV-1-infected cells and that this protection correlated with downregulation of MHC-I. This may explain how HTLV-1-infected cells escape immune surveillance without reducing Tax expression, which is important for the process of ATL development.
Acknowledgments
We thank Ko Okumura and Hideo Yagita (Juntendo University, Tokyo, Japan) for providing anti-rat CD80 and CD86 antibodies, Takashi Yoshiki (Hokkaido University, Sapporo, Japan) for TARS-1 cells, and Kayoko Matsumoto (Osaka Red Cross Blood Center, Osaka, Japan) for wild-type Tax expression vector and human sera containing Tax antibody. We are grateful to Mitsuhiko Yanagisawa and Shu Endo for cooperation with the maintenance of animals at the P3 level facilities.
This work was supported in part by grants from the Ministry of Education, Science, Culture, and Sports of Japan and the Japan Science and Technology Corporation.
REFERENCES
- 1.Arai, M., M. Kannagi, M. Matsuoka, T. Sato, N. Yamamoto, and M. Fujii. 1998. Expression of FAP-1 (Fas-associated phosphatase) and resistance to Fas-mediated apoptosis in T-cell lines derived from human T cell leukemia virus type 1-associated myelopathy/tropical spastic paraparesis patients. AIDS Res. Hum. Retrovir. 14:261-267. [DOI] [PubMed] [Google Scholar]
- 2.Bangham, C. R. 2000. The immune response to HTLV-I. Curr. Opin. Immunol. 12:397-402. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 4.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 5.Daenke, S., S. Nightingale, J. K. Cruickshank, and C. R. Bangham. 1990. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J. Virol. 64:1278-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duyao, M. P., D. J. Kessler, D. B. Spicer, C. Bartholomew, J. L. Cleveland, M. Siekevitz, and G. E. Sonenshein. 1992. Transactivation of the c-myc promoter by human T cell leukemia virus type 1 tax is mediated by NF kappa B. J. Biol. Chem. 267:16288-16291. [PubMed] [Google Scholar]
- 7.Felber, B. K., H. Paskalis, C. Kleinman-Ewing, F. Wong-Staal, and G. N. Pavlakis. 1985. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science 229:675-679. [DOI] [PubMed] [Google Scholar]
- 8.Fujii, M., P. Sassone-Corsi, and I. M. Verma. 1988. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 85:8526-8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa, Y., R. Kubota, M. Tara, S. Izumo, and M. Osame. 2001. Existence of escape mutant in HTLV-I tax during the development of adult T-cell leukemia. Blood 97:987-993. [DOI] [PubMed] [Google Scholar]
- 10.Gazzolo, L., and M. Duc Dodon. 1987. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature 326:714-717. [DOI] [PubMed] [Google Scholar]
- 11.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 12.Hall, W. W., C. R. Liu, O. Schneewind, H. Takahashi, M. H. Kaplan, G. Roupe, and A. Vahlne. 1991. Deleted HTLV-I provirus in blood and cutaneous lesions of patients with mycosis fungoides. Science 253:317-320. [DOI] [PubMed] [Google Scholar]
- 13.Hanabuchi, S., T. Ohashi, Y. Koya, H. Kato, A. Hasegawa, F. Takemura, T. Masuda, and M. Kannagi. 2001. Regression of human T-cell leukemia virus type I (HTLV-I)-associated lymphomas in a rat model: peptide-induced T-cell immunity. J. Natl. Cancer Inst. 93:1775-1783. [DOI] [PubMed] [Google Scholar]
- 14.Hanabuchi, S., T. Ohashi, Y. Koya, H. Kato, F. Takemura, K. Hirokawa, T. Yoshiki, H. Yagita, K. Okumura, and M. Kannagi. 2000. Development of human T-cell leukemia virus type 1-transformed tumors in rats following suppression of T-cell immunity by CD80 and CD86 blockade. J Virol. 74:428-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howcroft, T. K., K. Strebel, M. A. Martin, and D. S. Singer. 1993. Repression of MHC class I gene promoter activity by two-exon Tat of HIV. Science 260:1320-1322. [DOI] [PubMed] [Google Scholar]
- 17.Inoue, J., M. Seiki, T. Taniguchi, S. Tsuru, and M. Yoshida. 1986. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 5:2883-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson, S., H. Shida, D. E. McFarlin, A. S. Fauci, and S. Koenig. 1990. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature 348:245-248. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, J. M., C. Nicot, J. Fullen, V. Ciminale, L. Casareto, J. C. Mulloy, S. Jacobson, and G. Franchini. 2001. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12(I) protein. J. Virol. 75:6086-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannagi, M., S. Matsushita, and S. Harada. 1993. Expression of the target antigen for cytotoxic T lymphocytes on adult T-cell-leukemia cells. Int. J. Cancer 54:582-588. [DOI] [PubMed] [Google Scholar]
- 21.Kannagi, M., K. Sugamura, K. Kinoshita, H. Uchino, and Y. Hinuma. 1984. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia/lymphoma patient. J. Immunol. 133:1037-1041. [PubMed] [Google Scholar]
- 22.Kannagi, M., K. Sugamura, H. Sato, K. Okochi, H. Uchino, and Y. Hinuma. 1983. Establishment of human cytotoxic T cell lines specific for human adult T cell leukemia virus-bearing cells. J. Immunol. 130:2942-2946. [PubMed] [Google Scholar]
- 23.Kerkau, T., I. Bacik, J. R. Bennink, J. W. Yewdell, T. Hunig, A. Schimpl, and U. Schubert. 1997. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J. Exp. Med. 185:1295-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinoshita, T., M. Shimoyama, K. Tobinai, M. Ito, S. Ito, S. Ikeda, K. Tajima, K. Shimotohno, and T. Sugimura. 1989. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc. Natl. Acad. Sci. USA 86:5620-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita, T., A. Tsujimoto, and K. Shimotohno. 1991. Sequence variations in LTR and env regions of HTLV-I do not discriminate between the virus from patients with HTLV-I-associated myelopathy and adult T-cell leukemia. Int. J. Cancer 47:491-495. [DOI] [PubMed] [Google Scholar]
- 26.Kishi, S., S. Saijyo, M. Arai, S. Karasawa, S. Ueda, M. Kannagi, Y. Iwakura, M. Fujii, and S. Yonehara. 1997. Resistance to fas-mediated apoptosis of peripheral T cells in human T lymphocyte virus type I (HTLV-I) transgenic mice with autoimmune arthropathy. J. Exp. Med. 186:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koya, Y., T. Ohashi, H. Kato, S. Hanabuchi, T. Tsukahara, F. Takemura, K. Etoh, M. Matsuoka, M. Fujii, and M. Kannagi. 1999. Establishment of a seronegative human T-cell leukemia virus type 1 (HTLV-1) carrier state in rats inoculated with a syngeneic HTLV-1-immortalized T-cell line preferentially expressing Tax. J. Virol. 73:6436-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaGrenade, L., B. Hanchard, V. Fletcher, B. Cranston, and W. Blattner. 1990. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet 336:1345-1347. [DOI] [PubMed] [Google Scholar]
- 29.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 30.Maeda, K., T. Sato, M. Azuma, H. Yagita, and K. Okumura. 1997. Characterization of rat CD80 and CD86 by molecular cloning and mAb. Int. Immunol. 9:993-1000. [DOI] [PubMed] [Google Scholar]
- 31.Mangasarian, A., M. Foti, C. Aiken, D. Chin, J. L. Carpentier, and D. Trono. 1997. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity 6:67-77. [DOI] [PubMed] [Google Scholar]
- 32.Mann, D. L., P. DeSantis, G. Mark, A. Pfeifer, M. Newman, N. Gibbs, M. Popovic, M. G. Sarngadharan, R. C. Gallo, J. Clark, and W. Blattner. 1987. HTLV-I-associated B-cell CLL: indirect role for retrovirus in leukemogenesis. Science 236:1103-1106. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto, K., H. Shibata, J. I. Fujisawa, H. Inoue, A. Hakura, T. Tsukahara, and M. Fujii. 1997. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J. Virol. 71:4445-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niewiesk, S., S. Daenke, C. E. Parker, G. Taylor, J. Weber, S. Nightingale, and C. R. Bangham. 1995. Naturally occurring variants of human T-cell leukemia virus type I Tax protein impair its recognition by cytotoxic T lymphocytes and the transactivation function of Tax. J. Virol. 69:2649-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishioka, K., I. Maruyama, K. Sato, I. Kitajima, Y. Nakajima, and M. Osame. 1989. Chronic inflammatory arthropathy associated with HTLV-I. Lancet i:441. [DOI] [PubMed] [Google Scholar]
- 36.Ohashi, T., S. Hanabuchi, H. Kato, Y. Koya, F. Takemura, K. Hirokawa, T. Yoshiki, Y. Tanaka, M. Fujii, and M. Kannagi. 1999. Induction of adult T-cell leukemia-like lymphoproliferative disease and its inhibition by adoptive immunotherapy in T-cell-deficient nude rats inoculated with syngeneic human T-cell leukemia virus type 1-immortalized cells. J. Virol. 73:6031-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi, T., S. Hanabuchi, H. Kato, H. Tateno, F. Takemura, T. Tsukahara, Y. Koya, A. Hasegawa, T. Masuda, and M. Kannagi. 2000. Prevention of adult T-cell leukemia-like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T-cell leukemia virus type 1 Tax-coding DNA vaccine. J. Virol. 74:9610-9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 39.Overbaugh, J., and C. R. Bangham. 2001. Selection forces and constraints on retroviral sequence variation. Science 292:1106-1109. [DOI] [PubMed] [Google Scholar]
- 40.Parker, C. E., S. Daenke, S. Nightingale, and C. R. Bangham. 1992. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology 188:628-636. [DOI] [PubMed] [Google Scholar]
- 41.Ploegh, H. L. 1998. Viral strategies of immune evasion. Science 280:248-253. [DOI] [PubMed] [Google Scholar]
- 42.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawada, M., A. Suzumura, M. Yoshida, and T. Marunouchi. 1990. Human T-cell leukemia virus type I trans activator induces class I major histocompatibility complex antigen expression in glial cells. J. Virol. 64:4002-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 45.Seiki, M., A. Hikikoshi, T. Taniguchi, and M. Yoshida. 1985. Expression of the pX gene of HTLV-I: general splicing mechanism in the HTLV family. Science 228:1532-1534. [DOI] [PubMed] [Google Scholar]
- 46.Seiki, M., J. Inoue, T. Takeda, and M. Yoshida. 1986. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 5:561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siekevitz, M., M. B. Feinberg, N. Holbrook, F. Wong-Staal, and W. C. Greene. 1987. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc. Natl. Acad. Sci. USA 84:5389-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sodroski, J., C. Rosen, W. C. Goh, and W. Haseltine. 1985. A transcriptional activator protein encoded by the x-lor region of the human T-cell leukemia virus. Science 228:1430-1434. [DOI] [PubMed] [Google Scholar]
- 49.Sonoda, S., S. Yashiki, K. Takahashi, N. Arima, Y. Daitoku, M. Matsumoto, T. Matsumoto, M. Tara, K. Shinmyozu, K. Sato, H Inoko, A. Ando, and K. Tsuji. 1987. Altered HLA antigens expressed on T and B lymphocytes of adult T-cell leukemia/lymphoma patients and their relatives. Int. J. Cancer 40:629-634. [DOI] [PubMed] [Google Scholar]
- 50.Tateno, M., N. Kondo, T. Itoh, T. Chubachi, T. Togashi, and T. Yoshiki. 1984. Rat lymphoid cell lines with human T cell leukemia virus production. I. Biological and serological characterization. J. Exp. Med. 159:1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uchiyama, T., J. Yodoi, K. Sagawa, K. Takatsuki, and H. Uchino. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481-492. [PubMed] [Google Scholar]
- 52.Uno, H., H. Matsuoka, M. Suzuki, K. Tsuda, and H. Tsubouchi. 1995. Altered expression of class I HLA antigen on peripheral mononuclear cells in patients with adult T-cell leukemia: inverse relationship with natural killer susceptibility. Cancer Epidemiol. Biomark. Prev. 4:367-372. [PubMed] [Google Scholar]
- 53.Yamamoto, N., M. Okada, Y. Koyanagi, M. Kannagi, and Y. Hinuma. 1982. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science 217:737-739. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida, M., M. Osame, K. Usuku, M. Matsumoto, and A. Igata. 1987. Viruses detected in HTLV-I-associated myelopathy and adult T-cell leukaemia are identical on DNA blotting. Lancet i:1085-1086. [DOI] [PubMed] [Google Scholar]