Abstract
In vivo priming of cytotoxic T lymphocytes (CTL) by DNA injection predominantly occurs by antigen transfer from DNA-transfected cells to antigen-presenting cells. A rational strategy for increasing DNA vaccine potency would be to use a delivery system that facilitates antigen uptake by antigen-presenting cells. Exogenous antigen presentation through the major histocompatibility complex (MHC) class I-restricted pathway of some viral antigens is increased after adequate virus-receptor interaction and the fusion of viral and cellular membranes. We used DNA-based immunization with plasmids coding for human immunodeficiency virus type 1 (HIV-1) Gag particles pseudotyped with vesicular stomatitis virus glycoprotein (VSV-G) to generate Gag-specific CTL responses. The presence of the VSV-G-encoding plasmid not only increased the number of mice displaying anti-Gag-specific cytotoxic response but also increased the efficiency of specific lysis. In vitro analysis of processing confirmed that exogenous presentation of Gag epitopes occurred much more efficiently when Gag particles were pseudotyped with the VSV-G envelope. We show that the VSV-G-pseudotyped Gag particles not only entered the MHC class II processing pathway but also entered the MHC class I processing pathway. In contrast, naked Gag particles entered the MHC class II processing pathway only. Thus, the combined use of DNA-based immunization and nonreplicating pseudotyped virus to deliver HIV-1 antigen to the immune system in vivo could be considered in HIV-1 vaccine design.
Cytotoxic T lymphocytes (CTL) play a key role in the adaptative immune response by eliminating cells infected with intracellular pathogens or bearing tumor-related antigens. DNA-based vaccines are being evaluated as an attractive alternative to conventional protein vaccines, because they can induce potent CTL responses. Strong cellular and/or humoral immune responses have been elicited by injection of DNA vaccines in a variety of species, including humans (22, 56, 68). In vivo priming of CTL by DNA injection predominantly occurs by antigen transfer from DNA-transfected cells to antigen-presenting cells (APC) (15, 21). The injection of DNA into muscle results in the uptake of DNA, not only by myocytes, but also by the neighboring cells. These nonlymphoid tissues express the plasmid-encoded protein. Although directly transfected dendritic cells have been isolated following intradermal biolistic immunization (13), transfected APC probably play a minor role when the DNA is injected intramuscularly. After DNA-based immunization, the strength of the immune response is dependent on the nature of the antigen expressed by nonlymphoid tissues and on its transfer to bone marrow-derived APC (15). APC capture exogenous antigen through multiple pathways, which may influence the efficiency of antigen processing and presentation. It is well known that distinct antigen-processing pathways leading to antigen presentation by two separate major histocompatibility complex (MHC) classes (class I or II) are required for endogenous and exogenous antigens to stimulate either CD8+ or CD4+ T cells (25). During the past few years, this dichotomous processing pathway has become more complex, because it is now well demonstrated that exogenous antigens are processed for alternative MHC class I-restricted antigen presentation to CD8+ T cells by APC (32, 53, 74). The stimulation of naive CTL by peptides derived from exogenous proteins has been referred to as cross-priming (6, 29).
Enhancement of MHC-restricted antigen presentation and vaccine-elicited CTL responses has been demonstrated in mice and in nonhuman primates by cytokine administration (3, 34, 72), by triggering of costimulatory molecules (31, 33), and by induction of Fas-mediated apoptosis (12, 59). We recently demonstrated that human immunodeficiency virus type 1 (HIV-1) Gag epitopes are presented by MHC class I molecules in the absence of viral protein synthesis in primary human dendritic cells and macrophages in vitro after uptake of HIV-1 virions (10). This exogenous presentation requires interaction between viral envelopes and their receptors as well as the fusion activity of the viral envelope. This was observed with virions bearing either HIV-1 or VSV envelope glycoproteins (VSV-G). Thus, a rational strategy would be to take advantage of the VSV-G envelope fusogenic activity and receptor-mediated entry to increase antigen uptake in vivo after DNA-based immunization.
In this report, we investigate whether pseudotyping of Gag particles by the VSV-G envelope could enhance in vivo the Gag-specific immune response after DNA-based immunization. Our results show that injection of plasmids encoding VSV-G-coated HIV-1 Gag particles improved the Gag-specific CD8+ T-cell response in mice. This was confirmed by in vitro experiments indicating that VSV-G pseudotyping of Gag particles allowed the Gag protein to enter into the MHC class I pathway. Finally, we show that both CD4+ and CD8+ T-cell responses were improved after local recruitment of APC, confirming the predominant role of these cells in uptake of the released antigen and immune response induction.
MATERIALS AND METHODS
HIV-1 Gag and VSV-G expression vectors.
The HIV-1 Gag expression vector pCMV.ΔR8-2 is a kind gift of D. Trono (48, 75). It drives the synthesis of all HIV-1 proteins besides Env. The plasmid pCMV-VSV, a kind gift of A. Miyanohara, carries the VSV-G gene under the control of human cytomegalovirus (CMV) immediate-early gene promoter (73). pCMV.AS is a control plasmid carrying the VSV gene in an antisense orientation. It was constructed by inverting a BamHI-BamHI fragment encompassing the VSV-G gene in pCMV-VSV. Plasmid pCMV-VSV mut encodes a fusion-defective VSV-G protein (mutant Q117N) (8, 70).
HIV-1 Gag particles pseudotyped with the VSV-G glycoprotein were produced by cotransfecting pCMV.ΔR8-2 and pCMV-VSV plasmids (at a 3:1 ratio) in HeLa cells as previously described (45). “Naked” HIV-1 Gag particles were produced by using pCMV.AS instead of pCMV-VSV. Stocks of purified particles were obtained after concentration of supernatants from transfected HeLa cells by using membranes with a cutoff value of 100 kDa. Quantification of the particles was done according to their HIV-1 p24 content by enzyme-linked immunosorbent assay (ELISA) (Dupont de Nemours, Paris, France) and kept frozen at −70°C before use.
DNA-based immunization.
Female H-2d BALB/c mice (Iffa Credo, Les Oncins, France) 6 to 8 weeks old were used for immunogenicity studies. The HIV-1 Gag expression vector pCMV.ΔR8-2 was coinjected with either the VSV-G envelope-encoding plasmid DNA (pCMV.VSV) or with a control plasmid carrying the VSV gene in the antisense orientation (pCMV.AS). DNAs were injected into normal or regenerating (i.e., cardiotoxin treated) tibialis anterior (TA) muscles as previously described (43). Each TA received a total of 100, 10, or 1 μg of DNA composed of 3/4 pCMV.ΔR8-2 DNA and 1/4 pCMV.VSV or pCMV.AS DNA in a final volume of 100 μl. All intramuscular injections were carried out under anesthesia (sodium pentobarbital, 75 mg/kg of body weight, intraperitoneal). All DNA vectors used for immunization were purified with Qiagen (Hilden, Germany) Endofree kits.
CTL activity assay.
Immunized mice were sacrificed and spleens were removed 2 weeks after DNA-based immunization. Splenocytes were cultured (107 cells per well in 24-well plate) in 2 ml of a minimum essential medium (α-MEM; Gibco BRL, Cergy Pontoise, France) supplemented with 10 mM HEPES, nonessential amino acids, 1 mM sodium pyruvate, antibiotics, glutamine (Gibco BRL), 0.05 mM β-mercaptoethanol, and 10% fetal calf serum (Myoclone; Gibco BRL). Splenocytes were stimulated with (per milliliter) 1 μg of HIV-1 p24gag62-76 peptide (GHQAAMQMLKETINEE) containing an H-2d-restricted epitope (boldface residues) (63). Five days later, half of the medium was replaced with fresh medium, and 2 days later, cells were used as effectors for the measurement of specific cytolytic activity in a standard chromium release assay. The target cells were H-2d murine mastocytoma cells (P815) pulsed with the HIV-1 p24gag H-2d-restricted peptide (15 μg/ml) or P815 cells infected with a recombinant vaccinia virus encoding the HIV-1 Gag protein (rvv TG 1144) (52) at a multiplicity of infection (MOI) of 20/1. Unpulsed P815 cells or wild-type vaccinia virus-infected cells were used as control. Targets were labeled with 51Cr (3.7 MBq per 106 cells; Amersham, Little Chalfont, United Kingdom). After a 4-h incubation at 37°C, 50 μl of supernatants was collected and counted on a beta counter as described previously (9). Spontaneous and maximum releases were determined from targets incubated with either medium alone or lysis buffer (5% Triton X-100, 1% SDS). The percentage of specific release was calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. The specific lysis was determined for each point in triplicate.
ELISPOT assay.
Gamma interferon (IFN-γ)-releasing cells were quantified after peptide or Gag particle stimulation by cytokine-specific enzyme-linked immunospot (ELISPOT) assay. Flat-bottom nitrocellulose ELISA plates (Multiscreen, Millipore, Molsheim, France) were coated with 50 μl of rat anti-mouse IFN-γ (5 μg/ml; Pharmingen, San Diego, Calif.) overnight at 4°C and thereafter saturated for 2 h at 37°C with RPMI 1640 containing 10% fetal calf serum (FCS). Splenocytes (106 per well in 96-well plates) were incubated for 40 h in complete α-MEM (see CTL activity) at 37°C in 5% CO2 with different antigenic stimulations. Cells were incubated with HIV-1 Gag peptide (1 μg/ml), with VSV-G-pseudotyped HIV-1 Gag particles (100 ng/ml), with naked HIV-1 Gag particles (100 ng/ml), or with recombinant vaccinia virus encoding VSV-G envelope protein (rvv VSV; a kind gift of B. Moss) at the MOI of 1/1 (40). Wells containing cells in culture medium, in concentrated supernatants from untransfected HeLa cells or cells infected with wild-type vaccinia virus, were used as negative controls to evaluate background level. Cells were removed by flicking the plates and then were lysed with water. After washing with phosphate-buffered saline (PBS)-0.05% Tween 20, biotinylated rat anti-mouse IFN-γ antibody (1 μg/ml; Pharmingen, San Diego, Calif.) was added for a 90-min incubation at room temperature. Wells were washed as described above prior to incubation with streptavidin-alkaline phosphatase conjugate (Boehringer Mannheim, Mannheim, Germany) at a 1:1,000 dilution in PBS for 1 h 30 min. Then, a 2.3 mM solution of 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Promega, Madison, Wis.) diluted in alkaline buffer solution was added. When spots were visible, the reaction was stopped with water and air dried. The number of IFN-γ-secreting blue spots was counted, and the results were expressed as single spot-forming cells (SFC). Each cell population was titrated in triplicate, and spots were counted double blind.
The percentage of CD8+ and CD4+ T cells was determined by fluorescence-activated cell sorter (FACS) analysis of fresh splenocytes by direct staining with antimouse CD8+ fluorescein isothiocyanate (FITC)- and CD4+ phycoerythrin (PE)-conjugated antibodies (Pharmingen, San Diego, Calif.). Depletion of CD8+ and CD4+ T cells from mouse splenocytes was achieved by magnetic cell sorting (Miltenyi Biotec, Paris, France) as previously described (44). The percentage of undesired cells in the depleted fraction was less than 0.4%.
Statistical analysis.
Categorical variables were compared with the χ2 Pearson test. The minimal P value for rejection of the null hypothesis (i.e., no difference between VSV-immunized and control group) was 0.05.
RESULTS
Coinjection of a vector coding for HIV-1 Gag particles with a plasmid encoding the VSV-G envelope increases Gag-specific cytotoxic responses in vivo.
To investigate the role of VSV-G envelope pseudotyping in the in vivo uptake of Gag particles by APC, mice were immunized with a vector encoding Gag particles and a plasmid encoding or not encoding the VSV-G envelope. Optimal results for the intracellular expression of antigens and the production of fusogenic HIV-VSV particles were obtained in vitro following cotransfection of Gag and VSV-G expression vectors in HeLa cells (45). Immunofluorescence and confocal microscopy analysis of transfected cells indicated that both Gag and VSV-G antigens partially colocalized within the same cell (data not shown). Quantification of p24gag in cell culture supernatant allowed us to choose a 3-to-1 DNA ratio of pCMV.ΔR8-2 and pCMV.VSV for in vivo injections. As a control for pCMV.VSV injection, we used a vector containing the VSV-G coding domain in an antisense orientation (pCMV.AS). The efficiency of coinjection of plasmids encoding naked Gag particles (pCMV.ΔR8-2 + pCMV.AS) or of plasmids coding for VSV-G envelope-pseudotyped Gag particles (pCMV.ΔR8-2 + pCMV.VSV) at inducing Gag-specific CTL in vivo was tested in mice. Groups of five BALB/c mice were injected once intramuscularly with 10 or 100 μg of total DNA into normal muscle. The cytotoxic CD8+ T-cell response was tested 2 weeks later using splenocytes from immunized mice as effector cells and P815 cells pulsed with an MHC class I-restricted Gag peptide or unpulsed cells as targets.
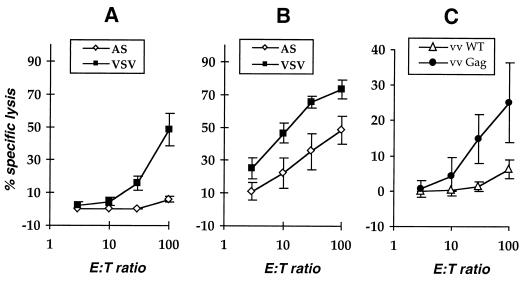
No specific lysis was observed for spleen cells derived from any of the five mice injected with 10 μg of plasmids encoding naked Gag particles. In contrast, cytotoxic T cells were found in the spleens of five out of five mice immunized with 10 μg of vectors coding for VSV-G-pseudotyped Gag particles (Fig. 1A). In addition, the Gag-specific cytotoxic activity of spleen T cells derived from mice immunized with 100 μg of pCMV.ΔR8-2 + pCMV.VSV was significantly higher than that detected after immunization with pCMV.ΔR8-2 + pCMV.AS (Fig. 1B). The number of effector cells required for a 50% lysis of target cells was 10 times lower for mice immunized with 100 μg of pCMV.ΔR8-2 + pCMV.VSV DNA than that for mice immunized with 100 μg of pCMV.ΔR8-2 + pCMV.AS DNA (Fig. 1B). The anti-Gag CTL response was also tested against P815 cells infected with recombinant vaccinia virus encoding the HIV-1 Gag protein. Spleen T cells derived from mice immunized with pCMV.ΔR8-2 + pCMV.VSV DNA and stimulated in vitro with Gag peptide specifically lysed target cells infected with recombinant vaccinia virus encoding the HIV-1 Gag protein. These results indicate that CTL induced after pCMV.ΔR8-2 + pCMV.VSV DNA injection recognized peptides derived from endogenously processed Gag protein (Fig. 1C). Moreover, these results show that coinjection of the plasmid encoding the VSV-G envelope significantly increases the magnitude of Gag-specific CTL response after pCMV.ΔR8-2 injection in vivo. This also indicates that Gag epitopes were better presented in vivo by the APC when Gag particles were pseudotyped with VSV-G envelope.
FIG. 1.
Efficiency of the Gag-specific cytotoxic T-cell response after DNA coinjection. Mice were immunized with 10 (A) or 100 (B) μg of pCMV.ΔR8-2 + pCMV.AS (open diamond) or pCMV.ΔR8-2 + pCMV.VSV (solid squares) plasmid DNA encoding naked or VSV-G-pseudotyped Gag particles, respectively. (C) Mice were immunized with 100 μg of pCMV.ΔR8-2 + pCMV.VSV. DNA was injected into normal muscle. Cytotoxic activity of in vitro-stimulated spleen T cells was measured 2 weeks after immunization. The specific lysis was calculated by subtracting the nonspecific lysis on P815 target cells from the specific lysis obtained on P815 cells pulsed with HIV-1 p24gag peptide (A and B). (C) Target cells were infected with either wild-type vaccinia virus (open triangles) or recombinant vaccinia virus expressing the HIV-1 Gag protein (solid circles). Specific lysis values represent mean values ± standard errors from three to five individual mice in each immunization group. E:T ratio, effector/target ratio.
Dose-dependent Gag-specific cytotoxic T-cell responses after DNA-based immunization in mice.
To evaluate whether the enhanced efficiency of Gag-specific immune response after coinjection of pCMV.VSV with pCMV.ΔR8-2 DNA would permit decreasing the dose of injected DNA, 1, 10, or 100 μg of DNA was injected into normal or regenerating muscle. The Gag-specific cytotoxic responses against P815 target cells pulsed with Gag peptide were evaluated 2 weeks after DNA injection as described above.
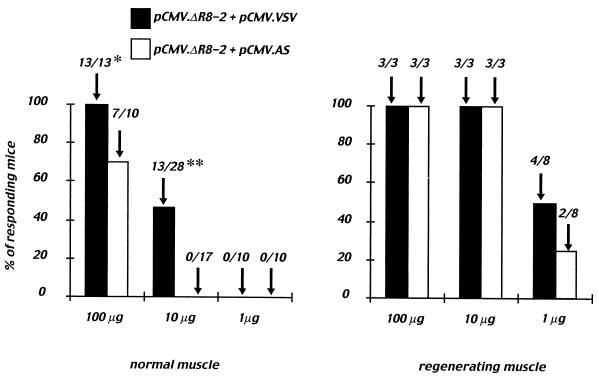
After coinjection into normal muscle of low doses of DNA plasmids (1 μg) encoding either the Gag particle alone (pCMV.ΔR8-2 + pCMV.AS) or the Gag particle pseudotyped with the VSV-G envelope (pCMV.ΔR8-2 + pCMV.VSV), no specific lytic activity against P815 cells pulsed with the Gag peptide was detected (see Fig. 2, left panel). At the dose of 10 μg of DNA, a significant number of mice with a Gag-specific response (13 of 28; P < 0.001) was observed following immunization with plasmids encoding the VSV-G-pseudotyped Gag particles. In contrast, none of the animals (0 of 17) injected with plasmids encoding the naked Gag particles responded at this dose. This result was further confirmed after immunization with 100 μg of DNA, since a significantly higher number of mice (P < 0.05) display a cytotoxic response after coinjection of plasmids encoding the Gag protein and VSV-G envelope (13 of 13 compared to 7 of 10; Fig. 2, left panel). It was previously shown that cardiotoxin allows destruction of muscle fibers followed by their regeneration. This results in a 10-fold more efficient gene transfer in regenerating than in normal muscle (17). Furthermore, the local inflammation leads to a better recruitment of APC to the site of injection, thus improving the immune response induced after DNA injection (41). The numbers of responding mice following injection in cardiotoxin-pretreated muscles of either 10 or 100 μg of vectors coding for naked or VSV-G-pseudotyped Gag particles were comparable and reached 100%. However, injection of 1 μg of DNA was sufficient to induce a specific CTL response in the spleens from 4 of 8 mice immunized with plasmids encoding the Gag protein and the VSV-G envelope and in 2 of 8 mice immunized with plasmids encoding the Gag protein alone (Fig. 2, right panel).
FIG. 2.
Dose-dependent cytotoxic T-cell responses after coinjection of DNAs coding for naked or VSV-G-pseudotyped Gag particles. Mice were immunized with 1, 10, or 100 μg of either pCMV.ΔR8-2 + pCMV.AS (open columns) or pCMV.ΔR8-2 + pCMV.VSV (solid columns) plasmid DNA. DNA was injected into either normal muscle (left panel) or cardiotoxin-pretreated muscle (regenerating muscle, right panel). Cytotoxic activity of spleen cells was measured by using peptide-loaded or unloaded P815 cells as targets. Cytolytic responses were considered positive after subtraction of the background when the specific lysis was 10% or more at an effector/target ratio of 100/1. The number of responding mice/tested mice is indicated at the top of each column and represents cumulative results obtained from three to five independent experiments. ∗, P < 0.05; ∗∗, P < 0.001 by χ2 Pearson test.
These results indicate that under normal conditions, when the number of APC present at the site of DNA injection is low, pCMV.ΔR8-2 is more immunogenic when coinjected with a vector encoding the VSV envelope. Thus, in normal muscle, the use of VSV-G envelope to pseudotype Gag particles could reduce the amount of injected DNA.
We also confirmed that recruitment of APC to the injection site strongly increases the number of mice displaying Gag-specific cytotoxic activity after DNA-based immunization.
Pseudotyping of HIV-1 Gag particles with VSV-G enhances the presentation of HIV-1 Gag epitopes in vitro.
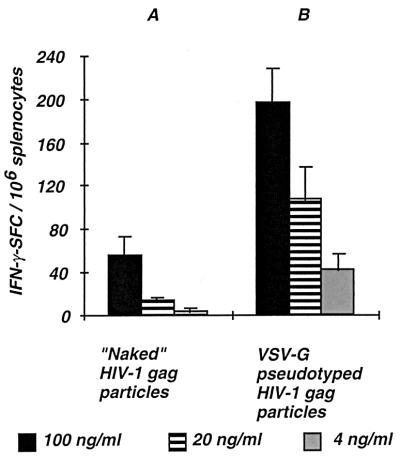
In order to get further insights in the mechanisms involved in the increased efficiency of DNA vectors encoding VSV-pseudotyped Gag particles for the induction of Gag-specific cytotoxic responses in vivo, we studied the involvement of the VSV-G envelope in the in vitro uptake and processing of HIV-1 Gag particles. Different concentrations of viral particles were tested for their ability to generate Gag epitopes after in vitro processing. As a readout for the detection of epitopes derived from the processing of either naked or VSV-G-pseudotyped Gag particles, we used Gag-specific effector T cells, which were obtained from mouse spleen taken 2 weeks after injection into regenerating muscle of 100 μg of DNA vector encoding the Gag protein only (pCMV.ΔR8-2). These spleen cells contained macrophages, dendritic cells, and B cells that could serve as APC for the processing of Gag particles and the presentation of Gag peptides to T cells. The number of epitope-specific T cells producing IFN-γ was measured in response to a short-term stimulation (40 h) of the splenocytes with either naked or VSV-G-pseudotyped Gag particles. The number of Gag-specific IFN-γ-producing T cells increased with the concentration of viral particles within the dose range studied (Fig. 3). Interestingly, the number of IFN-γ SFC was significantly higher when Gag particles were pseudotyped with VSV-G envelope (compare Fig. 3A and B). This result shows that the presentation of Gag epitopes derived from the in vitro processing of exogenous Gag particles is much more efficient when viral particles are pseudotyped with a heterologous viral envelope such as VSV-G than when they are in a naked form.
FIG. 3.
Analysis of in vitro processing of Gag particles. An IFN-γ ELISPOT assay was performed with Gag-specific effector T cells obtained from mice immunized with pCMV.ΔR8-2 DNA encoding naked Gag particles. The number of IFN-γ SFC per 106 splenocytes was measured in response to a short-term stimulation of splenocytes (40 h) with either naked or VSV-G-pseudotyped Gag particles. The number of specific SFC was calculated after subtracting the background obtained in wells containing splenocytes in culture medium. Different concentrations of viral particles were tested for their ability to present Gag epitopes (100, 20, and 4 ng of HIV-1 p24 per ml). Results are mean values ± standard errors from three individual mice. Please note that the number of IFN-γ SFC is expressed per 106 splenocytes.
VSV-G-pseudotyped Gag particles enter MHC class I and II pathways.
To determine if epitopes presented after in vitro processing of either naked or VSV-G-pseudotyped Gag particles were derived from the class I or class II processing pathway, we simultaneously performed ELISPOT assay on undepleted (Fig. 4A), CD4+ T-cell-depleted (Fig. 4B), and CD8+ T-cell-depleted (Fig. 4C) splenocytes taken from mice immunized with the DNA vector encoding the Gag protein only.
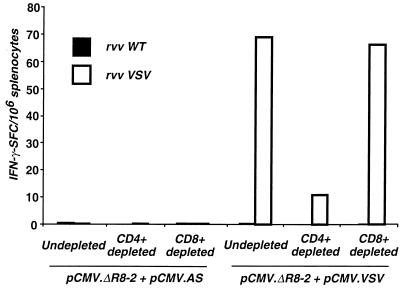
FIG. 4.
Analysis of T-cell subpopulations activated after in vitro processing of Gag particles. The IFN-γ ELISPOT assay was performed as described in the legend to Fig. 3. Effector T cells were pooled splenocytes from five mice immunized with pCMV.ΔR8-2 DNA encoding naked Gag particles. The number of Gag-specific IFN-γ SFC was measured in response to a short-term stimulation of the splenocytes with HIV-1 Gag peptide (1 μg/ml), VSV-G-pseudotyped HIV-1 Gag particles (p24; 100 ng/ml), or naked HIV-1 Gag particles (p24; 100 ng/ml). The ELISPOT assay was performed on undepleted (A), CD4+ T-cell-depleted (B), and CD8+ T-cell-depleted (C) splenocytes. Please note that IFN-γ SFC are expressed for, respectively, 106 T lymphocytes (A), 106 CD8+ T cells (B), and 106 CD4+ T cells (C) after staining and quantification of each cell population by FACS analysis.
Stimulation of undepleted Gag-primed spleen cells with VSV-G-pseudotyped Gag particle increased the number of IFN-γ-secreting T cells compared to stimulation with naked Gag particles (Fig. 4A). This confirms the more efficient presentation of Gag epitopes after in vitro uptake of VSV-pseudotyped particles by APC (Fig. 3).
The number of specific T cells producing IFN-γ after stimulation with naked HIV-1 Gag particles was reduced to the basal level following CD4+ T-cell depletion (Fig. 4B). This indicates that epitopes derived from in vitro processing of naked Gag particles were recognized by CD4+ T cells only. In contrast, after either CD4+ (Fig. 4B) or CD8+ (Fig. 4C) T-cell depletion, the number of specific T cells producing IFN-γ following stimulation with VSV-G-pseudotyped Gag particles was decreased compared to that of undepleted splenocytes (Fig. 4A), but was not significantly different between CD4+- or CD8+-depleted splenocytes (compare Fig. 4B and C). This indicates that, not only CD4+ T lymphocytes, but also CD8+ T cells secreted IFN-γ after recognition of Gag epitopes presented on APC pulsed with VSV-G-pseudotyped particles.
Stimulation of undepleted primed spleen cells with the HIV-1 p24gag peptide also resulted in the production of IFN-γ-secreting T cells. This suggests that some of the spots detected after stimulation with pseudotyped particles were due to the recognition of this epitope by specific T cells derived from pCMV.ΔR8-2-injected mice (Fig. 4A). The number of SFC stimulated with this peptide was decreased to a basal level in a CD8+ T-cell-depleted population (Fig. 4C), indicating that secretion of IFN-γ was due to recognition of the HIV-1 Gag epitope-MHC class I complex by CD8+ T cells. In contrast, after depletion of CD4+ T lymphocytes (Fig. 4B), the number of peptide-specific T cells producing IFN-γ was not significantly different, indicating that this 16-amino-acid Gag peptide was not recognized by CD4+ T cells obtained from pCMV.ΔR8-2 Gag-immunized mice.
Altogether, these results suggest that when Gag particles are pseudotyped with VSV-G envelope, the viral proteins enter both the MHC class I and class II processing pathways, whereas in the absence of VSV-G envelope, Gag particles gain access to the MHC class II processing pathway only.
Gag-specific CD4+ T-cell response in vivo is not dependent on the presence of the VSV-G envelope.
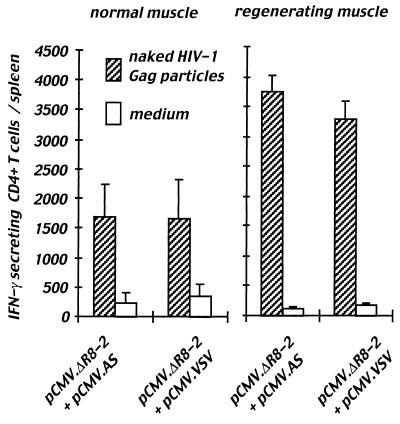
To confirm that the VSV-G pseudotyping of Gag particles had no effect on the Gag-specific CD4+ T-cell response, we immunized mice with vectors encoding either naked or VSV-pseudotyped Gag particles. Spleens were taken from mice 2 weeks after a single injection of 100 μg of DNA into normal or regenerating muscles. The CD4+ T-cell response was quantified by an IFN-γ ELISPOT assay after 40 h of stimulation with naked Gag particles that we have previously shown to be processed through the class II pathway only (described above). The frequency of Gag-specific CD4+ T cells producing IFN-γ was not significantly different in mice immunized with vectors coding or not coding for the VSV-G envelope (Fig. 5). The total number of specific CD4+ T cells per spleen was not different between these two groups either, but was two times higher in mice immunized following cardiotoxin pretreatment (Fig. 5). This indicates that pseudotyping Gag particles with the VSV-G envelope has no major effect on the generation of Gag-specific class II-restricted responses in vivo and underlines the importance of APC recruitment at the injection site for the induction of strong specific T-cell responses.
FIG. 5.
CD4+ T-cell responses induced in vivo by injection of DNAs encoding naked or VSV-G-pseudotyped particles. Groups of 5 or 11 mice were injected with 100 μg of DNA vectors encoding either naked (pCMV.ΔR8-2 + pCMV.AS) or VSV-G-pseudotyped Gag particles (pCMV.ΔR8-2 + pCMV.VSV) into normal muscle (left panel) or in regenerating muscle (right panel). Two weeks after DNA immunization, ex vivo ELISPOT assay was performed on splenocytes to measure Gag-specific IFN-γ-secreting CD4+ T cells. Splenocytes were incubated for 40 h with naked Gag particles (100 ng/ml) or in culture medium. Results are given as the mean number of specific IFN-γ-secreting CD4+ T cells per spleen ± standard error.
Role of the VSV-G envelope in enhancement of the cytotoxic response.
In order to determine if the increase in cytotoxic activity (Fig. 1) and in the frequency of responding mice (Fig. 2) observed after coimmunization of pCMV.ΔR8-2 with pCMV.VSV results from the fusogenic property of the VSV envelope or from a possible adjuvant effect of the VSV-G protein per se, cytotoxic T-cell responses were analyzed 2 weeks after injection of a total of 10 μg of DNA into normal muscle (Table 1). In these experiments, cytolytic responses were considered positive after subtraction of the background, when the specific lysis was 10% or more, and this cutoff value was used to calculate the number of responding mice in each group. Averaging of the results of three to five independent experiments is presented on Table 1. First, DNA expressing Gag particles was coinjected with a plasmid encoding a VSV-G envelope devoid of fusogenic activity (70). A significantly decreased number of responding mice (2 of 14 [14%]) was observed (P < 0.05; Table 1) compared to what was obtained following coinjection in the same leg with plasmid encoding the fusogenic VSV envelope (13 of 28 [46%]). This indicates that the fusogenic activity of the VSV-G envelope protein was necessary for the observed enhancement in cytotoxic responses in vivo. Next, to see whether the VSV-G protein could have an adjuvant effect per se, we injected the two plasmids into separate legs. Coinjection of pCMV-VSV with Gag-expressing DNA at different sites gave a 21% response rate (3 of 14 responding mice) compared to coinjection with control plasmid at the same site (0 of 17 responding mice). This indicated that VSV-G had an additional adjuvant effect on the Gag-specific immune response (P < 0.05).
TABLE 1.
Adjuvant effects of VSV-G on Gag-specific CTL response
| Injected DNA (10 μg)a | No. of responding mice/no. tested (%)b | P (χ2 Pearson test) |
|---|---|---|
| pCMV ΔR8-2 + pCMV.VSV (same paws) | 13/28 (46) | <0.001 |
| pCMV ΔR8-2 + pCMV.AS (same paws) | 0/17 (0) | |
| pCMV ΔR8-2 + pCMV.VSV (same paws) | 13/28 (46) | <0.05 |
| pCMV ΔR8-2 + pCMV.VSV mutc (same paws) | 2/14 (14) | |
| pCMV ΔR8-2 and pCMV.VSV (separate paws) | 3/14 (21) | <0.05 |
| pCMV ΔR8-2 + pCMV.AS (same paws) | 0/17 (0) |
DNA was injected into normal muscle.
Splenocyte cytotoxic activity was measured against P815 target cells pulsed or not pulsed with HIV-1 Gag peptide.
VSV mut is a nonfusogenic VSV-G envelope.
To further characterize the effect mediated by the VSV-G envelope on the Gag-specific cytotoxic response, we analyzed the T-cell response to VSV. This was performed in an IFN-γ ELISPOT assay by using spleen cells derived from mice coimmunized with either pCMV.ΔR8-2 and pCMV.VSV or pCMV.ΔR8-2 + pCMV.AS as effector T cells. Target cells were splenocytes infected with recombinant vaccinia virus expressing VSV-G or with wild-type vaccinia virus. IFN-γ-secreting T cells were detected for mice immunized with vectors encoding VSV-pseudotyped Gag particles only. ELISPOT assay performed on total splenocytes, on CD4+-depleted T cells, or on CD8+-depleted T cells indicated that the VSV-mediated T-cell response was mainly CD4+ dependent (Fig. 6). Thus, VSV-specific CD4+ T cells activated after pCMV.VSV immunization could provide help to the Gag-specific T-cell response observed when the nonfusogenic VSV-G envelope was used.
FIG. 6.
Analysis of VSV-specific T-cell responses induced in vivo by injection of DNAs encoding either naked or VSV-G-pseudotyped Gag particles. Groups of five mice were injected with 100 μg of either pCMV.ΔR8-2 + pCMV.AS DNA (left panel) or pCMV.ΔR8-2 + pCMV.VSV (right panel) DNA into normal muscle. Two weeks after DNA immunization, ex vivo ELISPOT assay was performed on splenocytes to measure VSV-specific IFN-γ-secreting T cells. Splenocytes were incubated for 40 h with wild-type vaccinia virus (rvv WT) or with recombinant vaccinia virus expressing VSV-G (rvv VSV) at an MOI of 1/1. The ELISPOT assay was performed on undepleted, CD4+ T-cell-depleted, and CD8+ T-cell-depleted splenocytes. The number of IFN-γ SFC is expressed per 106 splenocytes.
Altogether, these results suggest that the observed increase in Gag-specific responses after pCMV-VSV coinjection was due in large part to the fusogenic activity of the VSV-G envelope, which allows improved uptake and processing of the Gag particles by APCs, as well as to the intrinsic immunological properties of VSV.
DISCUSSION
In the present study, we show that induction of HIV-1 Gag-specific cytotoxic T cells can be increased in mice by using VSV-G-pseudotyped Gag particles administered by DNA immunization. This operates through an improved receptor-mediated uptake and processing of the Gag particles by APC after fusion with the VSV-G envelope, but also through the intrinsic adjuvant properties of VSV-G protein. In contrast, the efficiency of the class II processing and presentation remained unchanged whether Gag particles were pseudotyped or not.
DNA-based immunization represents an efficient strategy to induce CTL in vivo. Direct injection of a plasmid DNA expression vector into skeletal muscles results in the synthesis of plasmid-encoded antigens in the host cells (16, 71). These foreign proteins are then subjected to natural immune surveillance by dendritic cells, resulting in both MHC class I and II cellular responses. Studies using bone marrow chimeras showed that antigenic peptides involved in priming a CTL response are presented in the context of MHC class I molecules on bone marrow-derived cells and not by myocytes (14, 20, 24). Thus, immune responses are initiated by antigen expressed by transfected dendritic cells (direct priming) or by nonlymphoid cells (cross-priming). However, depending on the nature of the antigen and its localization in the transfected cells, immune responses could vary greatly (36, 39).
The VSV-G envelope allows HIV-1 entry through a pH-dependent endocytic pathway (1). The chimeric viruses composed of HIV-1 core and the VSV-G envelope, termed HIV-1(VSV) pseudotypes, have been shown to be much more infectious than nonpseudotyped HIV-1 virions due to the infection of a broad range of target cells through a fusion-dependent mechanism (48). It has been reported that nonreplicating HIV-1(VSV) virus efficiently transduced DC at an immature stage leading to further maturation and to efficient antigen presentation to CD4+ and CD8+ T cells from HIV-1-infected individuals (26). In addition, we have recently shown that human dendritic cells can present Gag epitopes upon exposure to incoming virions bearing either HIV-1 or VSV envelope glycoproteins and that this occurred in the absence of viral protein synthesis. However, a broader range of APC were targeted when incoming virions were coated with VSV-G rather than with HIV-1 envelope (10).
To increase the uptake efficiency of antigen produced in vivo after DNA-based immunization, we used the VSV-G envelope to pseudotype Gag particles. Our study was based on coimmunization of mice with DNA plasmids encoding either HIV-1 Gag particles only or Gag particles pseudotyped with the VSV-G envelope. We showed that, in vivo, the anti-Gag-specific CTL response was increased after coinjection with the VSV-G-encoding plasmid. The number of mice with Gag-specific CTL in spleen and the intensity of the cytotoxic response were significantly increased for two different doses of coinjected DNA. In contrast, coinjection of mice with a vector coding for a VSV envelope devoid of fusogenic activity significantly reduced the number of mice with Gag-specific CTL. Injection of the DNA coding for Gag and for VSV-G at different sites led to a twofold reduction in the number of mice with Gag-specific CTL. However, compared with mice receiving the pCMV.AS antisense vector, the number of responder mice was still significant. This suggests that production of VSV-G protein at a distant site induced an activation of the immune system that resulted, in turn, in an improvement in the Gag-specific CTL response. Indeed, we found that injection of a plasmid encoding VSV-G induced a high frequency of VSV-specific IFN-γ-secreting CD4+ T cells. Nevertheless, we cannot exclude that the adjuvant effect of VSV may require colocalization with the antigen. This point is difficult to address, because it would require the use of Gag mutants that are not pseudotyped by VSV. Recently, the requirement of CD4+ T-cell help for CTL priming was shown to act via cross-priming mechanisms involving APC (4, 54, 61). This CD4+ T-cell help was originally described as antigen specific; however, a nonspecific stimulus through CD40 was shown to restore APC conditioning, leading to CTL priming in MHC class II−/− mice (4, 61). Recent data indicate that dendritic cells in plasmid DNA-injected mice require conditioning signals from MHC class II-restricted T cells that are both CD40 dependent and independent. The signals required for priming CTL from plasmid injection may be antigen independent or nonspecific and may be provided by cytokine secretion (11, 42, 67). Thus, it is conceivable that the VSV-specific immune response provides nonspecific T-cell help for the generation of Gag-specific CD8+ T-cell responses.
Additionally, VSV-G could exert a positive effect on particle infectivity in various ways. VSV-G-carrying vesicles are produced and efficiently released into culture medium from cells expressing VSV-G in the absence of other viral components (50). VSV-G could thus increase the release of Gag particles when VSV-G and Gag proteins are coexpressed in the same cell. Moreover, it has been reported that VSV-G can be incorporated into naked HIV-1 particles after virion release (64), providing another mechanism for increasing viral infectivity. It is also conceivable that VSV- and HIV-encoding plasmids transfected different cells in vivo and that the VSV-G-induced cell-to-cell fusion resulted in a subsequent enhanced presentation of Gag antigen.
The enhancement in cytotoxic response observed following coinjection of Gag-encoding vector with VSV-encoding plasmid appears to operate by at least two different mechanisms: an activation of the immune system due to the nature of the VSV envelope itself and an increased processing of the secreted VSV-G-pseudotyped Gag particles.
The enhancement in antigen processing was further illustrated by in vitro experiments showing that exogenous presentation of Gag epitopes in APC was more efficient when Gag particles were pseudotyped with VSV-G. It is now well demonstrated that some exogenous antigens can be processed and presented to CD8+ T cells following the alternative class I antigen presentation pathway (32, 69). Our in vitro studies showed that when Gag particles were pseudotyped with VSV-G envelope, the Gag protein enters both MHC class I and II processing pathways. In contrast, naked Gag particles only enter the MHC class II pathway, and the derived epitopes are only recognized by CD4+ T cells.
Various successful strategies to prime MHC I-restricted CD8+ CTL responses to exogenous antigen have been described to date. These include the parvovirus virus-like particles (27, 62), the HIV-1 Gag core particle (19, 28), the hepatitis B surface antigen (60), and the yeast transposon-derived particle (37). Some of these approaches were combined with DNA-based immunization (30, 38, 76). Intramuscular administration of a DNA vaccine represents a simple and effective means of inducing both humoral and cellular immune responses, including cytotoxic T-cell responses (22). There are a number of strategies available to improve the potency of DNA vaccines. Such methods include (i) DNA delivery systems, such as cationic microparticles, that increase DNA transfer to APC (65); (ii) the use of adjuvants, either as a gene or as a coadministered agent (3, 66); (iii) the use of immunostimulatory sequences such as CpG in the plasmid or vector modification to enhance antigen expression (30); (iv) the use of peptides that target the antigen to sites of immune response induction (18); and (v) codelivery of plasmids activating the death pathway (12, 59).
Direct injection into muscle cells induces synthesis and, in some cases, secretion of recombinant protein (16, 47, 71). Targeting of the protein synthesized in the muscle to the dendritic cells operates through either cross-priming or secretion and capture of the DNA-encoded protein. Our results are in agreement with the latter pathway for antigen capture, since we obtained a greater number of mice with anti-Gag-specific cytotoxic activity and greater efficiency in the cytotoxic response when Gag particles were pseudotyped with a fusion-competent VSV-G envelope.
Because of the potential role of CTL in controlling HIV-1 infection (7, 35) and disease progression (46, 49), numerous approaches have been tested for activation of the cellular immune response, including, for example, nonpathogenic recombinant live vectors expressing HIV proteins, inactivated noninfectious virus particles, and DNA vaccines (2, 3). Recently, an AIDS vaccine based on live attenuated recombinant VSV was shown to be effective in protecting macaques after challenge with a pathogenic virus (57). There is increasing evidence that both CD4+ and CD8+ subsets are probably required for strong CTL memory and protection against HIV-1 (51, 55, 58). HIV-1 Gag is one of the most conserved viral proteins, and broad, cross-clade CTL responses recognizing conserved epitopes in HIV-1 Gag have been detected in HIV-1-infected individuals (5, 8, 23). Therefore, the induction of CTL and T-helper responses against conserved Gag epitopes via fusogenic HIV-1 or heterologous envelope-mediated targeting of Gag particles to APC in vivo could be significant for the development of a safe and effective HIV-1 DNA vaccine.
Acknowledgments
We thank F. Buseyne, Y. Rivière, J. Seeler, and L. Chakrabarti for critical reading of the manuscript and helpful discussions; M. Mancini for help with statistical analysis; and D. Trono, B. Moss, and A. Miyanohara for the kind gift of reagents.
This work was supported by grants from the Agence Nationale de Recherche contre le SIDA (ANRS) and from SIDACTION D. Marsac was supported by a fellowship from the ANRS.
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 97:4192-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett, S. R., F. R. Carbone, F. Karamalis, J. F. Miller, and W. R. Heath. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts, M. R., J. Krowka, C. Santamaria, K. Balsamo, F. Gao, G. Mulundu, C. Luo, N. N'Gandu, H. Sheppard, B. H. Hahn, S. Allen, and J. A. Frelinger. 1997. Cross-clade human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte responses in HIV-infected Zambians. J. Virol. 71:8908-8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bevan, M. J. 1976. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143:1283-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buseyne, F., M.-L. Chaix, C. Rouzioux, S. Blanche, and Y. Riviere. 2001. Patient-specific cytotoxic T-lymphocyte cross-recognition of naturally occurring variants of a human immunodeficiency virus type 1 (HIV-1) p24gag epitope by HIV-1-infected children. J. Virol. 75:4941-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buseyne, F., M. Février, S. Garcia, M. L. Gougeon, and Y. Rivière. 1996. Dual functions of a human immunodeficiency virus (HIV) specific cytotoxic T-lymphocyte (CTL) clone: inhibition of HIV replication by non-cytolytic mechanisms and lysis of HIV-infected CD4+ cells. Virology 225:248-253. [DOI] [PubMed] [Google Scholar]
- 10.Buseyne, F., S. Le Gall, C. Boccaccio, J. P. Abastado, J. D. Lifson, L. O. Arthur, Y. Riviere, J. M. Heard, and O. Schwartz. 2001. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat. Med. 7:344-349. [DOI] [PubMed] [Google Scholar]
- 11.Chan, K., D. J. Lee, A. Schubert, C. M. Tang, B. Crain, S. P. Schoenberger, and M. Corr. 2001. The roles of MHC class II, CD40, and B7 costimulation in CTL induction by plasmid DNA. J. Immunol. 166:3061-3066. [DOI] [PubMed] [Google Scholar]
- 12.Chattergoon, M. A., J. J. Kim, J. S. Yang, T. M. Robinson, D. J. Lee, T. Dentchev, D. M. Wilson, V. Ayyavoo, and D. B. Weiner. 2000. Targeted antigen delivery to antigen-presenting cells including dendritic cells by engineered Fas-mediated apoptosis. Nat. Biotechnol. 18:974-979. [DOI] [PubMed] [Google Scholar]
- 13.Condon, C., S. C. Watkins, C. M. Celluzzi, K. Thompson, and L. D. Falo, Jr. 1996. DNA-based immunization by in vivo transfection of dendritic cells. Nat. Med. 2:1122-1128. [DOI] [PubMed] [Google Scholar]
- 14.Corr, M., D. J. Lee, D. A. Carson, and H. Tighe. 1996. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J. Exp. Med. 184:1555-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corr, M., A. von Damm, D. J. Lee, and H. Tighe. 1999. In vivo priming by DNA injection occurs predominantly by antigen transfer. J. Immunol. 163:4721-4727. [PubMed] [Google Scholar]
- 16.Davis, H. L., M.-L. Michel, and R. G. Whalen. 1993. DNA based immunization for hepatitis B induces continuous secretion of antigen and high levels of circulating antibody. Hum. Mol. Genet. 2:1847-1851. [DOI] [PubMed] [Google Scholar]
- 17.Davis, H. L., R. G. Whalen, and B. A. Demeneix. 1993. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum. Gene Ther. 4:151-159. [DOI] [PubMed] [Google Scholar]
- 18.Deliyannis, G., J. S. Boyle, J. L. Brady, L. E. Brown, and A. M. Lew. 2000. A fusion DNA vaccine that targets antigen-presenting cells increases protection from viral challenge. Proc. Natl. Acad. Sci. USA 97:6676-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deml, L., R. Schirmbeck, J. Reimann, H. Wolf, and R. Wagner. 1997. Recombinant human immunodeficiency Pr55gag virus-like particles presenting chimeric envelope glycoproteins induce cytotoxic T-cells and neutralizing antibodies. Virology 235:26-39. [DOI] [PubMed] [Google Scholar]
- 20.Doe, B., M. Selby, S. Barnett, J. Baenziger, and C. M. Walker. 1996. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc. Natl. Acad. Sci. USA 93:8578-8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly, J. J., M. A. Liu, and J. B. Ulmer. 2000. Antigen presentation and DNA vaccines. Am. J. Respir. Crit. Care Med. 162:S190-S193. [DOI] [PubMed] [Google Scholar]
- 22.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 23.Durali, D., J. Morvan, F. Letourneur, D. Schmitt, N. Guegan, M. Dalod, S. Saragosti, D. Sicard, J.-P. Levy, and E. Gomard. 1998. Cross-reactions between the cytotoxic T-lymphocyte responses of human immunodeficiency virus-infected African and European patients. J. Virol. 72:3547-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu, T. M., J. B. Ulmer, M. J. Caulfield, R. R. Deck, A. Friedman, S. Wang, X. Liu, J. J. Donnelly, and M. A. Liu. 1997. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol. Med. 3:362-371. [PMC free article] [PubMed] [Google Scholar]
- 25.Germain, R. N. 1994. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell 76:287-299. [DOI] [PubMed] [Google Scholar]
- 26.Granelli-Piperno, A., L. Zhong, P. Haslett, J. Jacobson, and R. M. Steinman. 2000. Dendritic cells, infected with vesicular stomatitis virus-pseudotyped HIV-1, present viral antigens to CD4+ and CD8+ T cells from HIV-1-infected individuals. J. Immunol. 165:6620-6626. [DOI] [PubMed] [Google Scholar]
- 27.Greenstone, H. L., J. D. Nieland, K. E. de Visser, M. L. De Bruijn, R. Kirnbauer, R. B. Roden, D. R. Lowy, W. M. Kast, and J. T. Schiller. 1998. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc. Natl. Acad. Sci. USA 95:1800-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths, J. C., S. J. Harris, G. T. Layton, E. L. Berrie, T. J. French, N. R. Burns, S. E. Adams, and A. J. Kingsman. 1993. Hybrid human immunodeficiency virus Gag particles as an antigen carrier system: induction of cytotoxic T-cell and humoral responses by a Gag:V3 fusion. J. Virol. 67:3191-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang, A. Y., P. Golumbek, M. Ahmadzadeh, E. Jaffee, D. Pardoll, and H. Levitsky. 1994. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 264:961-965. [DOI] [PubMed] [Google Scholar]
- 30.Huang, Y., W.-P. Kong, and G. J. Nabel. 2001. Human immunodeficiency virus type 1-specific immunity after genetic immunization is enhanced by modification of Gag and Pol expression. J. Virol. 75:4947-4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwasaki, A., B. J. Stiernholm, A. K. Chan, N. L. Berinstein, and B. H. Barber. 1997. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J. Immunol. 158:4591-4601. [PubMed] [Google Scholar]
- 32.Jondal, M., R. Schirmbeck, and J. Reimann. 1996. MHC class I-restricted CTL responses to exogenous antigens. Immunity 5:295-302. [DOI] [PubMed] [Google Scholar]
- 33.Kim, J. J., M. L. Bagarazzi, N. Trivedi, Y. Hu, K. Kazahaya, D. M. Wilson, R. Ciccarelli, M. A. Chattergoon, K. Dang, S. Mahalingam, A. A. Chalian, M. G. Agadjanyan, J. D. Boyer, B. Wang, and D. B. Weiner. 1997. Engineering of in vivo immune responses to DNA immunization via codelivery of costimulatory molecule genes. Nat. Biotechnol. 15:641-646. [DOI] [PubMed] [Google Scholar]
- 34.Kim, J. J., J.-S. Yang, T. C. VanCott, D. J. Lee, K. H. Manson, M. S. Wyand, J. D. Boyer, K. E. Ugen, and D. B. Weiner. 2000. Modulation of antigen-specific humoral responses in rhesus macaques by using cytokine cDNAs as DNA vaccine adjuvants. J. Virol. 74:3427-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurts, C., J. F. Miller, R. M. Subramaniam, F. R. Carbone, and W. R. Heath. 1998. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J. Exp. Med. 188:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Layton, G. T., S. J. Harris, A. J. Gearing, M. Hill-Perkins, J. S. Cole, J. C. Griffiths, N. R. Burns, A. J. Kingsman, and S. E. Adams. 1993. Induction of HIV-specific cytotoxic T lymphocytes in vivo with hybrid HIV-1 V3:Ty-virus-like particles. J. Immunol. 151:1097-1107. [PubMed] [Google Scholar]
- 38.Le Borgne, S., M. Mancini, R. Le Grand, M. Schleef, D. Dormont, P. Tiollais, Y. Riviere, and M. L. Michel. 1998. In vivo induction of specific cytotoxic T lymphocytes in mice and rhesus macaques immunized with DNA vector encoding an HIV epitope fused with hepatitis B surface antigen. Virology 240:304-315. [DOI] [PubMed] [Google Scholar]
- 39.Lewis, P. J., H. van Drunen Littel-van den Hurk, and L. A. Babiuk. 1999. Altering the cellular location of an antigen expressed by a DNA-based vaccine modulates the immune response. J. Virol. 73:10214-10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loirat, D., F. A. Lemonnier, and M. L. Michel. 2000. Multiepitopic HLA-A∗0201-restricted immune response against hepatitis B surface antigen after DNA-based immunization. J. Immunol. 165:4748-4755. [DOI] [PubMed] [Google Scholar]
- 41.Loirat, D., Z. Li, M. Mancini, P. Tiollais, D. Paulin, and M. L. Michel. 1999. Muscle-specific expression of hepatitis B surface antigen: no effect on DNA-raised immune responses. Virology 260:74-83. [DOI] [PubMed] [Google Scholar]
- 42.Lu, Z., L. Yuan, X. Zhou, E. Sotomayor, H. I. Levitsky, and D. M. Pardoll. 2000. CD40-independent pathways of T cell help for priming of CD8+ cytotoxic T lymphocytes. J. Exp Med. 191:541-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancini, M., H. L. Davis, P. Tiollais, and M.-L. Michel. 1996. DNA-based immunization against the envelope proteins of the hepatitis B virus. J. Biotechnol. 44:47-57. [DOI] [PubMed] [Google Scholar]
- 44.Mancini, M., M. Hadchouel, P. Tiollais, and M. L. Michel. 1998. Regulation of hepatitis B virus mRNA expression in a hepatitis B surface antigen transgenic mouse model by IFN-gamma-secreting T cells after DNA-based immunization. J. Immunol. 161:5564-5570. [PubMed] [Google Scholar]
- 45.Maréchal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 47.Naffakh, N., C. Pinset, D. Montarras, Z. Li, D. Paulin, O. Danos, and J.-M. Heard. 1996. Long-term secretion of therapeutic proteins from genetically modified skeletal muscles. Hum. Gene Ther. 7:11-21. [DOI] [PubMed] [Google Scholar]
- 48.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 49.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 50.Okimoto, T., T. Friedmann, and A. Miyanohara. 2001. VSV-G envelope glycoprotein forms complexes with plasmid DNA and MLV retrovirus-like particles in cell-free conditions and enhances DNA transfection. Mol. Ther. 4:232-238. [DOI] [PubMed] [Google Scholar]
- 51.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518-525. [DOI] [PubMed] [Google Scholar]
- 52.Rautmann, G., M. P. Kieny, R. Brandely, K. Dott, M. Girard, L. Montagnier, and J. P. Lecocq. 1989. HIV-1 core proteins expressed from recombinant vaccinia viruses. AIDS Res. Hum. Retrovir. 5:147-157. [DOI] [PubMed] [Google Scholar]
- 53.Reimann, J., and R. Schirmbeck. 1999. Alternative pathways for processing exogenous and endogenous antigens that can generate peptides for MHC class I-restricted presentation. Immunol. Rev. 172:131-152. [DOI] [PubMed] [Google Scholar]
- 54.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 55.Riviere, Y., M. B. McChesney, F. Porrot, F. Tanneau-Salvadori, P. Sansonetti, O. Lopez, G. Pialoux, V. Feuillie, M. Mollereau, S. Chamaret et al. 1995. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res. Hum. Retrovir. 11:903-907. [DOI] [PubMed] [Google Scholar]
- 56.Robinson, H. L., and C. Torres. 1997. DNA vaccines. Semin. Immunol. 9:271-283. [DOI] [PubMed] [Google Scholar]
- 57.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki, S., R. R. Amara, A. E. Oran, J. M. Smith, and H. L. Robinson. 2001. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat. Biotechnol. 19:543-547. [DOI] [PubMed] [Google Scholar]
- 60.Schirmbeck, R., K. Melber, A. Kuhröber, Z. A. Janowicz, and J. Reimann. 1994. Immunization with soluble hepatitis B virus surface protein elicits murine H-2 class I-restricted CD8+ cytotoxic T lymphocyte responses in vivo. J. Immunol. 152:1110-1119. [PubMed] [Google Scholar]
- 61.Schoenberger, S. P., R. E. Toes, E. I. van der Voort, R. Offringa, and C. J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 62.Sedlik, C., M. Saron, J. Sarraseca, I. Casal, and C. Leclerc. 1997. Recombinant parvovirus-like particles as an antigen carrier: a novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc. Natl. Acad. Sci. USA 94:7503-7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selby, M. J., B. Doe, and C. M. Walker. 1997. Virus-specific cytotoxic T-lymphocyte activity elicited by coimmunization with human immunodeficiency virus type 1 genes regulated by the bacteriophage T7 promoter and T7 RNA polymerase protein. J. Virol. 71:7827-7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma, S., A. Miyanohara, and T. Friedmann. 2000. Separable mechanisms of attachment and cell uptake during retrovirus infection. J. Virol. 74:10790-10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh, M., M. Briones, G. Ott, and D. O'Hagan. 2000. Cationic microparticles: a potent delivery system for DNA vaccines. Proc. Natl. Acad. Sci. USA 97:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ulmer, J. B., C. M. DeWitt, M. Chastain, A. Friedman, J. J. Donnelly, W. L. McClements, M. J. Caulfield, K. E. Bohannon, D. B. Volkin, and R. K. Evans. 1999. Enhancement of DNA vaccine potency using conventional aluminum adjuvants. Vaccine 18:18-28. [DOI] [PubMed] [Google Scholar]
- 67.Wang, B., C. C. Norbury, R. Greenwood, J. R. Bennink, J. W. Yewdell, and J. A. Frelinger. 2001. Multiple paths for activation of naive CD8+ T cells: CD4-independent help. J. Immunol. 167:1283-1289. [DOI] [PubMed] [Google Scholar]
- 68.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, J. Ng, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 69.Watts, C. 1997. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol. 15:821-850. [DOI] [PubMed] [Google Scholar]
- 70.Whitt, M. A., P. Zagouras, B. Crise, and J. K. Rose. 1990. A fusion-defective mutant of the vesicular stomatitis virus glycoprotein. J. Virol. 64:4907-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolff, J. A., J. J. Ludtke, G. Acsadi, P. Williams, and A. Jani. 1992. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1:363-369. [DOI] [PubMed] [Google Scholar]
- 72.Xiang, Z., and H. C. Ertl. 1995. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity 2:129-135. [DOI] [PubMed] [Google Scholar]
- 73.Yee, J. K., A. Miyanohara, P. LaPorte, K. Bouic, J. C. Burns, and T. Friedmann. 1994. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 91:9564-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yewdell, J. W., C. C. Norbury, and J. R. Bennink. 1999. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol. 73:1-77. [DOI] [PubMed] [Google Scholar]
- 75.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15:871-875. [DOI] [PubMed] [Google Scholar]
- 76.zur Megede, J., M.-C. Chen, B. Doe, M. Schaefer, C. E. Greer, M. Selby, G. R. Otten, and S. W. Barnett. 2000. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J. Virol. 74:2628-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]