Abstract
Exposure of hematopoietic progenitors to gamma irradiation induces p53-dependent apoptosis. However, host responses to DNA damage are not uniform and can be modified by various factors. Here, we report that a split low-dose total-body irradiation (TBI) (1.5 Gy twice) to the host causes prominent apoptosis in bone marrow cells of Friend leukemia virus (FLV)-infected C3H mice but not in those of FLV-infected DBA mice. In C3H mice, the apoptosis occurs rapidly and progressively in erythroid cells, leading to lethal host anemia, although treatment with FLV alone or TBI alone induced minimal apoptosis in bone marrow cells. A marked accumulation of P53 protein was demonstrated in bone marrow cells from FLV-infected C3H mice 12 h after treatment with TBI. Although a similar accumulation of P53 was also observed in bone marrow cells from FLV-infected DBA mice treated with TBI, the amount appeared to be parallel to that of mice treated with TBI alone and was much lower than that of FLV- plus TBI-treated C3H mice. To determine the association of p53 with the prominent enhancement of apoptosis in FLV- plus TBI-treated C3H mice, p53 knockout mice of the C3H background (C3H p53−/−) were infected with FLV and treated with TBI. As expected, p53 knockout mice exhibited a very low frequency of apoptosis in the bone marrow after treatment with FLV plus TBI. Further, C3H p53−/− → C3H p53+/+ bone marrow chimeric mice treated with FLV plus TBI survived even longer than the chimeras treated with FLV alone. These findings indicate that infection with FLV strongly enhances radiation-induced apoptotic cell death of hematopoietic cells in host animals and that the apoptosis occurs through a p53-associated signaling pathway, although the response was not uniform in different host strains.
The specific induction of apoptosis to abnormal cells is one of the major treatment strategies for tumors as well as for virus-induced diseases. Therefore, trials to control apoptotic cell processes would be an attractive option for new therapeutic approaches to tumors or virus-induced diseases. To introduce apoptotic signals to the cell, many kinds of cell lines and stimuli have been employed in various experimental systems under physiological and pathological conditions. However, animal models still have priority when establishing the potential efficacy of a treatment in the sense that host reactions can be analyzed totally. In the present study, we investigated how retroviral infection modifies signals for DNA damage-induced apoptosis in hematopoietic cells by using an in vivo model of a Friend leukemia virus (FLV) infection system. The FLV is a murine retrovirus that can cause splenomegaly and induce erythroleukemia in susceptible mouse strains. The virus is a complex of two viruses: a replication-competent helper murine FLV and a replication-defective spleen focus-forming virus. It has been well established that FLV-induced leukemogenesis is genetically controlled by multiple non-H-2-linked virus susceptibility and/or resistance genes (Fv-1, Fv-2, Fv-3, Fv-4, Fv-5, and Fv-6) as well as H-2-linked and nonlinked immune response genes (Rfv-1, Rfv-2, and Rfv-3) (7, 23). Thus, it is known that C3H/He (C3H) (H-2k Fv-2s) mice and DBA/2 (DBA) (H-2d Fv-2s) mice are highly susceptible to FLV-induced leukemogenesis, whereas C57BL/6 (H-2b Fv-2r) mice are refractory to FLV (15).
Viral infection is known to have various effects on apoptotic cell signaling pathways of the cells. Viral Fas-associated death domain (FADD)-like interleukin-1β-converting enzyme (FLICE)-inhibitory proteins prevent apoptosis induced by death receptors (40), and the ets-2 transcription factor inhibits apoptosis through a bcl-xL-dependent mechanism (35). In contrast, human immunodeficiency virus caused apoptosis in CD4+ T cells via gp120 in combination with T cell receptor-enhanced (1) and Moloney murine leukemia virus-enhanced thymocyte apoptosis (4). FLV infection usually causes antiapoptotic features in transformed cell lines (14, 28, 30) as well as in primary erythroblasts (28), although the early effect of FLV infection on apoptotic signaling is still uncertain.
The DNA damage induced by gamma irradiation is known to cause p53-dependent apoptosis in many kinds of cells and tissues in vitro as well as in vivo (34). For the purpose of radiotherapy for FLV-induced disease, split low-dose total-body irradiation (TBI) treatment with 1.5 Gy of gamma irradiation has been performed and shown to exhibit a curative effect on FLV-induced leukemogenesis in DBA mice (36, 37, 38). However, the effectiveness of this system was not due to a direct radiation effect on the virus or tumor cells but was related to the enhancement of immune functions of the host by an increased number of CD4+ T cells and a decrease in CD8+ cells in the spleen. It is intriguing to speculate that the decrease in the number of immunosuppressive CD8+ T cells may be related to the efficacy of TBI treatment. Predicated on this report, clinical trials to treat AIDS patients with split low-dose TBI were initiated (8). However, the TBI effect on retrovirus-infected hematopoietic cells of the host animal remained unsolved. Therefore, to further examine the effect of FLV infection on TBI-induced signaling in hematopoietic cells, we infected C3H and DBA mice with FLV and treated them with low-dose TBI. We found that hematopoietic cells of FLV-infected C3H mice became highly sensitive to TBI-induced apoptosis, whereas FLV infection in DBA mice did not evoke a significant enhancement of apoptosis after treatment with TBI. The hematopoietic cells of FLV- plus TBI-treated C3H mice decreased rapidly, leading to lethal anemia, and revealed frequent apoptosis in the bone marrow. Thus, in the present study, we provided a model system for prominent enhancement of radiation-induced apoptotic signals in vivo by retroviral infection. We discuss the mechanism for modifying the apoptotic process by FLV infection.
MATERIALS AND METHODS
Mice.
Eight- to 10-week-old male C3H (H-2k Fv-2s) mice were bred from our colony at the Animal Production Facility of the National Institute of Radiological Sciences in Chiba, Japan. p53 knockout mice of a C3H background (C3H p53−/−) were also bred from our colony and kindly supplied by Kazuko Yoshida of the National Institute of Radiological Sciences. Specific-pathogen-free DBA (H-2d Fv-2s) mice of 6 to 8 weeks of age were purchased from the Shizuoka Laboratory Animal Cooperation (Shizuoka, Japan). All mice were maintained within a barrier-sustained specific-pathogen-free facility. All mouse work was performed in accordance with the guidelines established by the Animal Experiment Committee of the Tokyo Medical and Dental University.
Virus infection and TBI.
An NB-tropic FLV complex, originally a gift from C. Friend, was prepared as described earlier (16), and mice were inoculated intraperitoneally at a highly leukemogenic dose of 104 PFU/mouse (19). On days 5 and 12 after inoculation with FLV, 8- to 10-week-old C3H and DBA mice were treated with 1.5 Gy of TBI. In experiments with C3H p53−/− mice, the animals were also treated with 1.5 Gy of TBI 1 week after FLV inoculation. A dose of 1.5 Gy of TBI was delivered from a GAMMA-CELL-40 (Atomic Energy of Canada Ltd., Kanata, Ontario) at a rate of 1.12 Gy/min. Sham-treated mice that were not irradiated were also prepared in each experiment. Bone marrow specimens were taken from the femoral bone of each experimental group chronologically, fixed with 10% buffered formalin, and embedded in paraffin. Sections (6 to 8 μm thick) were decalcified with 5% trichloroacetic acid solution and stained with hematoxylin and eosin.
Detection of apoptotic cells.
Fresh bone marrow tissue was mounted in an OCT compound (Sakura, Tokyo, Japan), frozen with liquid nitrogen, and cut to make 8- to 10-μm-thick frozen sections. To determine the number of apoptotic cells on frozen tissue sections by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL), an in situ cell death detection kit and fluorescein (Boehringer Mannheim, Mannheim, Germany) were used as described previously (20). Briefly, frozen sections were fixed with a 4% paraformaldehyde solution for 20 min, washed with phosphate-buffered saline (PBS), incubated in 0.1% sodium citrate-0.1% Triton X-100 for 2 min, washed with PBS, and then incubated with fluorescein isothiocyanate (FITC)-labeled dUTP and terminal deoxytransferase at 37°C for 60 min. Sections were then observed by fluorescein microscopy, and the TUNEL-positive cell ratio was determined by dividing the number of positively stained cells by the total number of cells (counting more than 1,000 cells).
Apoptosis was also analyzed by quantifying the phosphatidylserine residues exposed on the cell membrane by using flow cytometry. Bone marrow cells were first stained with membrane-specific antibodies for erythroid cells (TER119; Pharmingen, San Diego, Calif.), myeloid cells (Gr-1; Pharmingen), T cells (Thy-1.2; Pharmingen), and B cells (B220; Pharmingen). After two washes with PBS, 5 μl of recombinant FITC-labeled annexin V (Takara, Kyoto, Japan) was added to cells resuspended in 100 μl of binding buffer (Takara). After 10 min of incubation in the dark at room temperature, 300 μl of binding buffer was added and the samples were analyzed on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, Mountain View, Calif.). In control samples with normal thymocytes and bone marrow cells, double staining for FITC-labeled annexin V and propidium iodide (2 μg/ml) was performed to help set the limit used to discriminate between annexin-V-positive and -negative cells. To determine the expression of viral antigen on the cell surface, FITC-conjugated goat anti-gp70 antibody (Quality Biotech, Camden, N.J.) was used for fluorescence-activated cell sorter analysis.
Western blot analysis for the P53 protein.
The bone marrow cells from each experimental group of mice were suspended in Iscove's modified Dulbecco's medium (Sigma Chemicals, St. Louis, Mo.) containing 30% fetal bovine serum at a concentration of 6 × 106 cells/tube and pelleted. Cell lysates were prepared by incubating cell pellets on ice for 15 min in 1 ml of a lysis buffer containing 10 mM Tris-HCl (pH 7.5), 5 mM EDTA, 1% Nonidet P-40, 0.02% NaN3, 1 mM phenylmethylsulfonyl fluoride, 0.1% aprotinin, 100 μM leupeptin, and 100 μM tosyl-l-phenylalanyl chloromethyl ketone (Sigma Chemicals). Supernatants were separated from debris by centrifugation at 12,000 rpm (9,000 × g) for 5 min at 4°C. Protein concentrations were determined by using a protein assay kit (Bio-Rad Laboratories, Hercules, Calif.). The whole-cell lysate of 30 μg was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10 or 12.5% acrylamide). Gels were transferred electrophoretically to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). The membranes were blocked in 10% skim milk in PBS and incubated with a mouse monoclonal antibody to P53 protein (Pab 240; Santa Cruz Biotechnology, Santa Cruz, Calif.). After washing, the membranes were incubated with a horseradish peroxidase-conjugated anti-mouse immunoglobulin G antibody (Dakopatts, Glostrup, Denmark). To confirm the equivalent loading of protein in each lane, the membranes were blocked, incubated in polyclonal rabbit anti-actin antisera (Sigma Chemicals), and after washing, incubated in horseradish peroxidase-conjugated anti-rabbit immunoglobulin antibody (Dakopatts). Bands in the washed membrane were detected with an enhanced chemiluminescence system (Amersham Life Science, Little Chalfont, Buckinghamshire, England) as described previously (18). The densities of the bands were measured by densitometric analysis with an ImageQuant scanning imager (Molecular Dynamics, Sunnyvale, Calif.). The relative intensities of the bands were calculated by comparing the density of the sample with that of the control.
Preparation of radiation bone marrow chimeras.
Eight- to 10-week-old C3H mice were irradiated with 12.5 Gy of 137Cs-gamma rays from a GAMMA-CELL-40 (Atomic Energy of Canada Ltd.) at a rate of 1.12 Gy/min to generate radiation bone marrow chimeras as described earlier (17). For the p53 knockout experiments, two groups of radiation bone marrow chimeras were constructed. They included irradiated C3H mice reconstituted with 1 × 107 bone marrow cells from p53 knockout mice of the C3H background (C3H p53−/−) and wild-type C3H mice (C3H p53+/+).
RT-PCR.
To determine the activation of the Spi-1 and Fli-1 genes, which is essential for the transformation of erythroid cells during the progression of FLV-induced disease (39), a reverse transcriptase PCR (RT-PCR) was performed in each experimental group. The RNA was extracted from the spleen and bone marrow with an RNeasy Mini kit (Qiagen, Valencia, Calif.) according to the manufacturer's directions. Tissue RNA (100 ng) was used as a template for the amplification reactions. cDNA was synthesized by using Rous-associated virus RT (Takara Biomedicals). The PCR was performed as described elsewhere (20). Oligonucleotides for use as specific primers for Spi-1 and Fli-1 were synthesized by a commercial laboratory (Life Technologies Oriental, Tokyo, Japan). As a PCR control, β-actin was also detected in each run. The sequences of the primers were as follows: Spi-1 5′ PCR primer, ATGGAAGGGTTTTCCCTCACCGCC; Spi-1 3′ PCR primer, CTGCACGCTCTGCAGCTCTGTGAA; Fli-1 5′ PCR primer, CCAGAACATGGATGGCAAGGA; Fli-1 3′ PCR primer, CCCAGGATCTGATAAGGATCTGGC; β-actin 5′ PCR primer, TGGAATCCTGTGGCATCCATGA; and β-actin 3′ PCR primer, ATCTTCATGGTGCTAGGAGCCAG. The expected sizes of the PCR products were 216 bp for Spi-1, 324 bp for Fli-1, and 175 bp for β-actin. φX174/HaeIII-cut DNA was run in parallel as a molecular size marker.
RESULTS
Effects of split low-dose TBI on the survival of FLV-infected C3H and DBA mice.
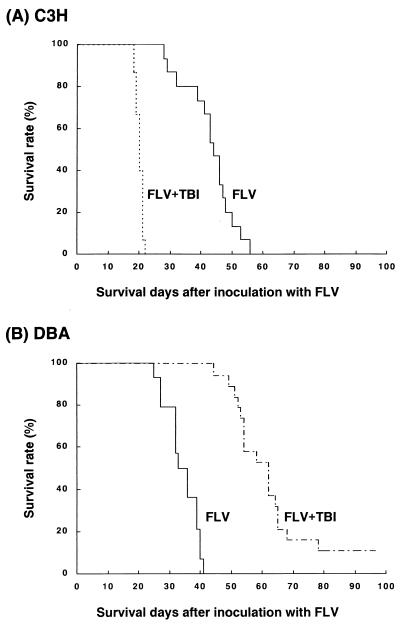
To determine the effects of split low-dose TBI on the survival of C3H and DBA mice infected with FLV, mice were irradiated with 1.5 Gy of gamma irradiation on day 5 or 12 after inoculation with FLV. The survival of TBI-treated mice was compared with that of sham-treated mice. In C3H mice, FLV inoculation induced marked splenomegaly and leukemia in all of the mice examined (15 of 15) and these mice died from days 28 to 56. However, FLV- plus TBI-treated C3H mice died on days 19 to 22 without exception (15 of 15) (Fig. 1A). These mice exhibited severe anemia. Strikingly, the FLV- plus TBI-treated C3H mice showed severe atrophy of the spleen in spite of the fact that treatment with FLV alone evoked FLV-induced splenomegaly by proliferation of erythroid cells in C3H mice.
FIG. 1.
(A) Survival curves of FLV-inoculated C3H mice and FLV-infected C3H mice treated with split low-dose TBI (1.5 Gy twice on days 5 and 12). Note that C3H mice treated with FLV alone died from days 28 to 56 after inoculation with FLV, whereas FLV- plus TBI-treated mice died suddenly on days 19 to 21 after inoculation with FLV. (B) Survival curves of FLV-inoculated DBA mice and FLV-infected DBA mice treated with split low-dose TBI (1.5 Gy twice). Treatment with FLV alone induced leukemic death of the DBA host from days 26 to 41, whereas TBI treatment markedly extended the survival of FLV-infected DBA mice.
In contrast, the survival of FLV-infected and TBI-treated DBA mice was markedly extended compared with that of FLV-infected and sham-treated DBA mice (Fig. 1B). All healthy C3H and DBA mice treated with low-dose TBI (1.5 Gy twice) alone lived for more than 200 days (data not shown).
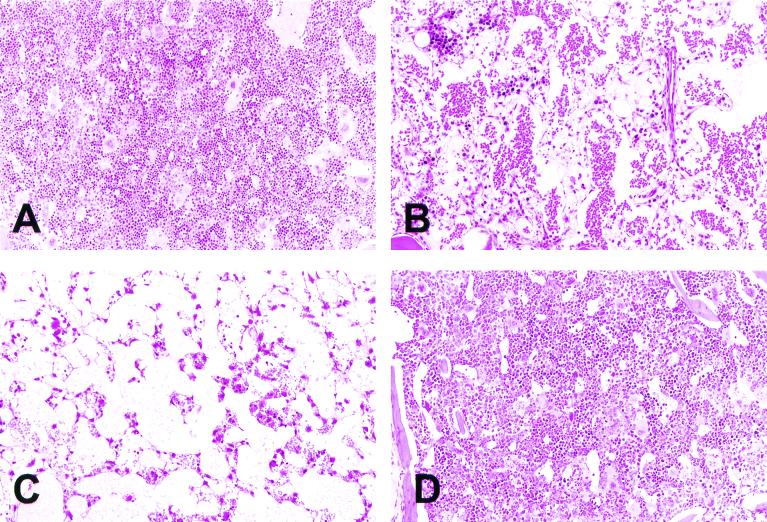
A histological examination of the bone marrow of mice receiving FLV was performed on days 13 and 20. In contrast to the bone marrow of control C3H mice, which was hypercellular (Fig. 2A), the bone marrow of TBI-treated C3H mice showed marked atrophy and revealed dilatation of the sinuses and hypoplastic hematopoiesis changes on day 13 (Fig. 2B). By day 20, the bone marrow hematopoietic cells were almost completely depleted, exhibiting a feature of fatty marrow (Fig. 2C). In contrast, the bone marrow of FLV-infected and TBI-treated DBA mice showed only a slight decrease in hematopoietic cells even on day 20 (Fig. 2D). These findings were consistent with the survival studies described above and shown in Fig. 1.
FIG. 2.
Histological features of the bone marrow of a control mouse (A) and of mice treated with FLV plus TBI on days 13 (B) and 20 (C) (hematoxylin and eosin staining). Note the marked decrease in the number of hematopoietic cells from the bone marrow after treatment. In contrast, the bone marrow of TBI- plus FLV-treated DBA mice (D) showed only a slight decrease in hematopoietic cells even on day 20. Magnification, ×130.
Frequency of apoptotic cells in bone marrow.
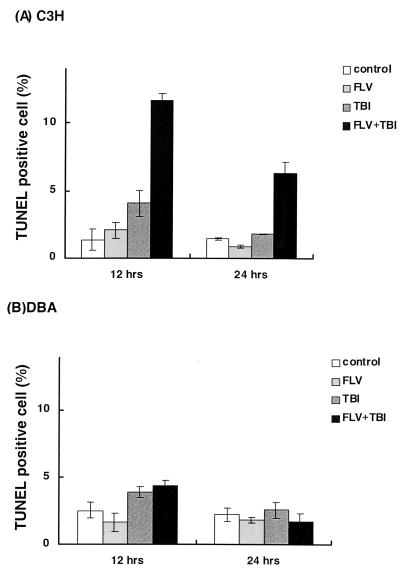
As the rapid decrease in the number of hematopoietic cells was prominent in TBI-treated C3H mice receiving FLV, the number of apoptotic bone marrow cells was determined with the TUNEL method. Figure 3 shows the TUNEL-positive cell ratio of the bone marrow from control mice, mice receiving FLV alone, mice treated with TBI alone, and FLV-infected mice treated with TBI 12 and 24 h after the second round of TBI on day 12. As indicated, TBI alone induced a slight increase in apoptotic cells in the bone marrow in both C3H and DBA mice, but no marked change in the TUNEL-positive cell ratio was observed in C3H or DBA mice receiving FLV alone. However, the bone marrow of FLV-infected C3H mice treated with TBI exhibited a significant increase in apoptotic cells, peaking 12 h after the last TBI treatment (Fig. 3A). These results indicate that the decrease in the number of hematopoietic cells in C3H mice treated with FLV plus TBI was caused by frequent apoptosis in the bone marrow.
FIG. 3.
The TUNEL-positive cell ratio of the bone marrow of C3H (A) and DBA (B) mice, 12 and 24 h after the last TBI treatment. Error bars indicate the standard errors of the means. Note that the marked increase in the TUNEL-positive cell ratio in the bone marrow of FLV- plus TBI-treated C3H mice peaked 12 h after the last TBI treatment. The differences were significant between the ratio of FLV- plus TBI-treated C3H mice and that of control (P < 0.01 by Student's t test), FLV-treated (P < 0.01), and TBI-treated (P < 0.01) C3H mice both 12 and 24 h after the last TBI treatment.
In contrast, the bone marrow of DBA mice treated with FLV plus TBI revealed only a slight increase in the number of apoptotic cells 12 h after TBI, as did the bone marrow of mice treated with TBI alone. At 24 h after TBI, differences in apoptotic frequency were not marked among bone marrow samples from each experimental group (Fig. 3B).
Cell typing of apoptotic cells in bone marrow of C3H mice.
To determine the cell type of apoptotic cells in the bone marrow of FLV-infected C3H mice treated with TBI, two-color fluorescence-activated cell sorter analysis was performed for cell markers and annexin V. Table 1 shows the annexin-V-positive cell ratio in the erythroid cells (TER119+ cells), a myeloid series (Gr-1+ cells) of bone marrow cells, T cells (Thy-1+ cells), and B cells (B220+ cells) in control C3H mice, mice treated with TBI alone, and FLV-infected mice treated with TBI 3 h after the last TBI treatment. First, Table 1 indicates the ratio of cell markers positive in each cell fraction in the bone marrow cells after TBI alone or FLV plus TBI treatment. The TER119+ erythroid cell ratio of C3H bone marrow exhibited a significant decrease after treatment with FLV plus TBI compared to the ratios of control mice or mice treated with TBI alone. The ratios of Gr-1+ myeloid cells and Thy-1+ T cells of C3H mice revealed relative increases after treatment with FLV plus TBI, probably due to the marked decrease of erythroid cells. In contrast, the B220+ B cells in the bone marrow of C3H mice also showed a decreased ratio after treatment with FLV plus TBI.
TABLE 1.
Effect of treatment with TBI or FLV plus TBI on the bone marrow cell subpopulation percentage and annexin-V-positive cell ratio in each cell fraction of C3H mice 3 h after TBI treatment
| Treatment | % of cells of cell fraction (phenotype) in bone marrowa
|
Annexin-V-positive cell ratio in cell fraction (phenotype)b
|
||||||
|---|---|---|---|---|---|---|---|---|
| Erythroid (TER119+) | Myeloid (Gr-1+) | T (Thy-1+) | B (B220+) | Erythroid (TER119+) | Myeloid (Gr-1+) | T (Thy-1+) | B (B220+) | |
| None (control) | 5.52 ± 0.76 | 19.2 ± 1.9 | 1.36 ± 0.30 | 12.4 ± 2.9 | 15.7 ± 3.2 | 19.5 ± 3.5 | 1.39 ± 1.20 | 2.29 ± 1.45 |
| TBI | 6.88 ± 3.2 | 18.0 ± 3.6 | 2.10 ± 0.16 | 12.2 ± 2.2 | 21.8 ± 4.7 | 26.0 ± 3.9 | 1.67 ± 0.16 | 2.15 ± 1.53 |
| FLV + TBI | 2.79 ± 0.78 | 41.9 ± 13.3 | 3.75 ± 0.85 | 8.2 ± 0.8 | 35.0 ± 1.7 | 7.1 ± 1.1 | 3.91 ± 2.04 | 4.99 ± 0.75 |
Values indicate the cell-marker-positive cell percentages in the total bone marrow cell population. Values are means ± standard deviations calculated from data for 3 mice in each experimental group. Note the significant decrease in the percentage of TER119+ erythroid cells in C3H mice treated with FLV plus TBI compared to that of the control mice (P < 0.05 by Student's t test) and mice treated with TBI alone (P < 0.05). The percentages of Gr-1+ myeloid cells and Thy-1+ T cells in the bone marrow showed relative increases after treatment with FLV plus TBI while the percentage of B220+ B cells decreased after the same treatment.
Values are means ± standard deviations. Note that TBI treatment induced a slight increase in the ratio of the annexin-V-positive cells in TER119+ erythroid cells, Gr-1+ myeloid cells, Thy-1+ T cells, and B220+ B cells. Further, TER119+ erythroid cells exhibited a prominent increase in the annexin-V-positive cell ratio after treatment with FLV plus TBI, whereas the ratio of annexin-V-positive cells in Gr-1+ myeloid cells was reduced in mice treated with FLV plus TBI. The differences were significant in TER119+ cells from control mice and mice treated with FLV plus TBI (P < 0.05), in TER119+ cells from mice treated with TBI alone and those treated with FLV plus TBI (P < 0.05), in Gr-1+ cells from control mice and those treated with TBI (P < 0.05), in Gr-1+ cells from control mice and those treated with FLV plus TBI (P < 0.01), and in Gr-1+ cells from mice treated with TBI alone and those treated with FLV plus TBI (P < 0.01) by Student's t test. Although the Thy-1+ T cells and B220+ B cells exhibited increases in annexin-V-positive cell ratio after treatment with FLV plus TBI, the differences were not significant.
Next, the annexin-V-positive apoptotic cell ratio was determined in each cell subpopulation. In erythroid cells, the annexin-V-positive cell ratio significantly increased in FLV- plus TBI-treated mice compared to the ratio of control mice as well as to the ratio of mice treated with TBI alone. In contrast, the annexin-V-positive cell ratio of the Gr-1+ cells in the FLV-plus-TBI group of mice was reduced in comparison to the control group or the group receiving TBI alone. The Thy-1+ T cells and B220+ B cells also exhibited increases in the annexin-V-positive cell ratio after treatment with FLV plus TBI, although the differences were not significant. Thus, apoptosis of bone marrow cells in C3H mice treated with FLV plus TBI occurred progressively in erythroid cells. The finding was consistent with the fact that C3H mice treated with FLV plus TBI died from severe anemia.
In addition, the majority of apoptotic cells (annexin-V-positive cells) expressed the viral gp70 antigen on the cell surface (data not shown), although it was very difficult to rule out the possibility that apoptosis also occurred in the non-FLV-infected cells.
Western blot analysis for P53 protein in bone marrow after treatment with FLV plus TBI.
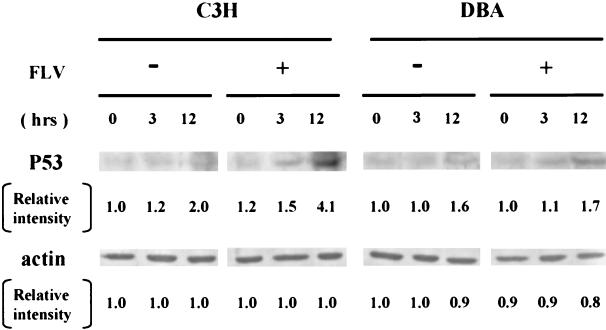
To address the molecular basis for this phenomenon, immunoblotting analysis was performed for the P53 protein in the bone marrow cells of FLV-free mice and FLV-infected mice treated with TBI. In FLV-free C3H and DBA mice, TBI treatment evoked a minimal increase in P53 protein expression 3 and 12 h after the last TBI treatment (Fig. 4). In contrast, bone marrow cells from FLV-infected C3H mice exhibited a marked accumulation of P53 protein 12 h after TBI. Although the degree was rather mild, bone marrow cells from FLV-infected DBA mice also revealed an accumulation of P53 protein 3 and 12 h after TBI, as observed in bone marrow cells from DBA mice treated with TBI alone. These findings suggested that a p53-associated signaling pathway after irradiation (21) was strongly activated in the bone marrow cells of FLV- plus TBI-treated C3H mice. Although a slight accumulation of P53 was observed in bone marrow cells from FLV- plus TBI-treated DBA mice, the amount did not appear to reach the threshold levels necessary to cause apoptosis, as these cells did not exhibit prominent apoptosis (Fig. 3B).
FIG. 4.
Western blotting for the P53 protein in the bone marrow from C3H and DBA mice 0, 3, and 12 h after the last TBI treatment. Cell lysates were prepared from the bone marrow of FLV-free or FLV-infected C3H and DBA mice. Bands for P53 protein were prominent in the sample from FLV-infected C3H mice 12 h after treatment with TBI. The relative intensity of each band was demonstrated as the ratio of the sample band intensity to the intensity of bands from FLV-free and TBI-free C3H or DBA mice. As expected, TBI induced an accumulation of P53 up to twofold in FLV-free C3H or DBA mice within 12 h of TBI. In contrast, the relative intensity in FLV- plus TBI-treated C3H mice increased to more than fourfold the intensity of control (FLV free and TBI free) mice 12 h after TBI, whereas FLV- plus TBI-treated DBA mice exhibited a pattern similar to that of DBA mice treated with TBI alone. As the bands for actin protein exhibited a similar density in each sample, levels of the amount of protein contained in cell lysates are similar in each lane. +, present; −, absent.
Effects of FLV infection and TBI on bone marrow cells from p53 knockout mice of the C3H background.
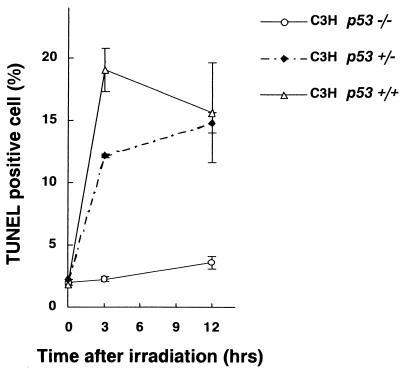
To further clarify whether the lethal anemia in FLV- plus TBI-treated C3H mice was associated with p53-dependent apoptosis of bone marrow hematopoietic cells, the apoptosis-inducing effects of FLV- plus TBI-treatment on bone marrow cells were determined in p53 knockout mice of the C3H background. Figure 5 shows the TUNEL-positive cell ratio of the bone marrow from p53−/−, p53+/−, and p53+/+ (wild type) mice of the C3H background after treatment with FLV plus TBI. In C3H p53+/+ wild-type mice and C3H p53+/− mice, the TUNEL-positive cell ratio markedly increased 3 and 12 h after treatment with TBI. The ratio in C3H p53+/− mice was a little lower than that in C3H p53+/+ wild-type mice. However, as expected, the apoptotic cell ratio was very low in the bone marrow cells of C3H p53−/− mice. These results clearly indicated that the enhanced apoptosis of C3H bone marrow cells by FLV plus TBI treatment was p53 dependent.
FIG. 5.
The TUNEL-positive cell ratio in bone marrow cells from FLV-infected C3H p53−/− mice, C3H p53+/− mice, and C3H p53+/+ wild-type mice after treatment with TBI. To simplify the effect of TBI, a single irradiation of 1.5 Gy was performed. Error bars indicate standard errors of the means calculated from the data from 3 to 5 mice at each point. Note that bone marrow cells from C3H p53−/− mice exhibited much lower values than those from C3H p53+/− mice or C3H p53+/+ mice.
Analysis of radiation bone marrow chimeric mice with bone marrow cells from p53 knockout mice of the C3H background.
To determine whether the lethality by FLV plus TBI treatment in C3H mice was p53 dependent or not, bone marrow cells from p53 knockout mice of the C3H background (C3H p53−/−) or wild-type C3H mice (C3H p53+/+) were transplanted to lethally irradiated C3H mice (C3H p53+/+ → C3H p53+/+ or C3H p53−/− → C3H p53+/+) and these mice were similarly treated with FLV and TBI.
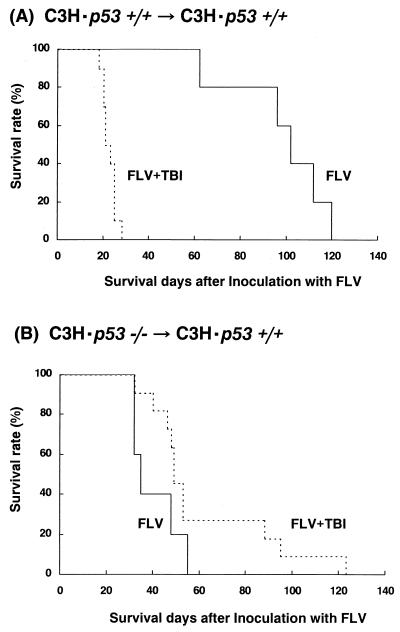
Survival curves of C3H p53+/+ → C3H p53+/+ mice treated with FLV alone or FLV plus TBI revealed results similar to those of C3H wild-type mice. Namely, those mice treated with FLV plus TBI evoked lethal anemia and died by day 30 after FLV inoculation (Fig. 6A), whereas mice treated with FLV alone lived longer and died from FLV-induced leukemia. In contrast, C3H p53−/− → C3H p53+/+ bone marrow chimeric mice treated with FLV plus TBI developed FLV-induced leukemia but lived longer than the mice treated with FLV alone (Fig. 6B). These results indicate that lethal anemia of C3H mice by FLV plus TBI treatment was also p53 dependent. The FLV-inoculated C3H p53−/− → C3H p53+/+ mice had shorter survival times than did the C3H p53+/+ → C3H p53+/+ mice, and all of them died from FLV-induced leukemia, as expected based on the recent findings that loss of p53 accelerates the progression of FLV-induced leukemogenesis (42).
FIG. 6.
Survival curves of FLV-infected C3H p53+/+ → C3H p53+/+ mice (A) and C3H p53−/− → C3H p53+/+ chimeras (B) treated with split low-dose TBI (1.5 Gy twice) (solid line) or sham TBI (dotted line).
Expression of Spi-1 and Fli-1 in the spleen and bone marrow.
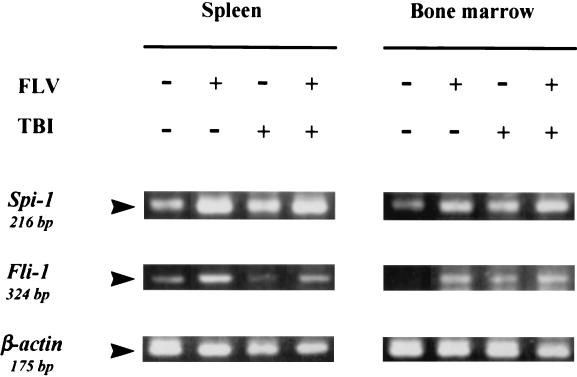
Activation of oncogenes is known to cause cellular apoptosis in several situations (9). Therefore, to eliminate the possibility that oncogene-associated apoptosis played a major role in C3H mice treated with FLV plus TBI, mRNA expression for Spi-1 and Fli-1 FLV-induced disease-specific transcription factors was detected in the spleen and bone marrow by using an RT-PCR technique. Expression of mRNA for Spi-1 and Fli-1 (Fig. 7) was slightly up-regulated in the group treated with FLV alone, as well as in the group treated with FLV plus TBI, compared to that in the group of control mice. These results confirmed that FLV infection actually occurred in the spleen and bone marrow cells in the present system, followed by the expression of FLV-specific transcription factors. A slight increase in Spi-1 and Fli-1 expression was also observed in mice treated with TBI alone, probably via nonspecific up-regulation of transcription factors due to TBI treatment. However, the difference in mRNA expression was not significant between FLV-treated and FLV- plus TBI-treated mice. Thus, in any event, the expression of these genes did not seem to regulate enhanced apoptotic signaling in FLV- plus TBI-treated mice.
FIG. 7.
RT-PCR analysis for expression of Spi-1 and Fli-1 mRNA in the spleen and bone marrow of C3H mice. RNA samples were prepared from the spleen and bone marrow of control, FLV-treated, TBI-treated, and FLV- plus TBI-treated C3H mice 12 h after the last TBI treatment. An RT-PCR technique revealed overexpression of Spi-1 and Fli-1 mRNA in FLV-infected mouse samples compared to that in the control samples. However, up-regulation was observed equally in the treatment (FLV plus TBI) or sham treatment (FLV alone) of TBI. +, present; −, absent.
DISCUSSION
The present data demonstrated a prominent enhancement of p53-associated radiation-induced apoptosis in FLV-infected C3H host cells. Frequent apoptosis of bone marrow erythroid cells occurred rapidly and severely, leading to lethal anemia in the host. It was surprising that the death of the host due to massive apoptosis of hematopoietic cells was caused by a combination of rather mild treatments for apoptosis such as low-dose TBI (34) and FLV infection. Thus, this study provides a unique model system of lethal apoptosis in vivo.
Levels of p53 increase markedly after ionizing radiation, and transmission of the DNA damage signal is associated with nuclear localization of the accumulated p53 (21). After transcriptional activation of the p53 gene, the expression of several genes is enhanced. In cases of signaling by radiation-induced apoptosis, the expression of several genes such as bax, NOXA, and PIG3 may be up-regulated to activate the final cascade of caspase networks and DNases (2, 5, 27, 29, 41). Recently, Pidd, a new death-domain containing protein, was found by using an FLV-transformed erythroleukemia cell line (24). This protein is induced by p53 activation and promotes apoptosis. Although apoptosis observed for liquid-cultured FLV-induced primary erythroleukemic cells has been reported to be p53 independent (12), the prominent apoptosis induced by FLV plus TBI treatment in the present study was associated with an overexpression of p53. And, as expected from this observation, up-regulation of a wide range of p53-associated downstream molecules was confirmed (S. Yamaguchi, M. Hasegawa, K. Hirokawa, S. Aizawa, and M. Kitagawa, unpublished data).
Several possibilities should be additionally mentioned as inducing apoptosis in C3H hematopoietic cells treated with FLV plus TBI. The first possibility is apoptosis by the withdrawal of erythropoietin (Epo), which is known to occur in erythroid progenitor cells. Radiation-induced apoptosis of erythroid cells is suppressed by treatment with Epo mediated by a Jak kinase-signaling pathway (31). Although this mechanism could serve as a cause of severe anemia in C3H mice, such a phenomenon would not occur so rapidly in vivo, as seen in the present model (3 to 6 h after TBI). In addition, levels of Epo in serum would not be markedly different between C3H and DBA mice. Further, evidence has been presented that apoptosis caused by Epo withdrawal does not have any association with conformational changes or stabilization of p53 (13). This point is also inconsistent with the present data that the P53 protein is accumulated in bone marrow cells from FLV- plus TBI-treated C3H mice. However, in any event, the influence of FLV infection on signaling after Epo-Epo receptor binding should be clarified in association with apoptotic signaling pathways.
Next, the association of the Fas-Fas ligand system should be considered. Using an lpr mouse system, Reap et al. (32) demonstrated that irradiation induced expression of Fas on C57BL/6 background cells, in contrast to several studies which suggested separate pathways for Fas-associated and radiation-induced apoptotic signaling (3, 10, 26). In the present study, the expression of Fas was not enhanced in C3H mice treated with FLV plus TBI, although TBI treatment alone induced a slight increase in the Fas expression level in bone marrow cells (data not shown). Therefore, enhanced apoptosis induced by FLV plus TBI in C3H mice may not require signals transduced by the Fas-Fas ligand system.
Oncogene-induced apoptosis has been reported in several situations (9). Activation of p53 can initiate apoptosis in response to the expression of a viral or cellular oncogene or the absence of a critical tumor suppressor gene product. The expression of the adenovirus E1A and human papilloma virus E7 proteins induces p53-mediated apoptosis (22). In FLV-induced leukemogenesis, the Spi-1 and Fli-1 genes of the host are known to be activated during oncogenesis. These molecules function as transcriptional factors in FLV-induced erythroid leukemic cells and usually inhibit apoptotic cell death in erythroblasts (28, 30). However, overexpression of Spi-1 induced growth inhibition, differentiation inhibition, and apoptotic cell death in FLV-induced erythroleukemia cells (43). As shown in the present study by RT-PCR analysis, the expression levels of mRNAs for Spi-1 and Fli-1 of bone marrow cells were similar in mice treated with FLV alone and in mice treated with FLV plus TBI. Thus, these genes may not be related to the frequent apoptosis in the bone marrow of FLV- plus TBI-treated mice.
It is important to understand why the hematopoietic cells of DBA mice escaped massive apoptosis. It has been noticed that DBA mice show immunological protection against FLV infection (36), whereas the immunological functions of C3H mice were severely deteriorated after infection with FLV (16, 17). In addition, DBA mice have a gene called Rmcf (11) which causes resistance to the replication of Friend mink cell focus-inducing virus (MCFV) (33), whereas C3H mice are susceptible to it. Therefore, the state of Friend MCFV-induced erythroid cell proliferation may differ between DBA and C3H mice. However, little is known about the strain difference in apoptotic signaling in response to DNA damage. As generally indicated in previous studies concerning radiation-induced apoptosis (29, 41), the apoptotic frequency in the bone marrow cells of DBA mice slightly increased after TBI treatment. The degree of TBI-induced apoptosis was almost the same as that in C3H mice. However, the difference was prominent when mice were preinoculated with FLV. C3H mice treated with FLV plus TBI died from apoptosis of the bone marrow cells, whereas DBA mice treated with FLV plus TBI did not show a marked increase in apoptosis compared to DBA mice treated with TBI alone. Possible interpretations of the difference between C3H mice and DBA mice may be (i) a difference in cell subpopulations of the bone marrow after FLV inoculation, possibly related to MCFV replication, which causes early erythroid cell proliferation after infection with FLV; (ii) a difference in the production of cytokines or expression of cytokine receptors in the bone marrow; (iii) a difference in the capacity of recovery from the loss of hematopoietic cells; (iv) a difference in the signaling pathway of apoptosis; or (v) a difference in the expression of FLV-reactive molecules modifying the signaling pathways of apoptosis. A more definitive answer regarding the precise mechanism of strain difference in this model will require studies in which the expression of specific molecules can be manipulated to see if the protein can affect p53-dependent apoptosis in various strains of mice.
One major difficulty in the implementation of radiotherapy is that healthy tissues are also sensitive to killing by radiation. Thus, methods that sensitize tumor cells or abnormal cells to apoptosis while sparing healthy tissues could potentially lead to greater success with radiation as a form of therapy (6, 25). The phenomenon presented here is potentially applicable to a therapeutic approach by specifically inducing apoptotic cell death in tumor cells as well as in virus-infected cells.
Acknowledgments
This work was supported in part by research grants from the National Institute of Radiological Sciences, Chiba, Japan, and by a grant-in-aid from the Ministry of Education, Science, and Culture of Japan.
We thank Kazuko Yoshida of the National Institute of Radiological Sciences for supplying the p53 knockout mice of the C3H background.
REFERENCES
- 1.Accornero, P., M. Radrizzani, A. Care, G. Mattia, C. Chiodoni, R. Kurrle, and M. P. Colombo. 1998. HIV/gp120 and PMA/ionomycin induced apoptosis but not activation induced cell death require PKC for Fas-L upregulation. FEBS Lett. 436:461-465. [DOI] [PubMed] [Google Scholar]
- 2.Attardi, L. D., E. E. Reczek, C. Cosmas, E. G. Demicco, M. E. McCurrach, S. W. Lowe, and T. Jacks. 2000. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 14:704-718. [PMC free article] [PubMed] [Google Scholar]
- 3.Belka, C., V. Heinrich, P. Marini, H. Faltin, O. K. Schulze, M. Bamberg, and W. Budach. 1999. Ionizing radiation and the activation of caspase-8 in highly apoptosis-sensitive lymphoma cells. Int. J. Radiat. Biol. 75:1257-1264. [DOI] [PubMed] [Google Scholar]
- 4.Bonzon, C., and H. Fan. 1999. Moloney murine leukemia virus-induced preleukemic thymic atrophy and enhanced thymocyte apoptosis correlate with disease pathogenicity. J. Virol. 73:2434-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvard, V., T. Zaitchouk, M. Vacher, A. Duthu, M. Canivet, C. Choisy-Rossi, M. Nieruchalski, and E. May. 2000. Tissue and cell-specific expression of the p53-target genes: bax, fas, mdm2 and waf1/p21, before and following ionizing irradiation in mice. Oncogene 19:649-660. [DOI] [PubMed] [Google Scholar]
- 6.Brown, J. M., and B. G. Wouters. 1999. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 59:1391-1399. [PubMed] [Google Scholar]
- 7.Chesebro, B., M. Miyazawa, and W. J. Britt. 1990. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu. Rev. Immmunol. 8:477-499. [DOI] [PubMed] [Google Scholar]
- 8.Del Regato, J. A. 1989. Trial of fractionated total-body irradiation in the treatment of patients with acquired immunodeficiency syndrome: a preliminary report. Am. J. Clin. Oncol. 12:365.. [DOI] [PubMed] [Google Scholar]
- 9.Evan, G., and T. Littlewood. 1998. A matter of life and cell death. Science 281:1317-1321. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs, E. J., K. A. McKenna, and A. Bedi. 1997. p53-dependent DNA damage-induced apoptosis requires Fas/APO-1-independent activation of CPP32β. Cancer Res. 57:2550-2554. [PubMed] [Google Scholar]
- 11.Hartley, J. W., R. A. Yetter, and H. C. Morse III. 1983. A mouse gene on chromosome 5 that restricts infectivity of mink cell focus-forming recombinant murine leukemia viruses. J. Exp. Med. 158:16-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard, J., Y. Ung, D. Adachi, and Y. Ben-David. 1996. p53-independent tumor growth and in vitro cell survival for F-MuLV-induced erythroleukemias. Cell Growth Differ. 7:1651-1660. [PubMed] [Google Scholar]
- 13.Kelley, L. L., W. F. Green, G. G. Hicks, M. C. Bondurant, M. J. Koury, and H. E. Ruley. 1994. Apoptosis in erythroid progenitors deprived of erythropoietin occurs during the G1 and S phases of the cell cycle without growth arrest or stabilization of wild-type p53. Mol. Cell. Biol. 14:4183-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley, L. L., G. G. Hicks, F. F. Hsieh, J. M. Prasher, W. F. Green, M. D. Miller, E. J. Eide, and H. E. Ruley. 1998. Endogenous p53 regulation and function in early stage Friend virus-induced tumor progression differs from that following DNA damage. Oncogene 17:1119-1130. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa, M., O. Matsubara, and T. Kasuga. 1983. Relation between Friend leukemia and genetic control of the host. Bull. Tokyo Med. Dent. Univ. 30:95-107. [PubMed] [Google Scholar]
- 16.Kitagawa, M., O. Matsubara, and T. Kasuga. 1986. Dynamics of lymphocytic subpopulations in Friend leukemia virus-induced leukemia. Cancer Res. 46:3034-3039. [PubMed] [Google Scholar]
- 17.Kitagawa, M., H. Kamisaku, T. Sado, and T. Kasuga. 1993. Friend leukemia virus-induced leukemogenesis in fully H-2 incompatible C57BL/6 → C3H radiation bone marrow chimeras. Leukemia 7:1041-1046. [PubMed] [Google Scholar]
- 18.Kitagawa, M., S. Aizawa, H. Kamisaku, T. Sado, H. Ikeda, and K. Hirokawa. 1996. Distribution of Fv-4 resistant gene product in Friend leukemia virus-resistant Fv-4r mouse strain. Exp. Hematol. 24:1423-1431. [PubMed] [Google Scholar]
- 19.Kitagawa, M., S. Aizawa, H. Kamisaku, K. Hirokawa, and H. Ikeda. 1999. Protection of retrovirus-induced disease by transplantation of bone marrow cells transduced with MuLV env gene via retrovirus vector. Exp. Hematol. 27:234-241. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa, M., M. Takahashi, S. Yamaguchi, M. Inoue, S. Ogawa, K. Hirokawa, and R. Kamiyama. 1999. Expression of inducible nitric oxide synthase (NOS) in bone marrow cells of myelodysplastic syndromes. Leukemia 13:699-703. [DOI] [PubMed] [Google Scholar]
- 21.Ko, L. J., and C. Prives. 1996. p53: puzzle and paradigm. Genes Dev. 10:1054-1072. [DOI] [PubMed] [Google Scholar]
- 22.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 23.Lilly, F., and T. Pincus. 1973. Genetic control of murine viral leukemogenesis. Adv. Cancer Res. 17:231-277. [Google Scholar]
- 24.Lin, Y., W. Ma, and S. Benchimol. 2000. Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nat. Genet. 26:124-127. [DOI] [PubMed] [Google Scholar]
- 25.Muschel, R. J., D. E. Soto, W. G. Mckenna, and E. J. Bernhard. 1998. Radiosensitization and apoptosis. Oncogene 17:3359-3363. [DOI] [PubMed] [Google Scholar]
- 26.Newton, K., and A. Strasser. 2000. Ionizing radiation and chemotherapeutic drugs induce apoptosis in lymphocytes in the absence of Fas or FADD/MORT1 signaling: implications for cancer therapy. J. Exp. Med. 191:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda, E., R. Ohki, H. Murasawa, J. Nemoto, T. Shibue, T. Yamashita, T. Tokino, T. Taniguchi, and N. Tanaka. 2000. Noxa, a BH3-only member of the bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053-1058. [DOI] [PubMed] [Google Scholar]
- 28.Pereira, R., C. T. Quang, I. Lesault, H. Dolznig, H. Beug, and J. Ghysdael. 1999. FLI-1 inhibits differentiation and induces proliferation of primary erythroblasts. Oncogene 18:1597-1608. [DOI] [PubMed] [Google Scholar]
- 29.Polyak, K., Y. Xia, J. L. Zweier, K. W. Kinzler, and B. Vogelstein. 1997. A model for p53-induced apoptosis. Nature 389:300-305. [DOI] [PubMed] [Google Scholar]
- 30.Quang, C. T., O. Wessely, M. Pironin, H. Beug, and J. Ghysdael. 1997. Cooperation of Spi-1/PU.1 with an activated erythropoietin receptor inhibits apoptosis and Epo-dependent differentiation in primary erythroblasts and induces their Kit ligand-dependent proliferation. EMBO J. 16:5639-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quelle, F. W., J. Wang, J. Feng, D. Wang, J. L. Cleveland, J. N. Ihle, and G. P. Zambetti. 1998. Cytokine rescue of p53-dependent apoptosis and cell cycle arrest is mediated by distinct Jak kinase signaling pathways. Genes Dev. 12:1099-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reap, E. A., K. Roof, K. Maynor, M. Borrero, J. Booker, and P. L. Cohen. 1997. Radiation and stress-induced apoptosis: a role for Fas/Fas ligand interactions. Proc. Natl. Acad. Sci. USA 94:5750-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruscetti, S., L. Davis, J. Field, A. Oliff. 1981. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J. Exp. Med. 154:907-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safwat, A. 2000. The immunobiology of low-dose total-body irradiation: more questions than answers. Radiat. Res. 153:599-604. [DOI] [PubMed] [Google Scholar]
- 35.Sevilla, L., C. Aperlo, V. Dulic, J. C. Chambard, C. Boutonnet, O. Pasquier, P. Pongnonec, and K. E. Boulukos. 1999. The ets2 transcription factor inhibits apoptosis induced by colony-stimulating factor 1 deprivation of macrophages through a bcl-xL-dependent mechanism. Mol. Cell. Biol. 19:2624-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen, R. N., N. B. Hornback, L. Lu, P. Young, Z. Brahmi, and H. E. Broxmeyer. 1988. Curative effect of split low dosage total-body irradiation on mice infected with the polycythemia-inducing strain of the Friend virus complex. Cancer Res. 48:2399-2403. [PubMed] [Google Scholar]
- 37.Shen, R. N., L. Lu, M. A. Harrington, C. Srivastava, Y. J. Kim, S. Z. Zhou, B. Wu, and S. Ruscetti. 1991. Effect of split low dose total body irradiation on SFFV mRNA, genomic DNA and protein expression in mice infected with the Friend virus complex. Leukemia 5:225-229. [PubMed] [Google Scholar]
- 38.Shen, R. N., L. Lu, H. E. Kaiser, and H. E. Broxmeyer. 1996. Curative effect of split low dosage total-body irradiation on murine AIDS induced by Friend virus: the results and the possible mechanism. In Vivo 10:191-199. [PubMed] [Google Scholar]
- 39.Starck, J., A. Doubeikovski, S. Sarrazin, C. Gonnet, G. Rao, A. Skoultchi, J. Godet, I. Dusanter-Fourt, and F. Morle. 1999. Spi-1/PU.1 is a positive regulator of the Fli-1 gene involved in inhibition of erythroid differentiation in Friend erythroleukemic cell lines. Mol. Cell. Biol. 19:121-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meinl, F. Neipel C. Mattmann, K. Burns, J. L. Bodmer, M. Schroter, C. Scaffidi, P. H. Krammer, M. E. Peter, and J. Tschopp. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517-521. [DOI] [PubMed] [Google Scholar]
- 41.Vogelstein, B., and K. W. Kinzler. 1994. X-rays strike p53 again. Nature 370:174-175. [DOI] [PubMed] [Google Scholar]
- 42.Wong, K. S., Y.-J. Li, J. Howard, and Y. Ben-David. 1999. Loss of p53 in F-MuLV induced-erythroleukemias accelerates the acquisition of mutational events that confers immortality and growth factor independence. Oncogene 18:5525-5534. [DOI] [PubMed] [Google Scholar]
- 43.Yamada, T., N. Kondoh, M. Matsumoto, M. Yoshida, A. Maekawa, and T. Oikawa. 1997. Overexpression of PU.1 induces growth and differentiation inhibition and apoptotic cell death in murine erythroleukemia cells. Blood 89:1383-1393. [PubMed] [Google Scholar]