Abstract
Phototransduction is initiated by the photoisomerization of rhodopsin (Rho) chromophore 11-cis-retinylidene to all-trans-retinylidene. Here, using Rho regenerated with retinal analogs with different ring sizes, which prevent isomerization around the C11=C12 double bond, the activation mechanism of this G-protein-coupled receptor was investigated. We demonstrate that 11-cis-7-ring-Rho does not activate G-protein in vivo and in vitro, and that it does not isomerize along other double bonds, suggesting that it fits tightly into the binding site of opsin. In contrast, bleaching 11-cis-6-ring-Rho modestly activates phototransduction in vivo and at low pH in vitro. These results reveal that partial activation is caused by isomerization along other double bonds in more rigid 6-locked retinal isomers and protonation of key residues by lowering pH in 11-cis-6-ring-Rhos. Full activation is not achieved, because isomerization does not induce a complete set of conformational rearrangements of Rho. These results with 6- and 7-ring-constrained retinoids provide new insights into Rho activation and suggest a potential use of locked retinals, particularly 11-cis-7-ring-retinal, to inactivate opsin in some retinal degeneration diseases.
In vertebrate retinal photoreceptor cells, isomerization of the visual pigment chromophore, 11-cis-retinal to all-trans-retinal, triggers a set of reactions collectively termed the phototransduction cascade (1, 2). The phototransduction events are initiated by activated rhodopsin (Rho*)1 and progress through a classical G-protein cascade, ultimately leading to neuronal signaling. Metarhodopsin II (or Meta II, Rho*), the catalytically active intermediate generated by photoisomerization of Rho chromophore, contains all-trans-retinal covalently bound to Lys296 of opsin via the deprotonated Schiff base. Subsequently, Meta II undergoes reprotonation, and the photolyzed chromophore is hydrolyzed and released from opsin (3-5). The precise mechanism of Rho activation by the photoisomerized chromophore is unknown (6).
The photobleaching process of rhodopsin has been investigated using retinal analogs that contained an extra ring between C10 and C13, making retinal non-isomerizable around the 11-cis double bond (7-12). An artificial visual pigment with restricted C9–C11 motion forms normal photolysis intermediates (13), suggesting an importance of C11=C12 bond isomerization in the activation of Rho. More recently, it was reported that after photoisomerization, the β-ionone ring of the chromophore moves to a new position during the transition to Meta II (7). Jang et al. (14) showed using 6-ring-constrained retinal isomers and the crystal structure of Rho in the ground state (15) that if this movement is restricted, only residual activity could be observed. Locked retinal analogs were also used to study visual transduction in vivo using vitamin A-deprived rats (16, 17). These animals had approximately half of the normal complement of rhodopsin, and injection of locked retinal led to the appearance of the analog pigment in the photoreceptors but without significant effect on the sensitivity of electroretinographic b-wave responses recorded from rat eye (16). Interference from wild-type rhodopsin prevented full interpretation of the results.
The light-triggered events in photoreceptors are intimately intertwined with the regeneration reactions that involve a two-cell system, photoreceptor cells and the retinal pigment epithelial cells (RPE). Every photoisomerization caused by absorption of a photon is counterbalanced by regeneration of Rho with newly synthesized 11-cis-retinal. The photoisomerized product all-trans-retinal released from Rho* is reduced to all-trans-retinol in photoreceptors and then converted back to 11-cis-retinal in the RPE in an enzymatic process referred to as the visual cycle or the retinoid cycle (reviewed in Ref. 18). Several components of the retinoid cycle have been identified, although major enzymatic and chemical transformations still remain poorly understood.
One of the proteins involved in the retinoid cycle is RPE65, a highly expressed membrane-associated (19) RPE protein with a molecular mass of 65 kDa (20). This protein appears to form a complex with 11-cis-retinol dehydrogenase (11-cis-RDH) (21, 22). The function of RPE65 is unknown, but it is believed to be involved in retinoid processing. RPE microsomes washed with high salt that removed >95% RPE65 still retained most of the isomerization activity (23). However, unexpectedly, Rpe65−/− mice had an overaccumulation of all-trans-retinyl esters in the RPE in the form of lipid-like droplets (24, 25). Further retinoid analysis revealed no detectable 11-cis-products in either ester or alcohol forms (25). Electroretinogram (ERG) measurements of Rpe65−/− mice revealed that the rod and cone functions were severely attenuated (25, 26). Small amounts of 11-cis-retinal are produced by photochemical reaction in situ in photoreceptor cells, and it was demonstrated that early intervention with cis-retinoids greatly attenuates retinyl ester accumulation (27). This animal model could be very useful for studying in vivo properties of rhodopsin regenerated with synthetic retinal analogs that undergo photoactivation processes differently from 11-cis-retinal without interference from wild-type Rho.
In this study, we provide evidence that Rho, regenerated with ring-constrained 11-cis-retinal isomers and containing a 3-carbon bridge between C10 and C13 that prevents isomerization around the C11–C12 double bond, does not undergo significant isomerization and activation in vivo or in vitro. In contrast, the bleaching of 11-cis-6-ring-Rho (2-carbon bridge) leads to isomerization along other double bonds and produces active species of Rho at low pH that trigger phototransduction events in vivo and in vitro as demonstrated by FTIR spectroscopy. These results provide new insights into Rho activation and concurrently suggest that 6- and 7-ring-constrained retinoids may be useful in retinoid therapy for retinal pathologies.
EXPERIMENTAL PROCEDURES
Synthesis of 11-cis-7-Ring-retinals—11-cis-7-ring-retinals were synthesized according to published procedures (12, 28, 29).
Photoisomerization of Rho Regenerated with 11-cis-7-Ring-retinals—Preparation of rod outer segment, opsin, Rho regeneration with retinals, and purification of Rho on a concanavalin A-Sepharose 4B column were conducted as described previously (14).
Phosphorylation of Rho Regenerated with 11-cis-Retinal and 11-cis-7-Ring-retinals—Regenerated Rho (2 mg/ml) was mixed in 100 μl of 100 mm sodium phosphate buffer, pH 7.2, containing 5 mm MgCl2, 0.5 mm [γ32P]ATP (35,000 to ∼50,000 cpm/nmol) and purified Rho kinase (∼5 μg of protein), and the assay was carried out as described previously (30). Experiments were performed in triplicate.
HPLC Activity Assay for RDH with 11-cis-7-Ring-retinal Isomers—Activities of 11-cis-RDH and photoreceptor all-trans-retinal-specific RDH (prRDH) or all-trans-RDH were assayed by monitoring the production of [15-3H]retinol isomers (reduction of retinal isomers) (31). The reaction mixture (100 μl) contained MES (final concentration, 66 mm, pH 5.5), 1 mm DTT, pro-S-[4-3H]NADH (16 μm) for purified 11-cis-RDHHis6 (0.31 μg), (31) or pro-S-[4-3H]NADPH (12 μm) for prRDH (expressed in Sf9 cells and suspended in 20 mm BTP, 1 mm dithiothreitol, 1 μm leupeptin at a 1:49 cell pellet/buffer ratio), and 2 μl of 11-cis-7-ring-retinal isomer (120 μm) substrate stock added last to initiate the reactions. The reactions were incubated at 33 °C for 10–20 min.
Lecithin:Retinol Acyltransferase (LRAT) Assay—Fresh bovine eyes were obtained from Schenk Packing Co., Inc. (Stanwood, WA). Preparation of bovine RPE microsomes was described previously (32). The microsomes were resuspended in 10 mm MOPS, 1 μm leupeptin, and 1 mm dithiothreitol to a total protein concentration of ∼5 mg/ml as determined photocolorimetrically (33). Aliquots were stored at −80 °C and were used within 1 month of preparation. To destroy endogenous retinoids, 200 μl of aliquots of RPE microsomes were irradiated in a quartz cuvette for 5 min at 0 °C using a ChromatoUVE-transilluminator (model TM-15 from UVP Inc.). All experiments were carried out under dim red light conditions. All-trans-retinol, 11-cis-retinol, and 11-cis-7-ring-retinols were dissolved in dimethylformamide to 1 mm concentration as determined spectrophotometrically. To a 1.5-ml polypropylene tube containing 130 μl of 10 mm BTP, pH 7.4, 20 μl of 10% bovine serum albumin, and 10 μl of 10 mm ATP (in 10 mm BTP, pH 7.4) was added 20 μl of UV-treated bovine RPE microsomes (∼100 μg of total protein). 2 μl of 1 mm dimethylformamide solution of 11-cis-7-ring-retinol then was added to the mixture and incubated at 37 °C for the indicated times. The reactions were quenched by the addition of 300 μl of MeOH and 300 μl of hexane. Retinoids were extracted by vigorous shaking on a vortex for 5 min and then centrifuged at 14,000 rpm for 4 min to separate hexane and aqueous layers. The hexane extract (100 μl) was analyzed by a normal phase HPLC (4% ethyl acetate/hexane). The experiments were performed in duplicate, and the amount of retinoids was normalized.
LRAT Inhibition Assay—The assay was performed as described above, but after preincubation with 11-cis-7-ring-retinols for 15 min at 37 °C, 2 μl of 1 mm solution of all-trans-retinol or 11-cis-retinol was added, and reactions were incubated for an additional 10 min. For control, the reactions were preincubated with 2 μl of dimethylformamide without 11-cis-7-ring-retinols.
FTIR Spectroscopy—Opsin membranes (24 μm) (34) were incubated overnight with 240 μm 11-cis-retinal or with the mixture of either 11-cis-6-ring- or 11-cis-7-ring-retinal isomers in the BTP buffer (20 mm BTP, pH 7.5, containing 1 mm MgCl2 and 130 mm NaCl). Suspensions of membranes were centrifuged for 25 min at 100,000 × g and resuspended in the BTP buffer to yield 2.2 mm Rho (from absorption at 500 nm). For the measurements with the high affinity analog of transducin (Gt)α-(340–350), 8 mm VLEDLKSCGLF was used. For H2O/D2O exchange, the pellet was resuspended three times in deuterated buffer. The buffer solution was removed, and the pellet transferred to a 30-mm-diameter temperature-controlled transmission cell with two BaF2 windows and a 3-μm polytetrafluoroethylen gasket. The spectra were recorded using a Bruker ifs 66-V spectrometer (35). For all samples, Meta II minus Rho difference spectra were produced.
Kinetic Light Scattering—Light-scattering changes were measured as previously described (36). Measurements were performed in 10-mm path cuvettes with 300-μl volumes in isotonic buffer (20 mm BTP, 130 mm NaCl, and 1 mm MgCl2, pH 6.4, as indicated in the Fig. 5) at 22 °C with a 5-ms dwell time of the A/D converter (Nicolet 400, Madison, WI). Samples contained membrane suspension (3 μm Rho) reconstituted with purified Gt (0.8 μm) and 1 mm GTP. Reactions were triggered by flash photolysis of Rho with a green (500 ± 20 nm) flash attenuated by appropriate neutral density filters. The flash intensity was quantified photometrically by the amount of Rho bleached and expressed in terms of the mole fraction of photoexcited Rho (Rho*/Rho).
Fig.5.
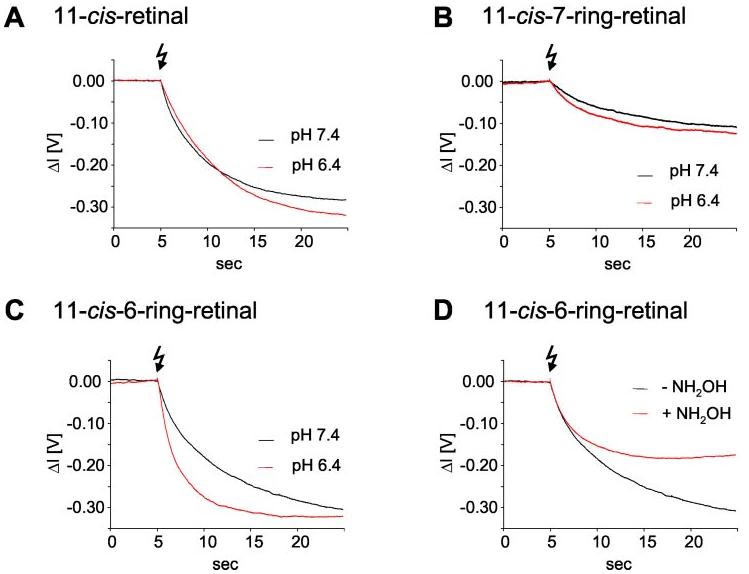
Dissociation of Gt in the presence of GTP as measured using light-scattering methods. A, the dissociation signal of the native sample at pH 7.4 and 6.4 evoked by a dim flash (Rho*/Rho = 2.4 × 10−4). These data show that according to the well known pH/rate profile at pH 7.4, a higher activity of receptor is observed compared with pH 6.4. B and C, the dissociation signal of the photoproduct of Rho regenerated with the 11-cis-7-ring (isomer 1) (B)or11-cis-6-ring (C) analogs at pH 6.4 and 7.4, respectively, evoked by a bright flash (1350-fold intensity as compared with A). D, sensitivity of 11-cis-6-ring-Rho to NH2OH (2.5 mm) at pH 7.4.
Animals—All animal experiments employed procedures approved by the University of Washington Animal Care Committee and conformed to recommendations of the American Veterinary Medical Association Panel on Euthanasia. All animals were maintained in complete darkness, and all manipulations were done under dim red light employing a Kodak No. 1 Safelight filter (transmittance >560 nm). Typically, 2–3-month-old mice were used in all experiments. RPE65-deficient mice were obtained from M. Redmond (National Eye Institute, National Institutes of Health, Bethesda, MD) and genotyped as described previously (24, 37).
Analyses of Retinoids and Visual Pigments—All procedures were performed under dim red light as described previously (25, 38, 39).
ERGs—Mice were anesthetized by intraperitoneal injection with 15 μl/g body weight of 6 mg/ml ketamine and 0.44 mg/ml xylazine diluted with 10 mm phosphate buffer, pH 7.2, containing 100 mm NaCl. The pupils were dilated with 1% tropicamide. A contact lens electrode was placed on the eye with a drop of methylcellulose, and a ground electrode (a reference electrode) was placed in the ear. ERGs were recorded with the universal testing and electrophysiologic system 3000 (UTAS E-3000) (LKC Technologies, Inc.). The mice were placed in a Ganzfield chamber, and responses to flash stimuli were obtained from both eyes simultaneously. Flash stimuli had a range of intensities (0.00020 – 41 candela s/m2), and white light flash duration was 10 ms. 2–4 recordings were made with >10-s intervals. Typically 4–8 animals were used for recording of each point in all conditions. All ERG measurements were done within 10–40 min after anesthesia.
Immunocytochemistry—The section preparation and immunolabeling using anti-phosphorylated Rho antibody, A11–82P (a generous gift from P. Hargrave), were carried out as described previously (27).
Modeling—Coordinates for bovine Rho were taken from the Protein Data Bank (1HZX). An addition of hydrogen atoms and all optimizations were done in Insight II (InsightII release 2000, Accelrys Inc, San Diego, CA) as described previously (14).
RESULTS
Synthesis of 11-cis-7-Ring-retinals and Modeling of the Active Site of Rho—The total synthesis of 11-cis-locked retinal analog incorporating a 7-membered ring was recently reported (28). We followed this method with modifications to synthesize 11-cis-7-ring-retinals (Fig. 1A). The compound was prepared as a mixture of four isomers (Fig. 1B). These isomers are well separated by normal phase HPLC and have UV-visible and 1HNMR spectra identical to those described earlier under “Experimental Procedures” (29, 40). The conformation of isomer 3, 11-cis-7-ring-retinal, overlaps to a high degree with 11-cis-retinal (Fig. 1C). All isomers fit into the binding site of Rho as demonstrated by molecular modeling using the x-ray structure of Rho (15, 41) and energy minimization algorithms (Fig. 1D).
Fig. 1.
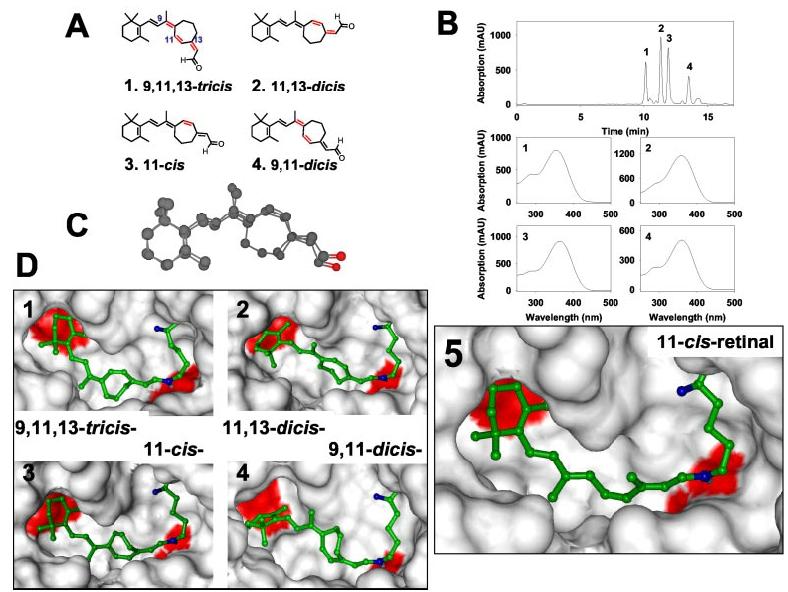
Chemical structure and HPLC separation of 11-cis-7-ring-retinal isomers and modeling these isomers within the binding site of Rho. A, chemical structures of the four 11-cis-7-ring-retinal isomers (cis-bonds are shown in red). B, chromatographic separation of the isomeric mixture of 11-cis-7-ring-retinal isomers using 2% ethyl acetate in hexane and normal phase HPLC on a silica column. Below are online UV-visible spectra of the isomers. C, overlapped structures of 11-cis-retinal and 11-cis-7-ring-retinal. Steric energies of the molecules were minimized by the Molecular Mechanics 2 method. D, 11-cis-7-Ring-retinal isomers and 11-cis-retinal in the binding pocket of Rho. In blue are nitrogen atoms of the peptide bond and a Schiff base with its hydrogen between Lys296 and the retinal. In red are two acid residues in the binding site, Glu113 and Glu122 close to the β-ionone ring. Some residues (268–269 and 292–295) were deleted to better see the retinal inside the binding pocket.
Susceptibility of 11-cis-7-Lock-Rho to Isomerization, Reduction, and Esterification—11-cis-7-Ring-retinals are more stable to thermal isomerization compared with 6-ring isomers. Bleaching these 7-ring-retinoids in solution produces a mixture of isomers with the least abundant isomer being 9,11,13-tricis-retinal 1 (Fig. 2A). Rho regenerated with these isomers was purified using concanavalin A column chromatography (Fig. 2B). When bound to opsin, 11-cis-7-ring-retinal (isomer 3) appears to be most stable to isomerization (Fig. 2C), whereas 11,13-dicis isomer 2 converts readily to isomer 3 even in the dark, suggesting that opsin promotes this isomerization. Overall, 7-ring-containing retinoids appear to be more stable in all conditions and undergo interconversion to a lower degree compared with 6-ring-containing retinals (14). With the exception of the tricis isomer 1, they are poor substrates for 11-cis-RDH. They are utilized by prRDH without discrimination among different isomers. These data again are different from 6-ring-retinoids (14). The activities of both dehydrogenases for the best substrates are only approximately one-tenth of that of native retinals (data not shown), suggesting that these substrates will be poorly utilized by dehydrogenases endogenous to the visual system. These retinoids are also poor substrates for LRAT, and only a fraction can be esterified (Fig. 3). 11-cis-7-Ring-retinals are ineffective inhibitors of LRAT when assayed in the presence of all-trans-retinol or 11-cis-retinol as substrates (data not shown). Our studies are consistent with the high specificity of LRAT observed earlier (42).
Fig. 2.
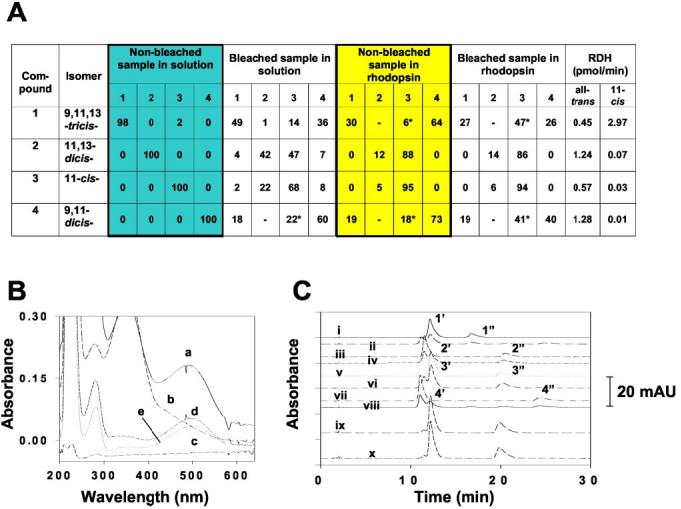
Photosensitivity of 11-cis-7-ring-retinal isomers and substrate specificity of eye-specific RDHs. A, light sensitivity of 11-cis-7-ring-retinals and 11-cis-7-ring-Rho. The bleaching experiments were carried out as described under “Experimental Procedures.” The conditions for oxime formation from each isomer are described in C. Activities of 11-cis-RDH (detergent-purified human recombinant 11-cis-RDHHis6) and all-trans-RDH (prRDH expressed in Sf9 cells) were determined by monitoring the production of the corresponding [15-3H]retinol analog from the reduction of the 11-cis-ring-retinal isomer and pro-S-[4-3H]NADH (for 11-cis-RDH) or pro-S-[4-3H]NADPH (for prRDH) (31) as described under “Experimental Procedures.” The product was analyzed by normal phase HPLC, collected, and quantified by scintillation counting. B, the purification of 11-cis-ring-Rho isomers was monitored by UV spectroscopy in each step. Trace a, the 71,700 × g supernatant of 11-cis-ring-Rho isomer 3 (solubilized by 10 mm n-dodecyl-β-d-maltoside); trace b, the flow-through fraction after the supernatant passed through a concanavalin A-Sepharose 4B column (see “Experimental Procedures”); trace c, the fraction after extensive wash of the concanavalin A-Sepharose 4B column; trace d, the purified 11-cis-7-ring-Rho isomer 3; and trace e, the photobleached 11-cis-7-ring-Rho isomer 3. C, normal phase HPLC analysis of oxime derivatives of 11-cis-7-ring-retinal isomers 1–4 in solution (HPLC traces i–viii, 1′and 1″:11-cis-7-ring-retinal isomer 1 oximes, syn and anti, respectively; 2′and 2″:11-7-cis-ring-retinal isomer 2 oximes, syn and anti, respectively; 3′and 3″:11-cis-7-ring-retinal isomer 3 oxime, syn and anti, respectively; and 4′and 4″:11-cis-7-ring-retinal isomer 4 oximes, syn and anti, respectively) and in Rho 3 (ix and x) without (i, iii, v, vii, and ix) or with (ii, iv, vi, viii, and x) photobleaching. The 11-cis-7-ring-Rho was solubilized with n-dodecyl-β-d-maltoside and purified over a concanavalin A-Sepharose 4B column. The purified fraction was subjected to photobleaching, and the chromophore(s) was derivatized with hydroxylamine and analyzed by HPLC as described under “Experimental Procedures.” As controls, isomers 1–4 were also derivatized in the same elution buffer with or without photobleaching. *, contains minor amounts of compound 2 because of the unresolved peaks between compounds 2 and 3; mAU, milliabsorption units.
Fig.3.
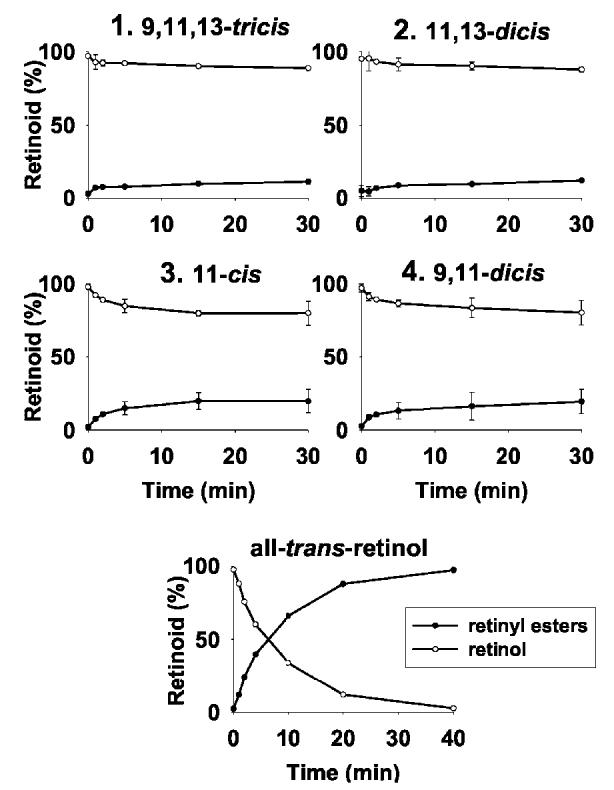
LRAT activity toward different retinoids. Time course of LRAT activity with four 11-cis-ring-7-ring-retinol isomers, an average of two independent experiments. Below is LRAT activity with all-trans-retinol, the native substrate for LRAT. Assays were performed as described under “Experimental Procedures.”
Activity of 11-cis-7-Ring-Rho in Vitro—To assess the light-induced transformation in Rho regenerated with 11-cis-7-ring-retinals, FTIR spectroscopy was employed to monitor spectral changes characteristic to different regions of opsin. Upon photoactivation, Rho regenerated with isomers 1 and 4 yielded only minor changes in the difference spectra (Fig. 4, A and B) compared with wild-type Rho, whereas isomers 2 and 3 were inactive (Fig. 4B). These changes reflect isomerization around other double bonds, excluding the locked C11=C12 double bond because the 11-cis-bond is locked. All of these spectra were pH-insensitive (data not shown). FTIR reveals that the chromophore is changing its geometry upon bleaching, but the movements of the chromophore do not cause significant changes in hydrogen bonding or in protonation states of carboxylic acids of Rho.
Fig.4.
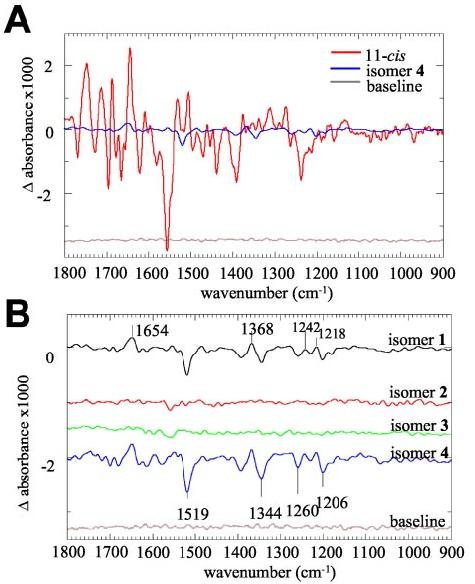
FTIR difference spectrum of Rho regenerated with 11-cis-retinals and 11-cis-7-ring-retinals. A, FTIR difference spectrum of Rho regenerated with 11-cis-retinals (red line) and regenerated with 9,11-dicis-7-ring-retinal (blue line). This figure compares the intensities of the bands caused by illumination of the dark-adapted products. B, the difference spectra between dark-adapted and bleached Rho regenerated with the four 7-locked isomers at pH 5.5 are shown with extended scale. Black line, Rho regenerated with isomer 1 (9,11,13-tricis); red line, isomer 2 (11,13-dicis); green line, isomer 3 (11-cis); and blue line, isomer 4 (9,11-dicis).
Consistent with the spectral data, light-scattering changes as a monitor of Gt activation yielded exceedingly low but measurable pH-independent activity (Fig. 5A).
In Vivo Regeneration of Rho with 6- and 7-Ring Isomers—To produce Rho regenerated with retinoid analogs for in vivo studies, we used Rpe65 mice generated by Redmond et al. (24). These mice are unable to produce substantial amounts of 11-cis-retinal (25). Rho in Rpe65+/+ mice has a chromophore that is light-sensitive (Fig. 6A). However, Rho regenerated with 11-cis-7-lock-retinals in vivo employing Rpe65−/− mice (Fig. 6B) produced light-insensitive Rho that could be detected in the difference spectra by the addition of 1% SDS (data not shown). The retinoid analysis revealed the presence of the expected amount of visual pigment (Fig. 6C). Although the injection of the mixture of 7-ring isomers yielded only one isomeric product (11-cis-7-ring-retinal, isomer 3), the mixture of 6-ring-containing retinal produced three isomers as could be predicted from previous work (14). These results suggest that opsin is readily and preferentially regenerated in vivo with 11-cis-7-ring-retinal, which mimics the structure of 11-cis-retinal (Fig. 1C).
Fig.6.
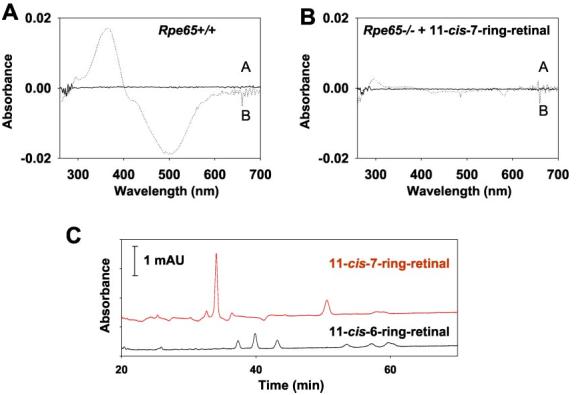
Analysis of Rho and retinoids in Rpe65 mice. A, difference spectra between unbleached (trace A) and bleached (trace B) Rpe65+/+ mouse Rho. B, similar spectra taken from Rpe65−/− mouse treated by intraocular injection of 1 μg of 11-cis-7-ring-retinal 3. C, HPLC retinoid analysis of Rpe65−/− mice treated with 11-cis-7-ring-retinal (top, red) and 11-cis-6-ring-retinal (bottom, black). mAU, milliabsorption unit.
Lack of Activity for 11-cis-7-Ring-Rho in Vivo—To increase the sensitivity of the assay and to assess the properties of Rho regenerated with 7-lock isomers in vivo, we employed Rpe65−/− mice treated with isomer 3. The activity (a- and b-wave) was unaffected by the treatment as compared with samples with only Me2SO. In contrast, in a positive control experiment, 9-cis-retinal significantly increased sensitivity of treated mice, even at low light intensities (Fig. 7). Furthermore, 11-cis-7-ring-Rho was inactive in vitro in the phosphorylation assay using purified Rho kinase (Fig. 8).
Fig.7.
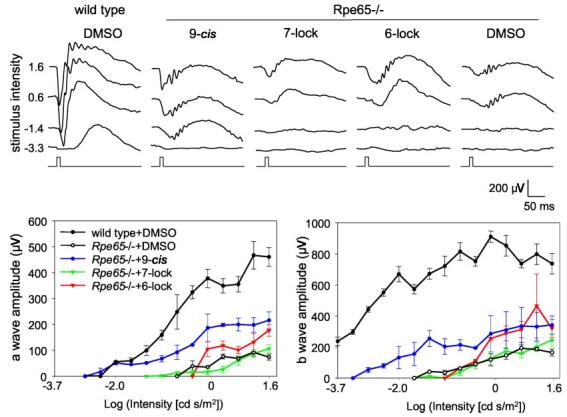
ERG flash responses in RPE 65−/− mice injected intraocularly with retinal analogs. The responses to flash stimuli were obtained with a range of intensities (0.00020 − 41 candela (cd) s/m2) in the dark from RPE 65−/− mice or wild-type mice treated with retinal analogs (upper panel). The amplitudes of a-waves (lower left panel) and b-waves (lower right panel) from these treated mice at different intensities were plotted. Five mice were used for each experiment. The difference between Me2SO (DMSO) and 11-cis-6-ring-retinal yielded p < 0.03.
Fig.8.
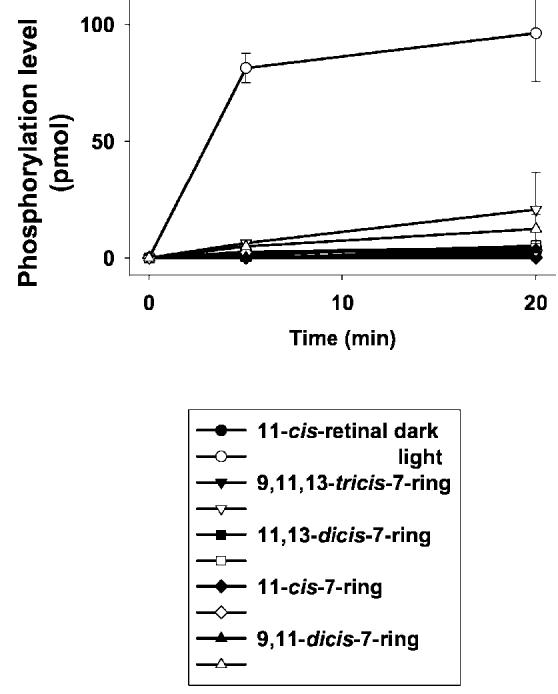
Phosphorylation of Rho in vitro. Phosphorylation of in vitro Rho was regenerated with retinal analogs and phosphorylated by purified rhodopsin kinase under light (open symbols) or in the dark (closed symbols) (see “Experimental Procedures”). Phosphorylation of Rho was measured at 0, 5, and 20 min after incubation, and phosphorylation levels (pmol/min) were plotted, respectively.
In summary, our results demonstrate that 7-ring-retinal produces Rho, which for the most part is inactive in all tested conditions. This finding was confirmed using complementary methods of different sensitivities in vivo and in vitro such as FTIR, ERG, Gt activation, and phosphorylation as detection assays.
11-cis-6-Ring-Rho Is Active in Vivo and in Vitro—Surprisingly, Rho regenerated in vivo with 6-ring-containing retinal is active at higher bleaches. The a- and b-waves are clearly elevated compared with the Me2SO control (Fig. 7). This result is consistent with the minor activity of Rho as previously measured (8, 9, 14). The relative activity of Rho and the pigments with locked analogs is compared in Fig. 5. With membranes containing Rho regenerated with 6-locked analogs, a 1350-fold intensity of the activating light flash is needed to evoke Gt activation rates comparable to wild-type rhodopsin. Consistent with the pH dependence observed in the FTIR spectra (Fig. 9C), the activity is enhanced at acidic pH (Fig. 5C). This is in contrast to the well known pH/rate profile of native Rho (higher activity at pH 7.4 as compared with pH 6.4) (Fig. 5A). Besides the mechanistic implications of this result (see “Discussion”), these data allow the exclusion of the idea that the activity of the locked analogs is merely because of trace amounts of endogenous 11-cis-retinal. Moreover, the activity of Rho regenerated with 6-locked analogs is sensitive to hydroxylamine (Fig. 5D), indicating a similar “open” conformation of the light-activated photo-product as compared with native Rho*. Consistent with the findings in vivo (Fig. 7), the activity of 11-cis-6-ring-Rho is markedly higher than that of the 7-locked pigment (Fig. 5B).
Fig.9.
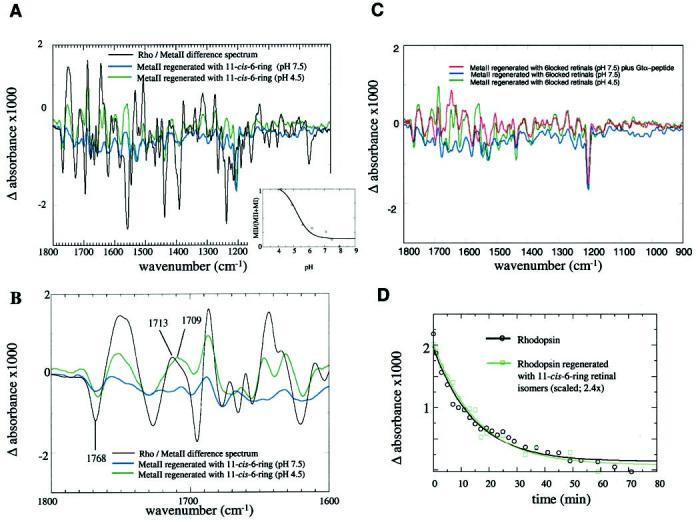
FTIR difference spectrum of Rho regenerated with 11-cis-retinals and 11-cis-6-ring-retinals. A, comparison of the FTIR Rho/Meta II difference spectra of Rho regenerated with 11-cis-retinal (native sample, black line) and with Rho regenerated with 11-cis-6-ring-retinals at pH 7.5 (blue line) and at pH 4.8 (green line); Inset, the relative amount of the photoproduct formed from 11-cis-6-ring-Rho as a function of pH. A hyperbolic fit to the data yields a titration curve with an apparent pKa of 5.4. B, the extended FTIR spectra in the region of 1800–1600 cm−1 with the same color code used in Fig. 8A. The pH dependence of the bands indicates structural changes of Rho. C, stabilization of a Meta II-like photoproduct by interaction with a Gtα C-terminal derived peptide, Rho regenerated with 11-cis-6-ring-retinals at pH 7.5 (blue line), pH 4.5 (green), and pH 7.5 in the presence of 8 mm Gtα high affinity peptide. D, comparison of the time course of the decay of Meta II (11-cis-retinal, black line) and the Meta II-like product regenerated with 6-ring isomers (green line). The decrease of the 1768/1751 cm−1 difference band was used to follow the decay. The flash was applied at time zero, and the measurements were done at pH 5.0 at 22 °C every 2 min up to a decay time of 20 min and then in longer time intervals. The half-width times for the decay of Meta II and the Meta II with 6-ring isomers are similar.
The FTIR spectra indicate different protein-chromophore interactions of the ground state of 11-cis-6-ring-Rho compared with the bleached sample (Fig. 9). At pH 7.5, the change in chromophore-protein interaction, indicated by a band at 1206 cm−1, did not lead to significant changes in the protein, and only residual activity could be detected (14). However, at pH 4.5, the same movements led to reorientation of hydrogen bonds and changes in secondary structure, forming a Meta II-like product (Fig. 9, A and B) that is able to bind Gtα-(340–350)-derived peptide (Fig. 9C). This finding suggests that pH induces structural changes in opsin that render possible the interaction of the chromophore with the protein environment in the binding site. The pKa for this change is 5.4 (Fig. 9B, inset) and the Meta II-like structure decays with a half-width time comparable to Meta II regenerated with 11-cis-retinal (Fig. 9D). A band at 1713 cm−1 in the Rho/Meta II difference spectrum assigned to the protonation of Glu 113 (43) appears to be shifted to 1708 cm−1 in the Meta II-like product regenerated with 11-cis-6-ring isomer at pH 4.5. In contrast to the Meta II band at 1713 cm−1, this band is not shifted significantly when the sample is treated with D2O,
but the bond shape has slightly changed (Fig. 10, A and B). Interestingly, this band is still observed in the Meta II-like photoproduct of the E113Q mutant of Rho regenerated with 11-cis-6-ring isomer (data not shown).
Fig. 10.
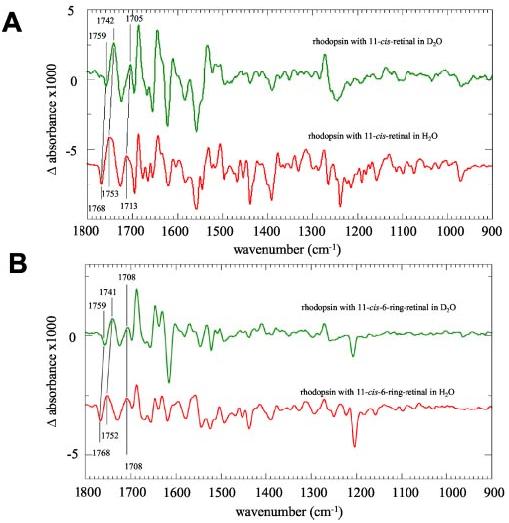
FTIR difference spectrum of Rho regenerated with 11-cis-retinals and 11-cis-6-ring-retinals in H2O and D2O. A, Rho/Meta II difference spectra in H2O(red line) and D2O(green line. In the deuterated sample, several bands were assigned to changes in hydrogen bonding and changes of the protonation state by the shift toward smaller wave numbers because of deuteration. B, the difference spectra (photoproduct minus dark-adapted) of Rho regenerated with 11-cis-6-ring-retinals in H2O(red line) and D2O(green line). As in the spectra of the native sample, there is a deuterium-induced shift of the 1768/1752 cm−1 doublet assigned to changes in hydrogen bonding of protonated carboxyl groups. The band at 1708 cm−1, however, is not shifted toward smaller wave numbers, indicating an important difference between the active Meta II-like product with 11-cis-6-ring-retinal and Meta II with the native chromophore (see “Results”).
DISCUSSION
The results of this study lead to conclusions on two different although related topics, namely the mechanism of activation of Rho and the utilization of the retinoid analogs in vivo.
Rho Activation: New Lessons Learned from the Studies of Retinoid Analogs—This study revealed new important information on the activation process. We have found three sharply distinct classes of the chromophore-protein interaction for 11-cis-7-ring- and 11-cis-6-ring-containing retinals and 11-cis-retinal. 1) Rho regenerated with 11-cis-7-ring isomer has only 0.1% of wild-type activity (Fig. 5); it is also inactive in both sensitive ERG (Fig. 7) and FTIR experiments (Fig. 4). This low activity could be a result of the presence of a small amount of free opsin and consistent with the estimated activation ratio of free opsin:Rho*, i.e. 106:1 (44). Therefore, it appears that 11-cis-7-ring-retinal is stabilized by the opsin binding pocket, forming stable 11-cis-7-ring-Rho. The bleaching of 9,11-dicis and 9,11,13-tricis results in a conversion to the most stable isomer, isomer 3, and FTIR spectra that are consistent with the lack of Gt activation. 2) In contrast, the possible movements of 11-cis-6-ring-retinal along C9=C10 and C13=C14 double bonds are sufficient to overcome the trigger barrier in the activation of Rho. This activity can be clearly detected in vivo (Fig. 7) and in the light-scattering assays (Fig. 5), advancing previous measurements using nucleotide uptake and phosphorylation assays (8, 9, 14). This active state resembles Meta II in its sensitivity to hydroxylamine, in features of the FTIR spectrum, and in its interaction with Gt peptide. However, the bleaching of 11-cis-6-ring-Rho leads to a Gt activation that is pH-dependent, whereas Meta II has a broad high activity over a wide range of pH values. This result suggests that isomerization along C9–C10 and C13–C14 causes sufficient relaxation of Rho around the chromophore to allow activation as was observed for chromophore-free opsin. However, the activation of the Rho is not achieved with proper “forced” protonation of key residues (6). The full activation occurs only when all-trans-retinylidene assumes the most extended conformation and that the β-ionone ring of the chromophore can act on its protein environment, possibly by moving to a new position as proposed previously (7, 14). Although the mechanism of activation is still unclear, we suspect a profound relationship with another surprising observation, namely that the FTIR spectrum indicated an active species, whereas a spectral motif indicating protonation of the counterion Glu113 and salt bridge breaking was altered in its spectral properties.
The most obvious explanation for the pH-dependent activity would be that H+ adjusts the retinal binding site in a way that is partially photoactivatable and resembles a state generated during photoactivation of wild-type Rho. Another non-exclusive explanation is that the restricted photochemistry of the 6-ring-retinals is sufficient to partially remove their reverse agonist-like property in a way that eventually allows protonation of key residue(s) (including Glu134) and the formation of the active conformation (similar to the opsin ↔ opsin* equilibrium (45)). 3) In native Meta II, the energy content is high enough to force the receptor into the active conformation, even at neutral and basic pH values. The apparent pKa of the light-induced active species of 11-cis-6-ring-Rho is 5.4, only one pH unit lower and thus ∼1.5 kcal off the pKa of a free Glu residue, whereas the protonated species of Meta II has pKa of 6.7 (46 - 48). Energy is required to protonate a residue at a pH higher than its native pKa of the observed active species in native and 11-cis-6-ring-retinal-regenerated Rho (49).
In conclusion, it appears that the isomerization of retinal in the Rho binding pocket of native or 6-locked retinals leads to conformational changes of the protein that allow coupling with Gt. Interestingly, this property is specific and even distinguishes between closely similar 6-ring- and 7-ring-containing retinals. This difference probably resides in the conformation of both retinals in the active site, their rigid nature imposed by the ring, and in the susceptibility of 6-ring-containing retinal to isomerization.
Use of 6- and 7-Ring-containing Analogs in Leber Congenital Amaurosis—Mutations in the RPE65 gene have been identified in patients diagnosed with Leber congenital amaurosis (LCA) (50, 51), an autosomal recessive childhood-onset severe retinal dystrophy (52), and autosomal recessive retinitis pigmentosa (53). LCA is characterized by congenital blindness or by poor central vision, slight fundus changes, nearly absent electroretinogram signal, nystagmus, reduced papillary reactions, occasional photophobia (54), eventual pigmentary degeneration of the retina, the absence of rod photoreceptors, remnants of cones, clumping of pigment in RPE, and an absence of chorioretinal adhesions (50, 55). The genetic abnormalities of LCA involve genes from different physiological pathways (56), and RPE65 gene mutations account for ∼12% of all LCA cases (57, 58).
Several therapeutical approaches to treating LCA have been proposed. These methods include RPE transplantation, gene replacement therapy, and pharmacological intervention. To date, most experimental therapeutical interventions for inherited degenerations in animals are aimed to slow down the progression of degeneration. Encouragingly, the block in the retinoid cycle caused by RPE65 gene mutations may be overcome pharmacologically by the oral addition of 9-cis-retinal, thereby creating iso-Rho (25). Within 48 h after cis-retinoid administration, rod photopigment was formed and rod physiology was improved dramatically, thus demonstrating that pharmacological intervention has the potential to restore vision when RPE65 is not present. The long term study on the effectiveness of the 9-cis-retinal intervention on restoration of visual function further lends support to this idea (27). The defect has also been corrected by gene therapy, which restored vision in a canine model of LCA using recombinant adeno-associated virus carrying wild-type RPE65 (59). This important study indicated that visual function was restored in this large animal model of childhood blindness.
Rods and cones are critical neurons in the vertebrate retina that translate a light signal into a chain of biochemical events (1, 18). There is a strong interdependency for the survival of cones during degenerative processes that affect rods (reviewed in Ref. 60), and gap junctions between both photoreceptors were implicated in this process (61). Blocking signal transduction of rods and sparing their demise at the cellular level, even if they are inactive in phototransduction, would prolong the survival of cones. The mouse model of LCA is useful in studying this relationship. Is it possible to inactivate rods but still have cone function? Although these experiments are outside the scope of the current studies, some predictions are worth considering. From a practical point of view, Rho binds 11-cis-retinal irreversibly (62). In contrast, an exchange of chromophore within the binding site of cone readily occurs (63). Therefore, a combination of 9-cis-retinal (25, 27) with locked photostable retinoids could be an alternative approach for restoring and maintaining useful vision in LCA patients. This notion is consistent with the observation of phosphorylation of Rho in Rpe65−/− mice when treatment with 7-locked retinals eliminated constitutive activity but not light-induced phosphorylation. These results suggest that all-trans-retinal coupled to a fraction of opsin (27) is not competed out by exogenous locked retinal. 7-Ring-containing isomers are poor substrates for RDH and for LRAT. This property may reduce the amount of these retinoids stored in the RPE in the form of retinyl esters, because they need to be reduced prior to esterification. One may take advantage of this property by not overloading this RPE system with 7-ring-containing retinoids before 9-cis-retinal is provided. Interestingly, 11-cis-6-ring-Rho has residual light-sensitive activity in vivo that can be a part of light-driven responses. This work provides the first step in understanding the chemistry and enzymology of these retinoids in vivo and in vitro toward achievable although difficult steps in restoring vision or preventing degeneration in otherwise incurable blinding diseases.
Acknowledgments
We thank Dr. P. Henklein for providing the peptide. We are grateful to Yunie Kim for help with the manuscript preparation. Computational tasks were partly done in the ICM Computer Center (University of Warsaw, Warsaw, Poland).
Footnotes
This research was supported by National Institutes of Health Grants EY09339 and EY13385 (to K. P.), a grant from Research to Prevent Blindness, Inc. (RPB) (to the Dept. of Ophthalmology at the University of Washington), and grants from the Foundation Fighting Blindness, Inc., the Ruth and Milton Steinbach Fund, the E. K. Bishop Foundation, and by a grant from the Deutsche Forschungsgemeinschaft (to K. P. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: Rho*, photoactivated rhodopsin; Rho, rhodopsin; Meta II, metarhodopsin II; RPE, retinal pigment epithelium; RDH, retinol dehydrogenase; ERG, electroretinogram; prRDH, all-trans-retinal-specific RDH; LRAT, lecithin:retinol acyltransferase; Gt, transducin; LCA, Leber congenital amaurosis; MOPS, 3-(N-morpholino)propanesulfonic acid; HPLC, high pressure liquid chromatography; BTP, 1,3-bis[tris(hydroxymethyl)-methylamino]propane; MES, 2-(N-morpholino)ethanesulfonic acid.
REFERENCES
- 1.Polans A, Baehr W, Palczewski K. Trends Neurosci. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- 2.Pugh EN, Jr., Nikonov S, Lamb TD. Curr. Opin. Neurobiol. 1999;9:410–418. doi: 10.1016/S0959-4388(99)80062-2. [DOI] [PubMed] [Google Scholar]
- 3.Matthews RG, Hubbard R, Brown PK, Wald G. J. Gen. Physiol. 1963;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wald G. Science. 1968;162:230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- 5.Baumann C. J. Physiol. (Lond.) 1972;222:643–663. doi: 10.1113/jphysiol.1972.sp009819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada T, Ernst OP, Palczewski K, Hofmann KP. Trends Biochem. Sci. 2001;26:318–324. doi: 10.1016/s0968-0004(01)01799-6. [DOI] [PubMed] [Google Scholar]
- 7.Borhan B, Souto ML, Imai H, Shichida Y, Nakanishi K. Science. 2000;288:2209–2212. doi: 10.1126/science.288.5474.2209. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya S, Ridge KD, Knox BE, Khorana HG. J. Biol. Chem. 1992;267:6763–6769. [PubMed] [Google Scholar]
- 9.Ridge KD, Bhattacharya S, Nakayama TA, Khorana HG. J. Biol. Chem. 1992;267:6770–6775. [PubMed] [Google Scholar]
- 10.Kandori H, Matuoka S, Shichida Y, Yoshizawa T, Ito M, Tsukida K, Balogh-Nair V, Nakanishi K. Biochemistry. 1989;28:6460–6467. doi: 10.1021/bi00441a045. [DOI] [PubMed] [Google Scholar]
- 11.Hu S, Franklin PJ, Wang J, Ruiz Silva BE, Derguini F, Nakanishi K, Chen AH. Biochemistry. 1994;33:408–416. doi: 10.1021/bi00168a004. [DOI] [PubMed] [Google Scholar]
- 12.Akita A, Tanis SP, Adams M, Balogh-Nair V, Nakanishi K. J. Am. Chem. Soc. 1980;102:6372–6376. [Google Scholar]
- 13.Albeck A, Friedman N, Ottolenghi M, Sheves M, Einterz CM, Hug SJ, Lewis JW, Kliger DS. Biophys. J. 1989;55:233–241. doi: 10.1016/S0006-3495(89)82798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang GF, Kuksa V, Filipek S, Bartl F, Ritter E, Gelb MH, Hofmann KP, Palczewski K. J. Biol. Chem. 2001;276:26148–26153. doi: 10.1074/jbc.M102212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 16.Crouch R, Nodes BR, Perlman JI, Pepperberg DR, Akita H, Nakanishi K. Invest. Ophthalmol. Vis. Sci. 1984;25:419–428. [PubMed] [Google Scholar]
- 17.Crouch R, Katz S, Nakanishi K, Gawinowicz MA, Balogh-Nair V. Photochem. Photobiol. 1981;33:91–95. doi: 10.1111/j.1751-1097.1981.tb04302.x. [DOI] [PubMed] [Google Scholar]
- 18.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Prog. Retin. Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 19.Tsilou E, Hamel CP, Yu S, Redmond TM. Arch. Biochem. Biophys. 1997;346:21–27. doi: 10.1006/abbi.1997.0276. [DOI] [PubMed] [Google Scholar]
- 20.Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. J. Biol. Chem. 1993;268:15751–15757. [PubMed] [Google Scholar]
- 21.Bavik CO, Busch C, Eriksson U. J. Biol. Chem. 1992;267:23035–23042. [PubMed] [Google Scholar]
- 22.Simon A, Hellman U, Wernstedt C, Eriksson U. J. Biol. Chem. 1995;270:1107–1112. [PubMed] [Google Scholar]
- 23.Choo DW, Cheung E, Rando RR. FEBS Lett. 1998;440:195–198. doi: 10.1016/s0014-5793(98)01459-8. [DOI] [PubMed] [Google Scholar]
- 24.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 25.Van Hooser JP, Aleman TS, He YG, Cideciyan AV, Kuksa V, Pittler SJ, Stone EM, Jacobson SG, Palczewski K. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeliger MW, Grimm C, Stahlberg F, Friedburg C, Jaissle G, Zrenner E, Guo H, Reme CE, Humphries P, Hofmann F, Biel M, Fariss RN, Redmond TM, Wenzel A. Nat. Genet. 2001;29:70–74. doi: 10.1038/ng712. [DOI] [PubMed] [Google Scholar]
- 27.Van Hooser JP, Liang Y, Maeda T, Kuksa V, Jang GF, He YG, Rieke F, Fong HK, Detwiler PB, Palczewski K. J. Biol. Chem. 2002;277:19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimoto Y, Xie R, Tully SE, Berova N, Nakanishi K. Chirality. 2002;14:340–346. doi: 10.1002/chir.10076. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell CG, Derguini F, Bigge CF, Chen AH, Hu S, Sastry L, Nakanishi K. J. Org. Chem. 1993;58:3533–3537. [Google Scholar]
- 30.Palczewski K, Buczylko J, Kaplan MW, Polans AS, Crabb JW. J. Biol. Chem. 1991;266:12949–12955. [PubMed] [Google Scholar]
- 31.Jang GF, McBee JK, Alekseev AM, Haeseleer F, Palczewski K. J. Biol. Chem. 2000;275:28128–28138. doi: 10.1074/jbc.M004488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stecher H, Gelb MH, Saari JC, Palczewski K. J. Biol. Chem. 1999;274:8577–8585. doi: 10.1074/jbc.274.13.8577. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Sachs K, Maretzki D, Meyer CK, Hofmann KP. J. Biol. Chem. 2000;275:6189–6194. doi: 10.1074/jbc.275.9.6189. [DOI] [PubMed] [Google Scholar]
- 35.Bartl F, Ritter E, Hofmann KP. FEBS Lett. 2000;473:259–264. doi: 10.1016/s0014-5793(00)01544-1. [DOI] [PubMed] [Google Scholar]
- 36.Heck M, Pulvermuller A, Hofmann KP. Methods Enzymol. 2000;315:329–347. doi: 10.1016/s0076-6879(00)15852-5. [DOI] [PubMed] [Google Scholar]
- 37.Redmond TM, Hamel CP. Methods Enzymol. 2000;316:705–724. doi: 10.1016/s0076-6879(00)16758-8. [DOI] [PubMed] [Google Scholar]
- 38.Jang GF, Van Hooser JP, Kuksa V, McBee JK, He YG, Janssen JJ, Driessen CA, Palczewski K. J. Biol. Chem. 2001;276:32456–32465. doi: 10.1074/jbc.M104949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palczewski K, Van Hooser JP, Garwin GG, Chen J, Liou GI, Saari JC. Biochemistry. 1999;38:12012–12019. doi: 10.1021/bi990504d. [DOI] [PubMed] [Google Scholar]
- 40.Akito H, Tanis SP, Adams M, Balogh-Nair V, Nakajima K. J. Am. Chem. Soc. 1980;102:6370–6372. [Google Scholar]
- 41.Teller DC, Okada T, Behnke CA, Palczewski K, Stenkamp RE. Biochemistry. 2001;40:7761–7772. doi: 10.1021/bi0155091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canada FJ, Law WC, Rando RR, Yamamoto T, Derguini F, Nakanishi K. Biochemistry. 1990;29:9690–9697. doi: 10.1021/bi00493a026. [DOI] [PubMed] [Google Scholar]
- 43.Jager F, Fahmy K, Sakmar TP, Siebert F. Biochemistry. 1994;33:10878–10882. doi: 10.1021/bi00202a005. [DOI] [PubMed] [Google Scholar]
- 44.Melia TJ, Jr., Cowan CW, Angleson JK, Wensel TG. Biophys. J. 1997;73:3182–3191. doi: 10.1016/S0006-3495(97)78344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel R, Siebert F. J. Biol. Chem. 2001;276:38487–38493. doi: 10.1074/jbc.M105423200. [DOI] [PubMed] [Google Scholar]
- 46.Arnis S, Fahmy K, Hofmann KP, Sakmar TP. J. Biol. Chem. 1994;269:23879–23881. [PubMed] [Google Scholar]
- 47.Arnis S, Hofmann KP. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7849–7853. doi: 10.1073/pnas.90.16.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parkes JH, Liebman PA. Biochemistry. 1984;23:5054–5061. doi: 10.1021/bi00316a035. [DOI] [PubMed] [Google Scholar]
- 49.Hofmann KP. Novartis Found. Symp. 1999;224:158–180. doi: 10.1002/9780470515693.ch10. [DOI] [PubMed] [Google Scholar]
- 50.Leber T. Arch. Ophthalmol. (Paris) 1869;15:1–25. [Google Scholar]
- 51.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Nat. Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 52.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Nat. Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 53.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schappert-Kimmijser J, Henkes HE, Van Den Bosch J. Ophthalmologica. 1949;137:420–422. [Google Scholar]
- 55.Kroll AJ, Kuwabara T. Arch. Ophthalmol. 1964;71:683–690. doi: 10.1001/archopht.1964.00970010699016. [DOI] [PubMed] [Google Scholar]
- 56.Cremers FP, Van Den Hurk JA, Den Hollander AI. Hum. Mol. Genet. 2002;11:1169–1176. doi: 10.1093/hmg/11.10.1169. [DOI] [PubMed] [Google Scholar]
- 57.Thompson DA, Gyurus P, Fleischer LL, Bingham EL, McHenry CL, Apfelstedt-Sylla E, Zrenner E, Lorenz B, Richards JE, Jacobson SG, Sieving PA, Gal A. Invest. Ophthal. Vis. Sci. 2000;41:4293–4299. [PubMed] [Google Scholar]
- 58.Lorenz B, Gyurus P, Preising M, Bremser D, Gu S, Andrassi M, Gerth C, Gal A. Invest. Ophthalmol. Vis. Sci. 2000;41:2735–2742. [PubMed] [Google Scholar]
- 59.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Nat. Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 60.Hicks D, Sahel J. Invest. Ophthalmol. Vis. Sci. 1999;40:3071–3074. [PubMed] [Google Scholar]
- 61.Ripps H. Exp. Eye Res. 2002;74:327–336. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- 62.Defoe DM, Bok D. Invest. Ophthalmol. Vis. Sci. 1983;24:1211–1226. [PubMed] [Google Scholar]
- 63.Kefalov VJ, Crouch RK, Cornwall MC. Neuron. 2001;29:749–755. doi: 10.1016/s0896-6273(01)00249-5. [DOI] [PubMed] [Google Scholar]


