Abstract
Self-reactive clones, estimated by anti-V beta monoclonal antibodies (mAb) in conjunction with the Mls system, are confined to a population of intermediate (int) T-cell receptor (TCR) (or CD3) cells (i.e. TCRint cells), but are not found among TCRhigh cells. The next questions to be answered are whether autologous killing is confined to TCRint cells and how such killing is mediated. In this study, 51Cr-labelled thymocytes of syngeneic or allogeneic origin were used as target cells (4-hr assay). When liver and splenic mononuclear cells (MNC) obtained from B6 mice were used as effector cells, prominent autologous killing was seen in liver MNC, but not splenic MNC. Such killing was not seen when thymocytes from B6-lpr/lpr mice (i.e. Fas-) were used as target cells, nor when liver MNC from MRL-gld/gld mice (i.e. Fas ligand-) were used as effector cells (target thymocytes of MRL(-)+/+ mice). Cell separation experiments using a cell sorter revealed that autologous killing was mediated for the most part by CD3int cells, while allogeneic killing was mediated entirely by natural killer (NK) cells, TCRint cells and TCRhigh cells. Among CD3int cells, the NK1.1+ subset (i.e. NK1.1+ T cells) manifested a higher level of autologous killing than did the NK1.1- subset. Consistent with the results of a functional assay, it was found by reverse-transcription-polymerase chain reaction (RT-PCR) assay that CD3int cells among liver MNC showed the expression of Fas ligand mRNA, while thymocytes expressed Fas mRNA. When class I major histocompatibility complex (MHC)- thymocytes (from beta 2-microglobulin-deficient mice) were used as target cells, NK cells, but not CD3int cells, showed potent cytotoxicity. These results suggest that autologous killing is a major function of TCRint cells with self-reactivity, and that such killing is mediated by means of Fas ligand/Fas molecules.
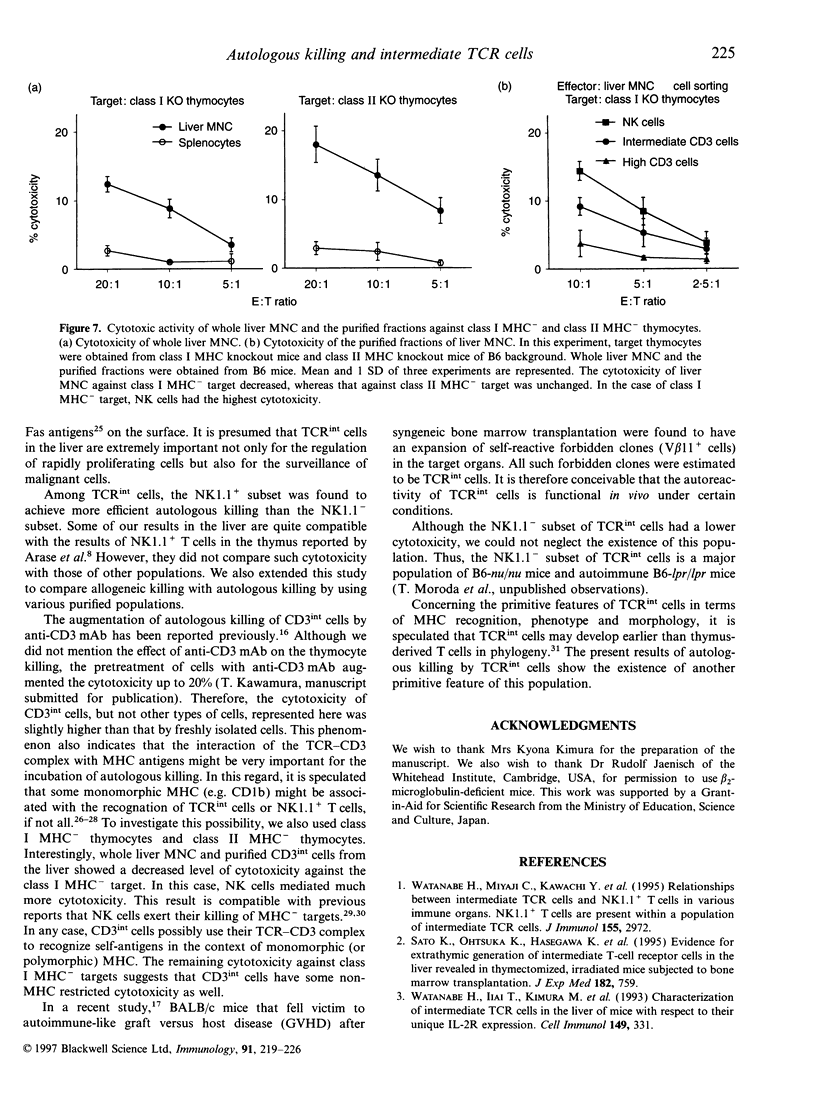
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Watanabe H., Iiai T., Kimura M., Ohtsuka K., Sato K., Ogawa M., Hirahara H., Hashimoto S., Sekikawa H. Extrathymic pathways of T-cell differentiation in the liver and other organs. Int Rev Immunol. 1994;11(1):61–102. doi: 10.3109/08830189409061717. [DOI] [PubMed] [Google Scholar]
- Arase H., Arase N., Ogasawara K., Good R. A., Onoé K. An NK1.1+ CD4+8- single-positive thymocyte subpopulation that expresses a highly skewed T-cell antigen receptor V beta family. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6506–6510. doi: 10.1073/pnas.89.14.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995 Dec 1;182(6):2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicher P. A., Balk S. P., Hagen S. J., Blumberg R. S., Flotte T. J., Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990 Nov 2;250(4981):679–682. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- D'Andrea A., Chang C., Franz-Bacon K., McClanahan T., Phillips J. H., Lanier L. L. Molecular cloning of NKB1. A natural killer cell receptor for HLA-B allotypes. J Immunol. 1995 Sep 1;155(5):2306–2310. [PubMed] [Google Scholar]
- Galle P. R., Hofmann W. J., Walczak H., Schaller H., Otto G., Stremmel W., Krammer P. H., Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995 Nov 1;182(5):1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens P. L., Jouin H., Marchal G., Milon G. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J Immunol Methods. 1990 Aug 28;132(1):137–144. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- Grusby M. J., Glimcher L. H. Immune responses in MHC class II-deficient mice. Annu Rev Immunol. 1995;13:417–435. doi: 10.1146/annurev.iy.13.040195.002221. [DOI] [PubMed] [Google Scholar]
- Iiai T., Watanabe H., Iwamoto T., Nakashima I., Abo T. Predominant activation of extrathymic T cells during melanoma development of metallothionein/ret transgenic mice. Cell Immunol. 1994 Feb;153(2):412–427. doi: 10.1006/cimm.1994.1039. [DOI] [PubMed] [Google Scholar]
- Kawachi Y., Arai K., Moroda T., Kawamura T., Umezu H., Naito M., Ohtsuka K., Hasegawa K., Takahashi-Iwanaga H., Iwanaga T. Supportive cellular elements for hepatic T cell differentiation: T cells expressing intermediate levels of the T cell receptor are cytotoxic against syngeneic hepatoma, and are lost after hepatocyte damage. Eur J Immunol. 1995 Dec;25(12):3452–3459. doi: 10.1002/eji.1830251237. [DOI] [PubMed] [Google Scholar]
- Kawachi Y., Watanabe H., Moroda T., Haga M., Iiai T., Hatakeyama K., Abo T. Self-reactive T cell clones in a restricted population of interleukin-2 receptor beta+ cells expressing intermediate levels of the T cell receptor in the liver and other immune organs. Eur J Immunol. 1995 Aug;25(8):2272–2278. doi: 10.1002/eji.1830250824. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Kawachi Y., Moroda T., Weerasinghe A., Iiai T., Seki S., Tazawa Y., Takada G., Abo T. Cytotoxic activity against tumour cells mediated by intermediate TCR cells in the liver and spleen. Immunology. 1996 Sep;89(1):68–75. doi: 10.1046/j.1365-2567.1996.d01-719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S. CD3+CD16+NK1.1+B220+ large granular lymphocytes arise from both alpha-beta TCR+CD4-CD8- and gamma-delta TCR+CD4-CD8- cells. J Exp Med. 1994 Jun 1;179(6):1957–1972. doi: 10.1084/jem.179.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994 Jul 22;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Kärre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today. 1990 Jul;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R. NK1.1+ T cell receptor-alpha/beta+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995 Sep 1;182(3):633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman Y., Watanabe T., Kawachi Y., Sato K., Ohtsuka K., Watanabe H., Hashimoto S., Moriyama Y., Shibata A., Abo T. Intermediate TCR cells with self-reactive clones are effector cells which induce syngeneic graft-versus-host disease in mice. Cell Immunol. 1995 Dec;166(2):172–186. doi: 10.1006/cimm.1995.9980. [DOI] [PubMed] [Google Scholar]
- Porcelli S. A., Modlin R. L. CD1 and the expanding universe of T cell antigens. J Immunol. 1995 Oct 15;155(8):3709–3710. [PubMed] [Google Scholar]
- Porcelli S., Morita C. T., Brenner M. B. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992 Dec 10;360(6404):593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Seaman M. S., Miller R. E., Tough T. W., Alderson M. R., Lynch D. H. gld/gld mice are unable to express a functional ligand for Fas. Eur J Immunol. 1994 Apr;24(4):928–933. doi: 10.1002/eji.1830240422. [DOI] [PubMed] [Google Scholar]
- Sato K., Ohtsuka K., Hasegawa K., Yamagiwa S., Watanabe H., Asakura H., Abo T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995 Sep 1;182(3):759–767. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Abo T., Sugiura K., Ohteki T., Kobata T., Yagita H., Okumura K., Rikiishi H., Masuda T., Kumagai K. Reciprocal T cell responses in the liver and thymus of mice injected with syngeneic tumor cells. Cell Immunol. 1991 Oct 1;137(1):46–60. doi: 10.1016/0008-8749(91)90055-g. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Takahashi-Iwanaga H., Iwanaga T., Sakamoto Y., Fujita T. Ultrastructural and time-lapse observations of intraepithelial lymphocytes in the small intestine of the guinea pig: their possible role in the removal of effete enterocytes. Cell Tissue Res. 1995 Jun;280(3):491–497. doi: 10.1007/BF00318353. [DOI] [PubMed] [Google Scholar]
- Vicari A. P., Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996 Feb;17(2):71–76. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Iiai T., Kimura M., Ohtsuka K., Tanaka T., Miyasaka M., Tsuchida M., Hanawa H., Abo T. Characterization of intermediate TCR cells in the liver of mice with respect to their unique IL-2R expression. Cell Immunol. 1993 Jul;149(2):331–342. doi: 10.1006/cimm.1993.1159. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Miyaji C., Kawachi Y., Iiai T., Ohtsuka K., Iwanage T., Takahashi-Iwanaga H., Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995 Sep 15;155(6):2972–2983. [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]



