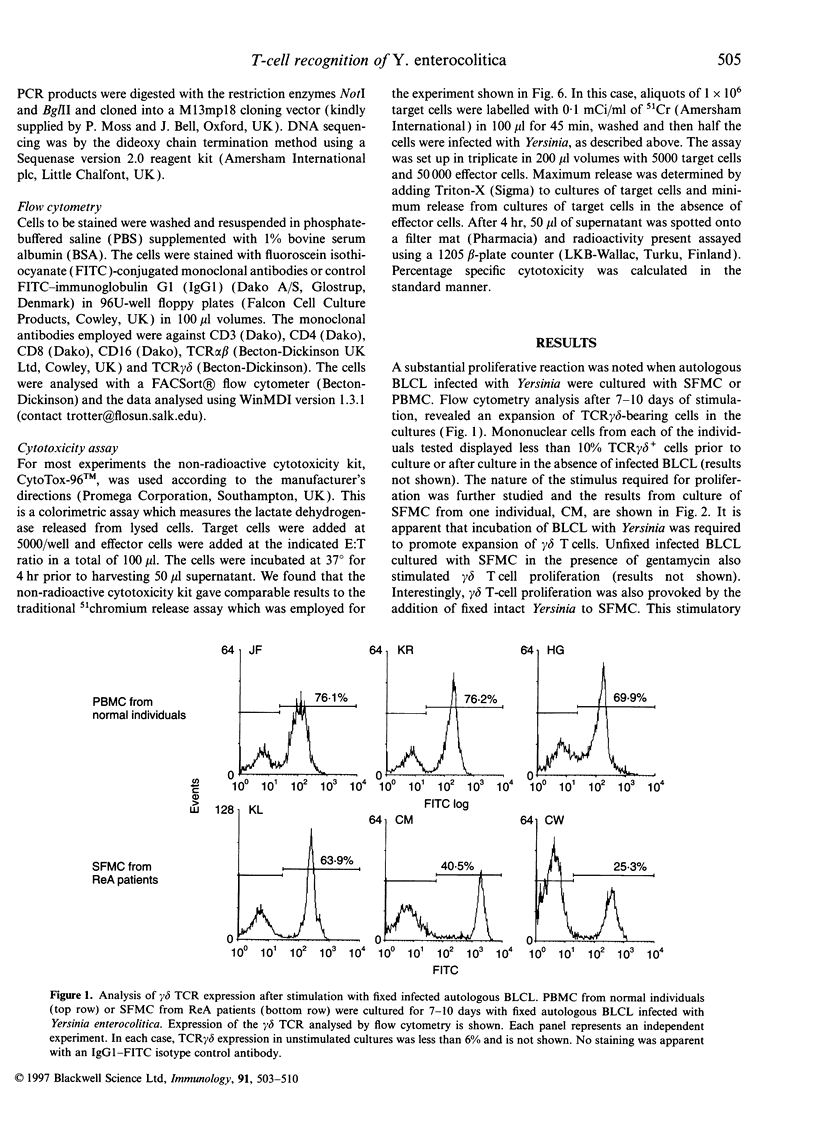
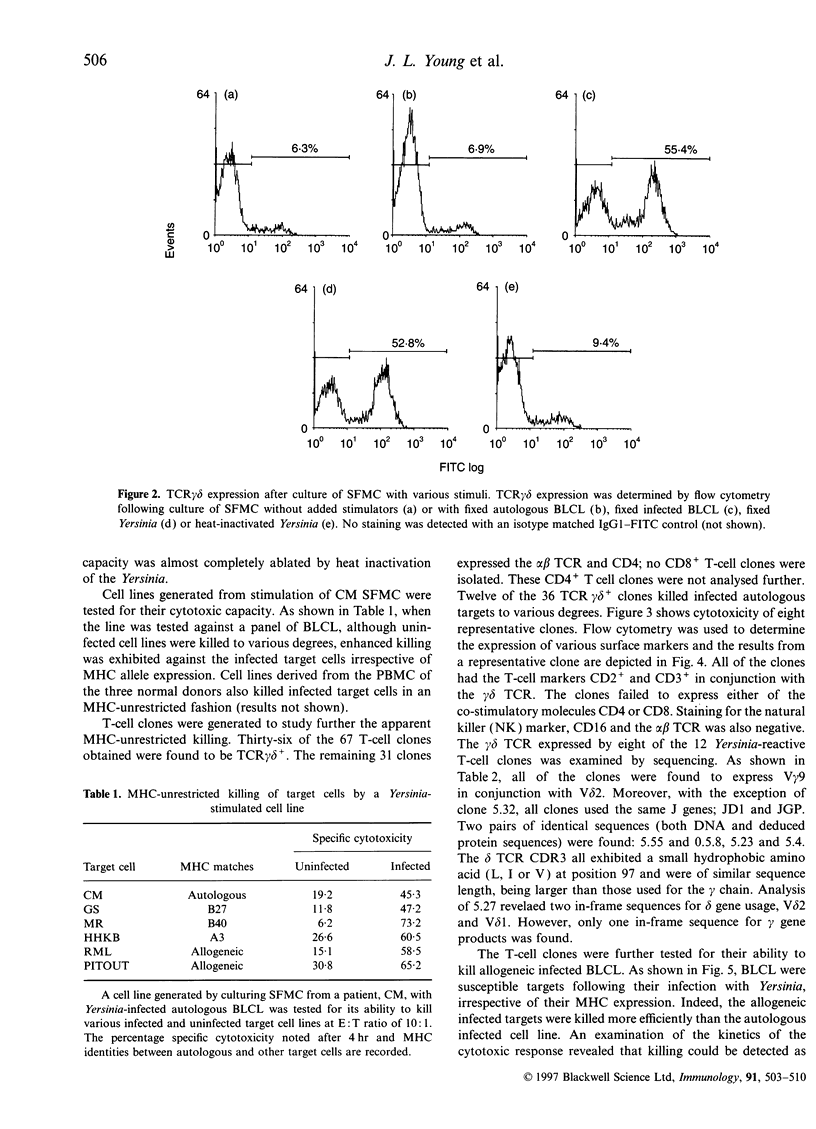
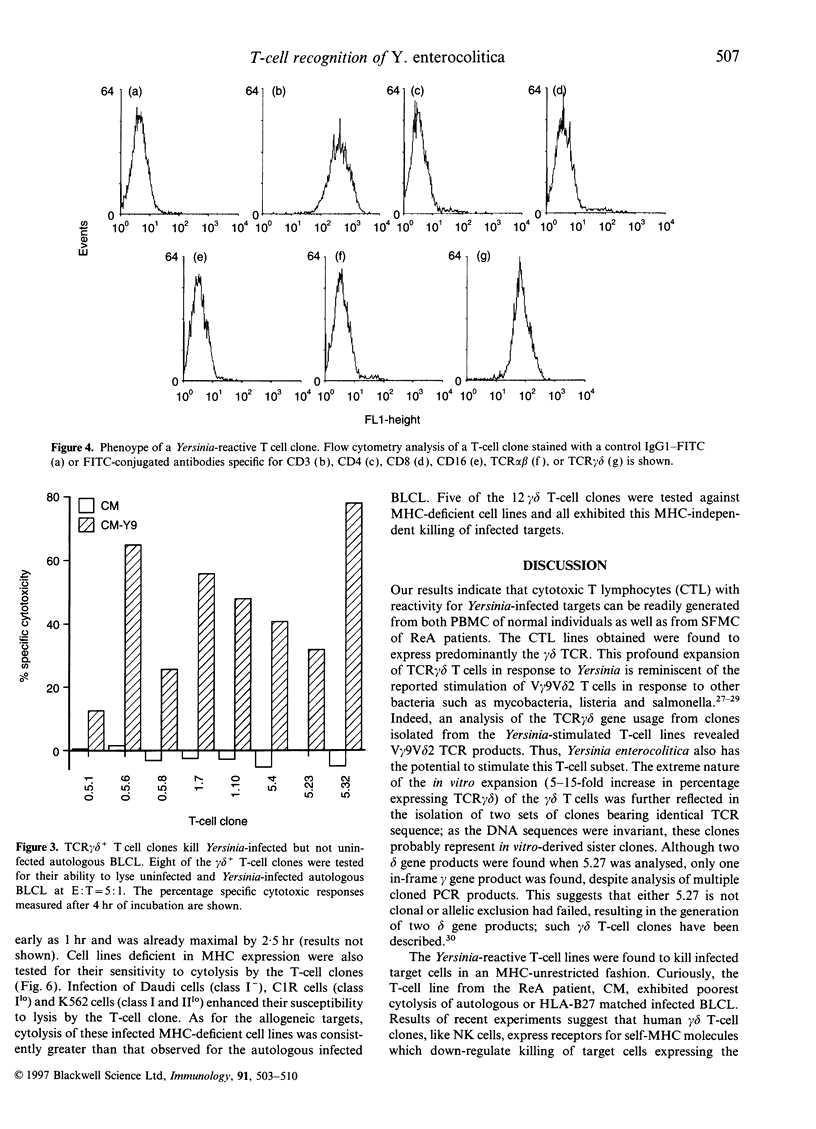
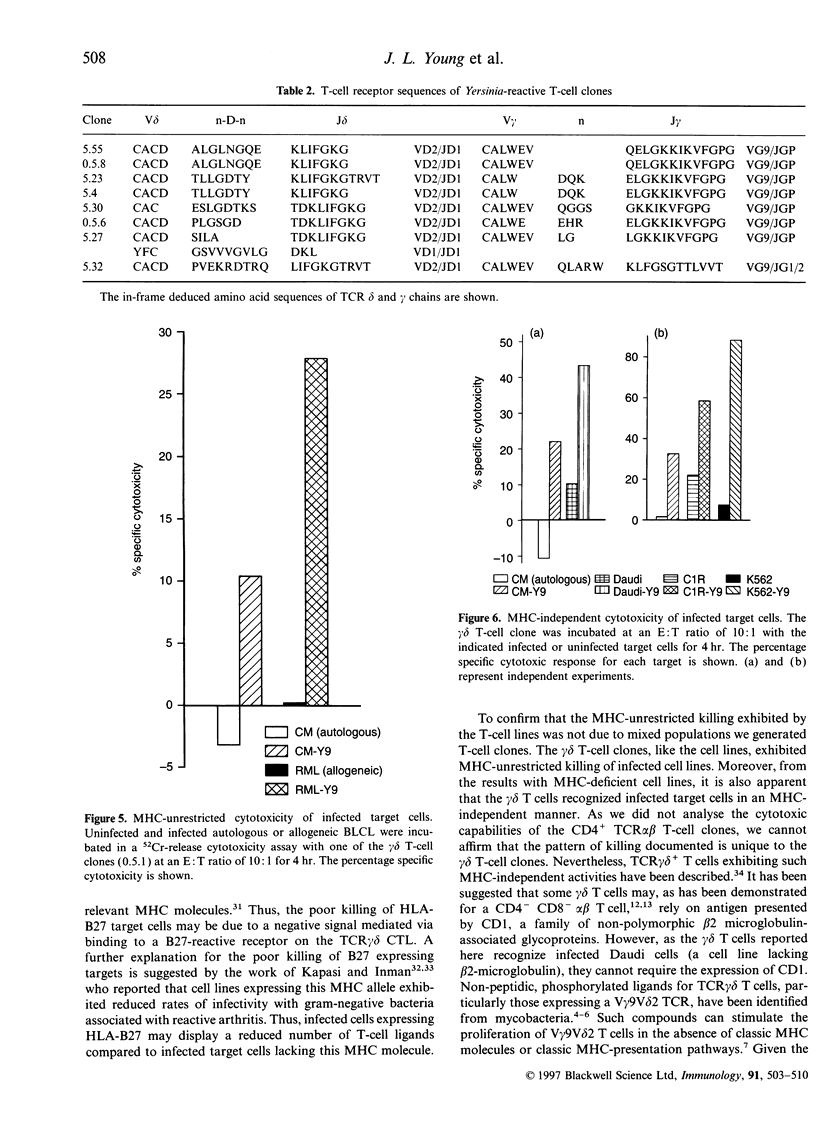
Abstract
We have studied the human gamma delta T-cell response to Yersinia enterocolitica, a facultative intracellular bacterium which causes gastroenteritis and, particularly in human leucocyte antigen (HLA)-B27+ individuals, reactive arthritis (ReA). A marked proliferation of that cytotoxic gamma delta T cells is seen when Yersinia-infected lymphoblastoid cell lines or fixed intact Yersinia are added to cultures of mononuclear cells derived from the synovial fluid of ReA patients or from the peripheral blood of healthy donors. In contrast, heat-inactivated Yersinia fail to stimulate the gamma delta T-cell response. The gamma delta T-cell lines generated killed both autologous and allogeneic infected cell lines. Interestingly, a T-cell line generated from synovial fluid mononuclear cells (SFMC) killed infected autologous cell lines and a cell line matched for HLA-B27 less well than infected allogeneic target cells. gamma delta T-cell clones isolated from this line were found to express V gamma 9V delta 2 T-cell receptor (TCR) and also killed infected mismatched cells more efficiently than autologous targets. Moreover, from experiments using major histocompatability complex (MHC)-deficient cell lines, it was apparent that target cell recognition was MHC independent. Our results suggest that gamma delta T cells can be involved in immunity to Yersinia enterocolitica and should be taken into account when considering immunopathological mechanisms leading to reactive arthritis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balk S. P., Ebert E. C., Blumenthal R. L., McDermott F. V., Wucherpfennig K. W., Landau S. B., Blumberg R. S. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991 Sep 20;253(5026):1411–1415. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- Beckman E. M., Brenner M. B. MHC class I-like, class II-like and CD1 molecules: distinct roles in immunity. Immunol Today. 1995 Jul;16(7):349–352. doi: 10.1016/0167-5699(95)80154-5. [DOI] [PubMed] [Google Scholar]
- Breit T. M., Wolvers-Tettero I. L., van Dongen J. J. Unique selection determinant in polyclonal V delta 2-J delta 1 junctional regions of human peripheral gamma delta T lymphocytes. J Immunol. 1994 Mar 15;152(6):2860–2864. [PubMed] [Google Scholar]
- Burmester G. R., Daser A., Kamradt T., Krause A., Mitchison N. A., Sieper J., Wolf N. Immunology of reactive arthritides. Annu Rev Immunol. 1995;13:229–250. doi: 10.1146/annurev.iy.13.040195.001305. [DOI] [PubMed] [Google Scholar]
- Constant P., Davodeau F., Peyrat M. A., Poquet Y., Puzo G., Bonneville M., Fournié J. J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994 Apr 8;264(5156):267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Chien Y. Issues concerning the nature of antigen recognition by alpha beta and gamma delta T-cell receptors. Immunol Today. 1995 Jul;16(7):316–318. doi: 10.1016/0167-5699(95)80143-x. [DOI] [PubMed] [Google Scholar]
- Davodeau F., Peyrat M. A., Hallet M. M., Houde I., Vie H., Bonneville M. Peripheral selection of antigen receptor junctional features in a major human gamma delta subset. Eur J Immunol. 1993 Apr;23(4):804–808. doi: 10.1002/eji.1830230405. [DOI] [PubMed] [Google Scholar]
- De Libero G., Casorati G., Giachino C., Carbonara C., Migone N., Matzinger P., Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral gamma/delta T cells. J Exp Med. 1991 Jun 1;173(6):1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure F., Jitsukawa S., Miossec C., Hercend T. CD1c as a target recognition structure for human T lymphocytes: analysis with peripheral blood gamma/delta cells. Eur J Immunol. 1990 Mar;20(3):703–706. doi: 10.1002/eji.1830200336. [DOI] [PubMed] [Google Scholar]
- Ferrick D. A., Schrenzel M. D., Mulvania T., Hsieh B., Ferlin W. G., Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995 Jan 19;373(6511):255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Deane K. H., Jecock R. M., Pearce J. H. Identification of 2 Chlamydia trachomatis antigens recognized by synovial fluid T cells from patients with Chlamydia induced reactive arthritis. J Rheumatol. 1996 Jan;23(1):130–136. [PubMed] [Google Scholar]
- Goodall J. C., Henwood J., Bacon P. A., Gaston J. S. Marked conservation of complementarity-determining region 3 of the beta-chain of TCRs recognizing a mycobacterial heat shock protein 60-derived peptide with strong sequence similarity to human heat shock protein 60. J Immunol. 1995 Sep 1;155(5):2329–2338. [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Hara T., Mizuno Y., Takaki K., Takada H., Akeda H., Aoki T., Nagata M., Ueda K., Matsuzaki G., Yoshikai Y. Predominant activation and expansion of V gamma 9-bearing gamma delta T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest. 1992 Jul;90(1):204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann E., Ackermann B., Duchmann R., Meyer zum Büschenfelde K. H. Synovial fluid MHC-unrestricted gamma delta-T lymphocytes contribute to antibacterial and anti-self cytotoxicity in the spondylarthropathies. Clin Exp Rheumatol. 1995 Mar-Apr;13(2):187–191. [PubMed] [Google Scholar]
- Hermann E., Lohse A. W., Mayet W. J., van der Zee R., Van Eden W., Probst P., Poralla T., Meyer zum Büschenfelde K. H., Fleischer B. Stimulation of synovial fluid mononuclear cells with the human 65-kD heat shock protein or with live enterobacteria leads to preferential expansion of TCR-gamma delta+ lymphocytes. Clin Exp Immunol. 1992 Sep;89(3):427–433. doi: 10.1111/j.1365-2249.1992.tb06975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann E., Yu D. T., Meyer zum Büschenfelde K. H., Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993 Sep 11;342(8872):646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- Hsieh B., Schrenzel M. D., Mulvania T., Lepper H. D., DiMolfetto-Landon L., Ferrick D. A. In vivo cytokine production in murine listeriosis. Evidence for immunoregulation by gamma delta+ T cells. J Immunol. 1996 Jan 1;156(1):232–237. [PubMed] [Google Scholar]
- Kapasi K., Inman R. D. HLA-B27 expression modulates gram-negative bacterial invasion into transfected L cells. J Immunol. 1992 Jun 1;148(11):3554–3559. [PubMed] [Google Scholar]
- Kapasi K., Inman R. D. ME1 epitope of HLA-B27 confers class I-mediated modulation of gram-negative bacterial invasion. J Immunol. 1994 Jul 15;153(2):833–840. [PubMed] [Google Scholar]
- Kronenberg M. Antigens recognized by gamma delta T cells. Curr Opin Immunol. 1994 Feb;6(1):64–71. doi: 10.1016/0952-7915(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Ladel C. H., Blum C., Dreher A., Reifenberg K., Kaufmann S. H. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur J Immunol. 1995 Oct;25(10):2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- Lahesmaa R., Shanafelt M. C., Steinman L., Peltz G. Immunopathogenesis of human inflammatory arthritis: lessons from Lyme and reactive arthritis. J Infect Dis. 1994 Oct;170(4):978–985. doi: 10.1093/infdis/170.4.978. [DOI] [PubMed] [Google Scholar]
- Morita C. T., Beckman E. M., Bukowski J. F., Tanaka Y., Band H., Bloom B. R., Golan D. E., Brenner M. B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995 Oct;3(4):495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Tomiyama H., Takiguchi M. Inhibition of gamma delta T cell recognition by receptors for MHC class I molecules. J Immunol. 1995 Nov 1;155(9):4139–4142. [PubMed] [Google Scholar]
- Panchamoorthy G., McLean J., Modlin R. L., Morita C. T., Ishikawa S., Brenner M. B., Band H. A predominance of the T cell receptor V gamma 2/V delta 2 subset in human mycobacteria-responsive T cells suggests germline gene encoded recognition. J Immunol. 1991 Nov 15;147(10):3360–3369. [PubMed] [Google Scholar]
- Parham P. Antigen presentation. Chewing the fat. Nature. 1994 Dec 15;372(6507):615–616. doi: 10.1038/372615a0. [DOI] [PubMed] [Google Scholar]
- Peyrat M. A., Davodeau F., Houde I., Romagné F., Necker A., Leget C., Cervoni J. P., Cerf-Bensussan N., Vié H., Bonneville M. Repertoire analysis of human peripheral blood lymphocytes using a human V delta 3 region-specific monoclonal antibody. Characterization of dual T cell receptor (TCR) delta-chain expressors and alpha beta T cells expressing V delta 3J alpha C alpha-encoded TCR chains. J Immunol. 1995 Sep 15;155(6):3060–3067. [PubMed] [Google Scholar]
- Porcelli S., Brenner M. B., Greenstein J. L., Balk S. P., Terhorst C., Bleicher P. A. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature. 1989 Oct 5;341(6241):447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- Probst P., Hermann E., Meyer zum Büschenfelde K. H., Fleischer B. Identification of the Yersinia enterocolitica urease beta subunit as a target antigen for human synovial T lymphocytes in reactive arthritis. Infect Immun. 1993 Oct;61(10):4507–4509. doi: 10.1128/iai.61.10.4507-4509.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst P., Hermann E., Meyer zum Büschenfelde K. H., Fleischer B. Multiclonal synovial T cell response to Yersinia enterocolitica in reactive arthritis: the Yersinia 61-kDa heat-shock protein is not the major target antigen. J Infect Dis. 1993 Feb;167(2):385–391. doi: 10.1093/infdis/167.2.385. [DOI] [PubMed] [Google Scholar]
- Rock E. P., Sibbald P. R., Davis M. M., Chien Y. H. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994 Jan 1;179(1):323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H., Mavaddat N., Litzenberger C., Ehrich E. W., Davis M. M., Bluestone J. A., Matis L., Draper R. K., Chien Y. H. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994 Jan 14;76(1):29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Sciammas R., Johnson R. M., Sperling A. I., Brady W., Linsley P. S., Spear P. G., Fitch F. W., Bluestone J. A. Unique antigen recognition by a herpesvirus-specific TCR-gamma delta cell. J Immunol. 1994 Jun 1;152(11):5392–5397. [PubMed] [Google Scholar]
- Sieling P. A., Chatterjee D., Porcelli S. A., Prigozy T. I., Mazzaccaro R. J., Soriano T., Bloom B. R., Brenner M. B., Kronenberg M., Brennan P. J. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995 Jul 14;269(5221):227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Morita C. T., Tanaka Y., Nieves E., Brenner M. B., Bloom B. R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995 May 11;375(6527):155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sano S., Nieves E., De Libero G., Rosa D., Modlin R. L., Brenner M. B., Bloom B. R., Morita C. T. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]


