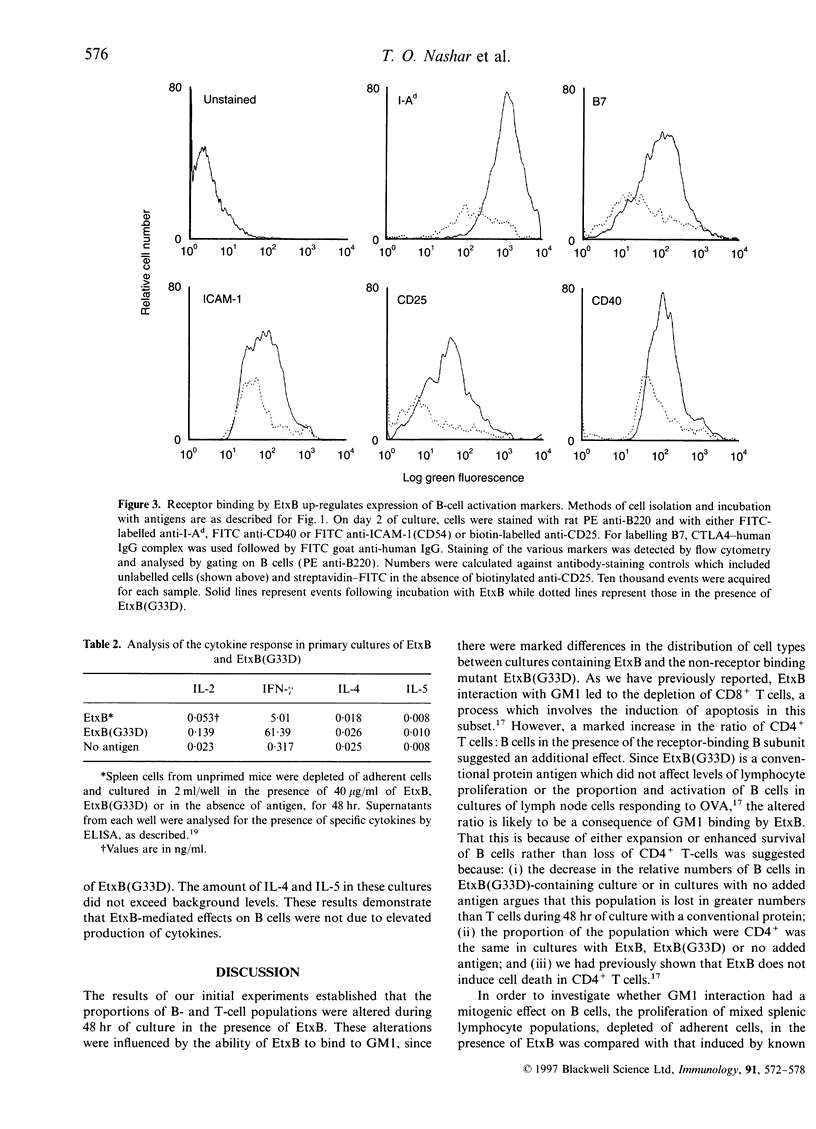
Abstract
The B subunits of cholera toxin (CtxB) and Escherichia coli heat-labile enterotoxin (EtxB) are non-toxic lectins that bind and cross-link a ubiquitous cell glycolipid receptor, ganglioside GM1, and are recognized as potent mucosal and systemic immunogens. Here we examine the role of EtxB receptor occupancy in modulating the activation of B cells, in vitro, in primary lymphocyte cultures containing B and T cells. When 48-hr spleen cell cultures containing EtxB were compared with those in the presence of a non-receptor binding mutant, EtxB(G33D), a marked shift in the ratio of CD4+ T cells: B cells was noted. Evidence suggested that this was the result of either enhanced survival or proliferation of B cells associated with receptor occupancy by EtxB. Investigation revealed that EtxB induced only a minimal increase in proliferation above that of EtxB(G33D), in mixed cell cultures, and failed to induce any cell division of purified B cells or T cells. In contrast, receptor-binding by EtxB markedly up-regulated the expression of major histocompatability complex (MHC) class II, B7, intracellular adhesion molecule-1 (ICAM-1), CD40 and CD25 on the B-cell surface. These results indicate that the polyclonal effects of EtxB on B cells are not associated with wide-scale proliferation, but more likely with maintenance of B-cell survival by activation of molecules essential for B-cell differentiation. The findings also highlight the essential role of GM1-interaction with EtxB in the regulation of lymphocyte responses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin T., Hirst T. R. Purification of the B-subunit oligomer of Escherichia coli heat-labile enterotoxin by heterologous expression and secretion in a marine vibrio. Protein Expr Purif. 1994 Apr;5(2):198–204. doi: 10.1006/prep.1994.1031. [DOI] [PubMed] [Google Scholar]
- Anastassiou E. D., Yamada H., Francis M. L., Mond J. J., Tsokos G. C. Effects of cholera toxin on human B cells. Cholera toxin induces B cell surface DR expression while it inhibits anti-mu antibody-induced cell proliferation. J Immunol. 1990 Oct 15;145(8):2375–2380. [PubMed] [Google Scholar]
- Bromander A., Holmgren J., Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991 May 1;146(9):2908–2914. [PubMed] [Google Scholar]
- Cooper M. D., Kearney J. F., Gathings W. E., Lawton A. R. Effects of anti-Ig antibodies on the development and differentiation of B cells. Immunol Rev. 1980;52:29–53. doi: 10.1111/j.1600-065x.1980.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Croft M. Activation of naive, memory and effector T cells. Curr Opin Immunol. 1994 Jun;6(3):431–437. doi: 10.1016/0952-7915(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Dugas B., Paul-Eugène N., Génot E., Mencia-Huerta J. M., Braquet P., Kolb J. P. Effect of bacterial toxins on human B cell activation. II. Mitogenic activity of the B subunit of cholera toxin. Eur J Immunol. 1991 Feb;21(2):495–500. doi: 10.1002/eji.1830210236. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984 Dec;133(6):2892–2897. [PubMed] [Google Scholar]
- Elson C. O., Solomon S. Activation of cholera toxin-specific T cells in vitro. Infect Immun. 1990 Nov;58(11):3711–3716. doi: 10.1128/iai.58.11.3711-3716.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M. L., Ryan J., Jobling M. G., Holmes R. K., Moss J., Mond J. J. Cyclic AMP-independent effects of cholera toxin on B cell activation. II. Binding of ganglioside GM1 induces B cell activation. J Immunol. 1992 Apr 1;148(7):1999–2005. [PubMed] [Google Scholar]
- Harper H. M., Cochrane L., Williams N. A. The role of small intestinal antigen-presenting cells in the induction of T-cell reactivity to soluble protein antigens: association between aberrant presentation in the lamina propria and oral tolerance. Immunology. 1996 Nov;89(3):449–456. doi: 10.1046/j.1365-2567.1996.d01-760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y., Tamura S. I., Shimada K., Kurata T. Involvement of antigen-presenting cells in the enhancement of the in vitro antibody responses by cholera toxin B subunit. Immunology. 1992 Mar;75(3):493–498. [PMC free article] [PubMed] [Google Scholar]
- Hörnqvist E., Goldschmidt T. J., Holmdahl R., Lycke N. Host defense against cholera toxin is strongly CD4+ T cell dependent. Infect Immun. 1991 Oct;59(10):3630–3638. doi: 10.1128/iai.59.10.3630-3638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Johnson J. G. Molecules involved in T-cell costimulation. Curr Opin Immunol. 1993 Jun;5(3):361–367. doi: 10.1016/0952-7915(93)90054-v. [DOI] [PubMed] [Google Scholar]
- Jertborn M., Svennerholm A. M., Holmgren J. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine. 1992;10(2):130–132. doi: 10.1016/0264-410x(92)90030-n. [DOI] [PubMed] [Google Scholar]
- Lederman S., Yellin M. J., Covey L. R., Cleary A. M., Callard R., Chess L. Non-antigen signals for B-cell growth and differentiation to antibody secretion. Curr Opin Immunol. 1993 Jun;5(3):439–444. doi: 10.1016/0952-7915(93)90066-2. [DOI] [PubMed] [Google Scholar]
- Lewis D. J., Castello-Branco L. R., Novotny P., Dougan G., Poulton T. A., Griffin G. E. Circulating cellular immune response to oral immunization of humans with cholera toxin B-subunit. Vaccine. 1993;11(2):119–121. doi: 10.1016/0264-410x(93)90005-i. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986 Oct;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Nashar T. O., Amin T., Marcello A., Hirst T. R. Current progress in the development of the B subunits of cholera toxin and Escherichia coli heat-labile enterotoxin as carriers for the oral delivery of heterologous antigens and epitopes. Vaccine. 1993;11(2):235–240. doi: 10.1016/0264-410x(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Nashar T. O., Webb H. M., Eaglestone S., Williams N. A., Hirst T. R. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashar T. O., Williams N. A., Hirst T. R., Nahar T. O. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of mature CD8+ T lymphocytes. Int Immunol. 1996 May;8(5):731–736. doi: 10.1093/intimm/8.5.731. [DOI] [PubMed] [Google Scholar]
- Noelle R., Krammer P. H., Ohara J., Uhr J. W., Vitetta E. S. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. A., Hill T. J., Hooper D. C. Murine epidermal antigen-presenting cells in primary and secondary T-cell proliferative responses to herpes simplex virus in vitro. Immunology. 1991 Jan;72(1):34–39. [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D., Bailey M., Williams N. A., Stokes C. R. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur J Immunol. 1991 Oct;21(10):2333–2339. doi: 10.1002/eji.1830211007. [DOI] [PubMed] [Google Scholar]
- Woogen S. D., Ealding W., Elson C. O. Inhibition of murine lymphocyte proliferation by the B subunit of cholera toxin. J Immunol. 1987 Dec 1;139(11):3764–3770. [PubMed] [Google Scholar]
- Xu-Amano J., Jackson R. J., Fujihashi K., Kiyono H., Staats H. F., McGhee J. R. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine. 1994 Aug;12(10):903–911. doi: 10.1016/0264-410x(94)90033-7. [DOI] [PubMed] [Google Scholar]


