Abstract
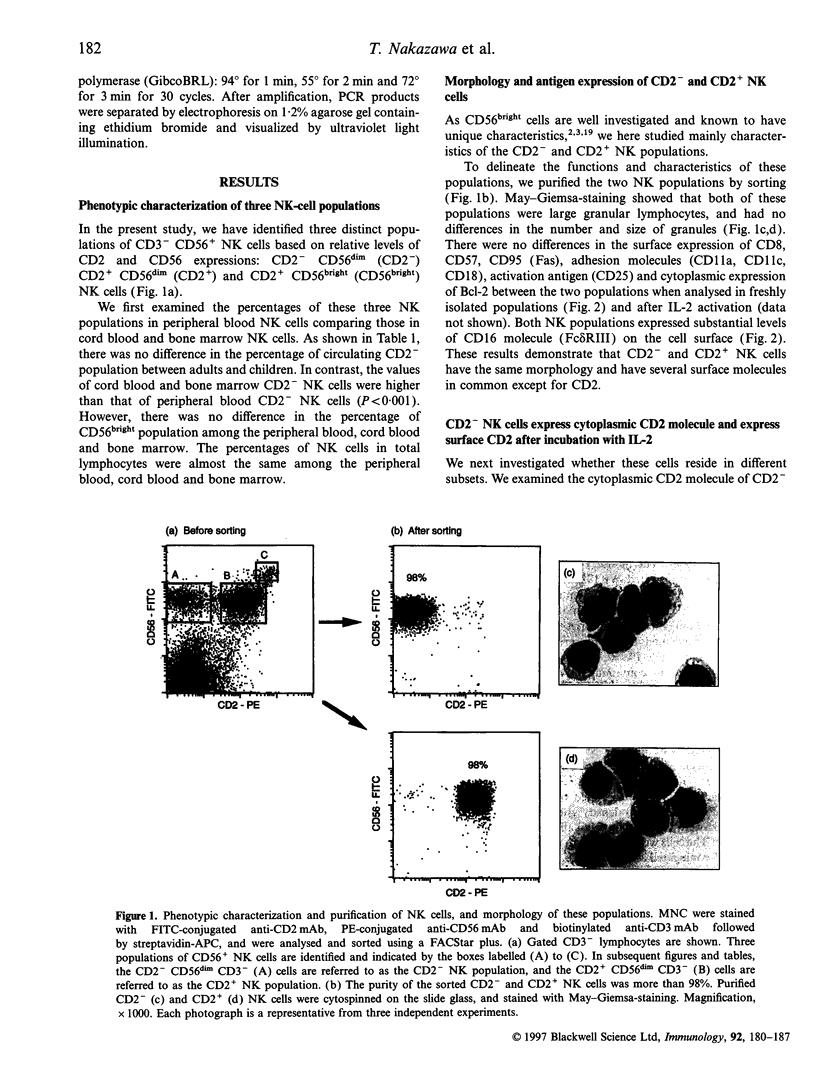
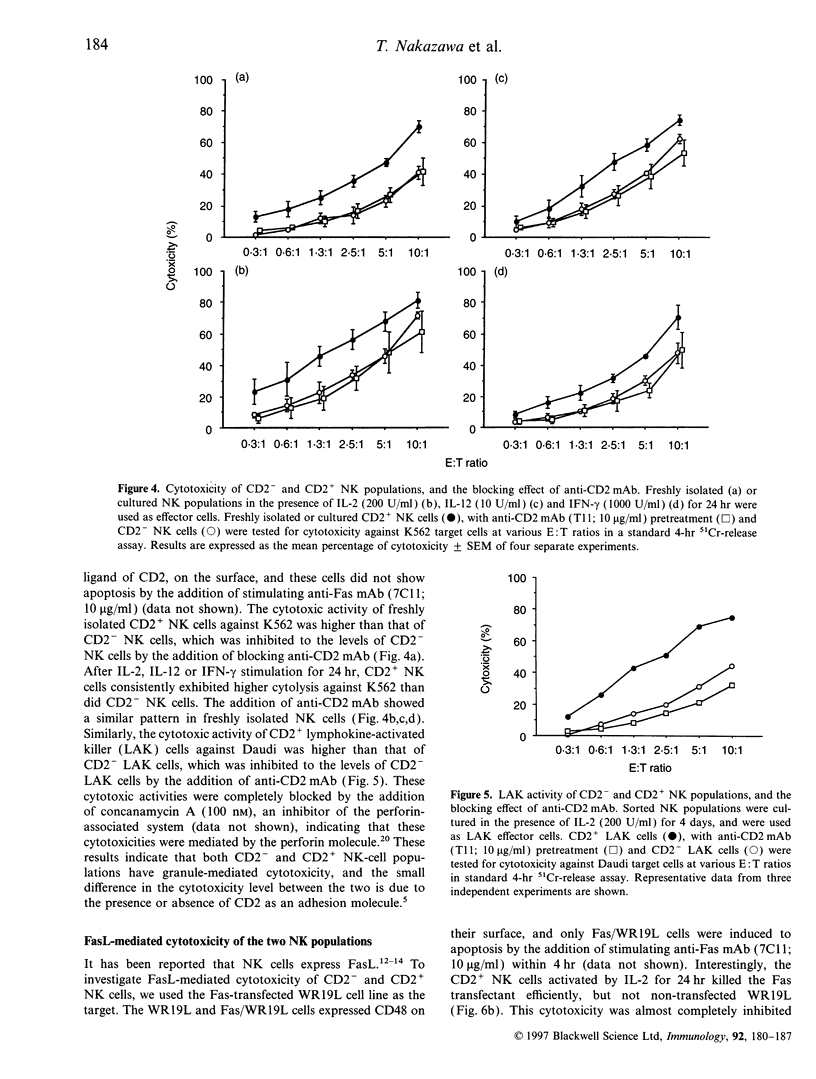
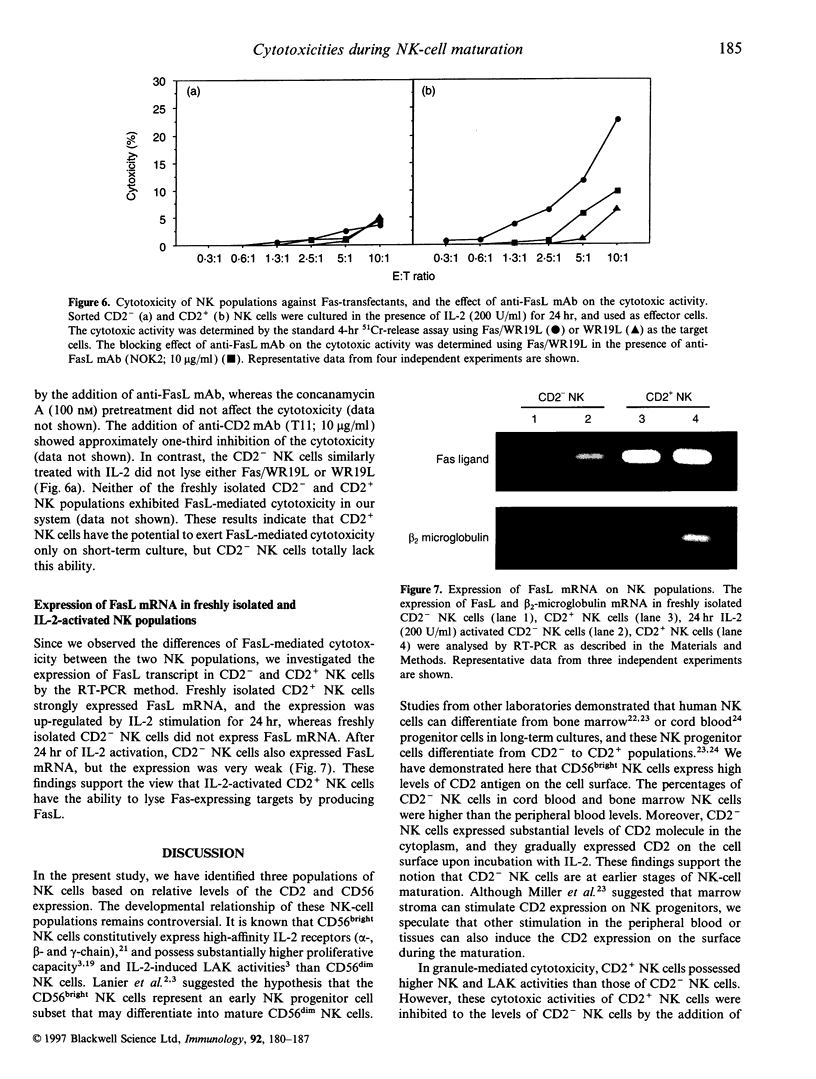
We classified CD56+ CD3- natural killer (NK) cells into CD2- CD56dim (CD2- NK), CD2+ CD56dim (CD2+ NK) and CD2+ CD56bright populations, and investigated mainly functional differences between the former two populations. CD2- and CD2+ NK cells were the same in their morphology and several surface molecules except for CD2. The percentages of CD2- NK cells in total NK cells were higher in the cord blood and marrow than in the peripheral blood of adults or children. Freshly isolated CD2- NK cells had CD2 in the cytoplasm, and gradually expressed it on the surface upon incubation with interleukin-2 (IL-2). These results demonstrated that CD2 is an antigen which appears on the surface during the maturation of NK cells. The granule-mediated cytotoxicities, which are mainly performed by the perforin molecule, of CD2+ NK cells against K562 and Daudi cells were higher than those of CD2- NK cells, and they were inhibited to the levels of CD2- NK cells by the addition of a blocking anti-CD2 monoclonal antibody (mAb). Fas ligand (FasL) mRNA was expressed in freshly isolated CD2+ NK cells but not in the CD2- NK cells. Neither freshly isolated NK populations showed FasL-mediated cytotoxicity, and only CD2+ NK cells lysed Fas-transfected targets after the 24-hr incubation with IL-2. Based on these results, CD2- NK cells have already developed granule-mediated cytotoxicity equal to that of CD2+ NK cells except for the CD2-associated activity, but they, unlike CD2+ NK cells, totally lack FasL-mediated cytotoxicity. These findings suggest that FasL-mediated cytotoxicity may be acquired at more mature stages of NK-cell maturation than granule-mediated cytotoxicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arase H., Arase N., Saito T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995 Mar 1;181(3):1235–1238. doi: 10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulanandam A. R., Moingeon P., Concino M. F., Recny M. A., Kato K., Yagita H., Koyasu S., Reinherz E. L. A soluble multimeric recombinant CD2 protein identifies CD48 as a low affinity ligand for human CD2: divergence of CD2 ligands during the evolution of humans and mice. J Exp Med. 1993 May 1;177(5):1439–1450. doi: 10.1084/jem.177.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri M. A., Zmuidzinas A., Manley T. J., Levine H., Smith K. A., Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990 May 1;171(5):1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güssow D., Rein R., Ginjaar I., Hochstenbach F., Seemann G., Kottman A., Ploegh H. L. The human beta 2-microglobulin gene. Primary structure and definition of the transcriptional unit. J Immunol. 1987 Nov 1;139(9):3132–3138. [PubMed] [Google Scholar]
- Kataoka T., Shinohara N., Takayama H., Takaku K., Kondo S., Yonehara S., Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996 May 15;156(10):3678–3686. [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Alley K. A., McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994 May 1;83(9):2594–2601. [PubMed] [Google Scholar]
- Miller J. S., Verfaillie C., McGlave P. The generation of human natural killer cells from CD34+/DR- primitive progenitors in long-term bone marrow culture. Blood. 1992 Nov 1;80(9):2182–2187. [PubMed] [Google Scholar]
- Montel A. H., Bochan M. R., Hobbs J. A., Lynch D. H., Brahmi Z. Fas involvement in cytotoxicity mediated by human NK cells. Cell Immunol. 1995 Dec;166(2):236–246. doi: 10.1006/cimm.1995.9974. [DOI] [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Cwirla S., Phillips J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989 Nov 15;143(10):3183–3191. [PubMed] [Google Scholar]
- Oshimi Y., Oda S., Honda Y., Nagata S., Miyazaki S. Involvement of Fas ligand and Fas-mediated pathway in the cytotoxicity of human natural killer cells. J Immunol. 1996 Oct 1;157(7):2909–2915. [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. J., Caligiuri M. A., Manley T. J., Levine H., Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990 Nov 15;145(10):3194–3201. [PubMed] [Google Scholar]
- Robertson M. J., Manley T. J., Donahue C., Levine H., Ritz J. Costimulatory signals are required for optimal proliferation of human natural killer cells. J Immunol. 1993 Mar 1;150(5):1705–1714. [PubMed] [Google Scholar]
- Robertson M. J., Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990 Dec 15;76(12):2421–2438. [PubMed] [Google Scholar]
- Schmidt R. E., Michon J. M., Woronicz J., Schlossman S. F., Reinherz E. L., Ritz J. Enhancement of natural killer function through activation of the T11 E rosette receptor. J Clin Invest. 1987 Jan;79(1):305–308. doi: 10.1172/JCI112800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Spits H., Lanier L. L., Phillips J. H. Development of human T and natural killer cells. Blood. 1995 May 15;85(10):2654–2670. [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995 Mar 10;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Inazawa J., Abe T., Suda T., Nagata S. Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol. 1994 Oct;6(10):1567–1574. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- Ullberg M., Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981 Mar 1;153(3):615–628. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuhara A., Kawai H., Komiyama A. Development of natural killer cytotoxicity during childhood: marked increases in number of natural killer cells with adequate cytotoxic abilities during infancy to early childhood. Pediatr Res. 1990 Oct;28(4):316–322. doi: 10.1203/00006450-199010000-00002. [DOI] [PubMed] [Google Scholar]
- Yang F. C., Agematsu K., Nakazawa T., Mori T., Ito S., Kobata T., Morimoto C., Komiyama A. CD27/CD70 interaction directly induces natural killer cell killing activity. Immunology. 1996 Jun;88(2):289–293. doi: 10.1111/j.1365-2567.1996.tb00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Hengartner H., Podack E. R., Cohn Z. A. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 1986 Mar 28;44(6):849–859. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]




