Abstract
Modulation of proliferative T-cell responses by n-butyrate has been suggested to result from direct interference with cell cycle progression. Considering the important role of antigen-presenting cells (APC) in T-cell activation, we were particularly interested in studying the impact of n-butyrate on these cells. We demonstrated that pretreatment of human peripheral blood mononuclear cells (PBMC) or monocytes with this agent resulted in a dose- and time-dependent downregulation of their capability to stimulate T-cell responses with a similar pattern of inhibition when this agent was present throughout the culture period. Pretreatment with n-butyrate was effective in preventing both alloresponses and T-cell proliferation to immobilized anti-CD3 monoclonal antibody (mAb) suggesting alteration of costimulatory function. Flow cytometric analysis revealed that interferon-gamma (IFN-gamma)-induced upregulation of B7-1 expression on monocytes was profoundly inhibited by n-butyrate. Furthermore, this agent significantly suppressed the expression of intercellular adhesion molecule-1 (ICAM-1) or lymphocyte function-associated antigen-3 (LFA-3). In contrast, constitutive as well as cytokine-induced expression of B7-2 was enhanced by n-butyrate. Additionally, in monocytes, but not in T cells, treatment with n-butyrate led to significant alteration of membrane integrity owing to apoptotic cell death. Our findings indicate that modulation of T-cell responses by n-butyrate could also result from altered APC function, possibly as a consequence of downregulating distinct adhesion and/or costimulatory receptors as well as of inducing apoptosis. A potential clinical relevance of short-chain fatty acids for reducing T-cell-mediated immune reactions via modulating APC function is speculated.
Full text
PDF

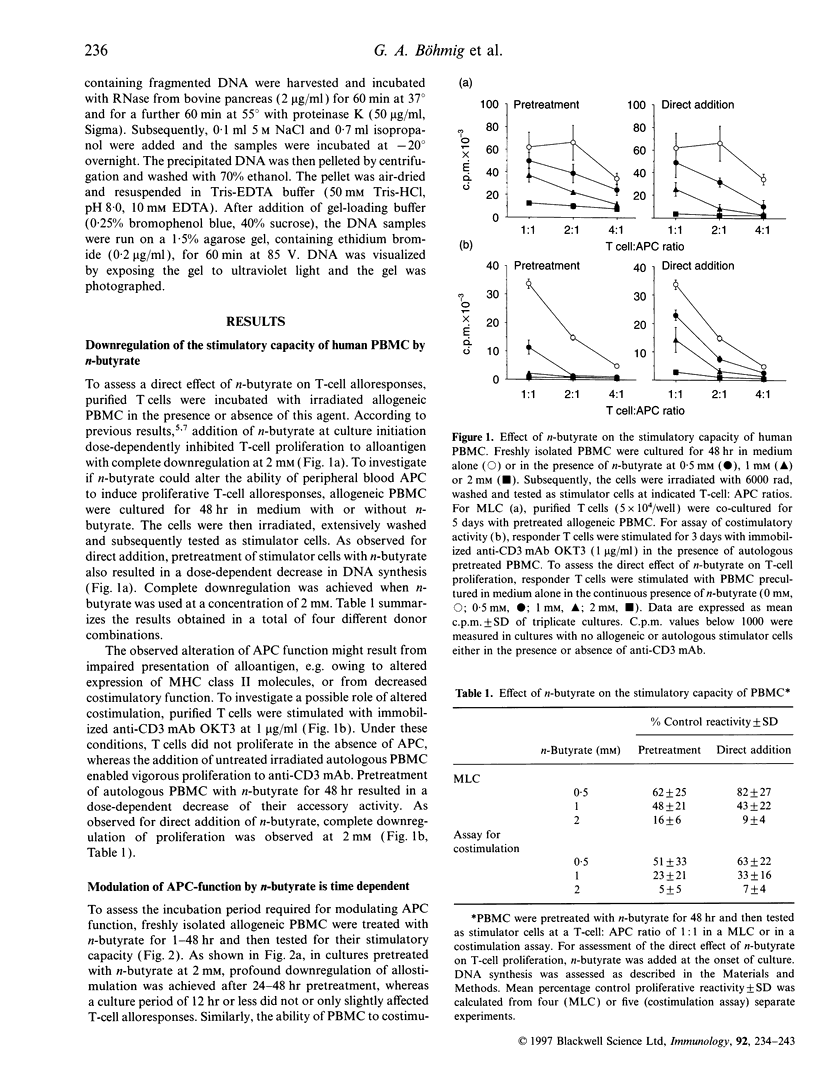



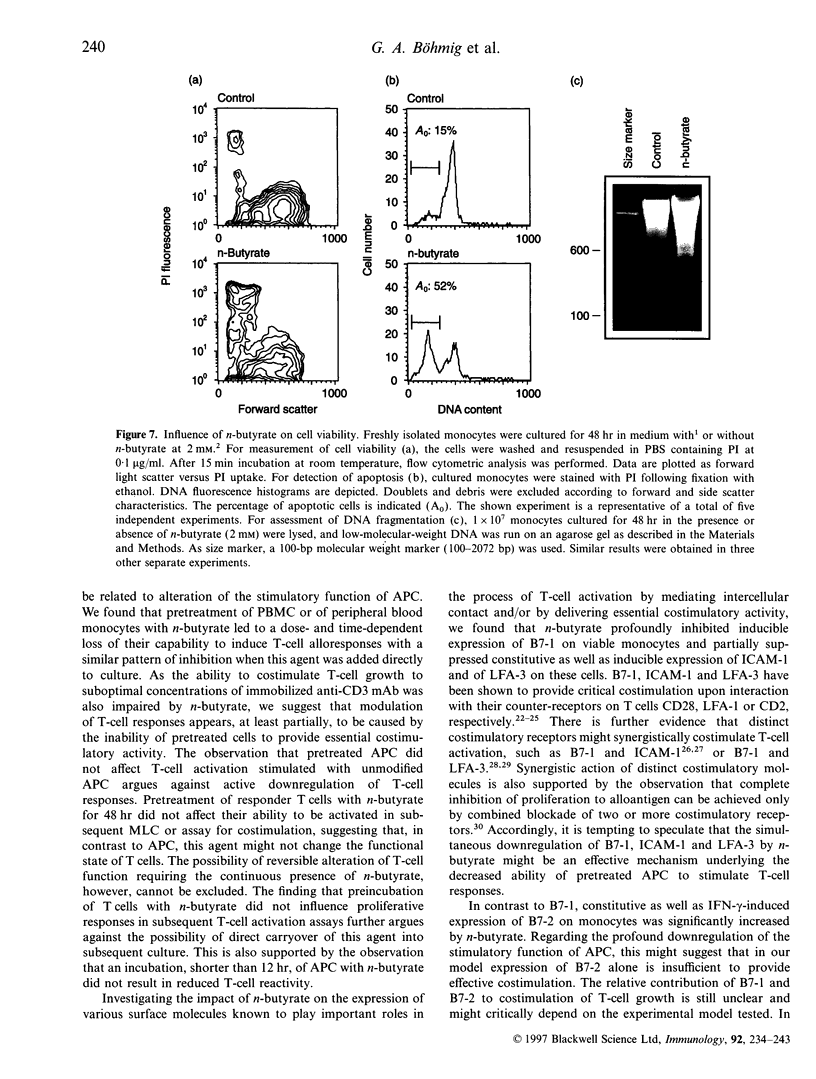
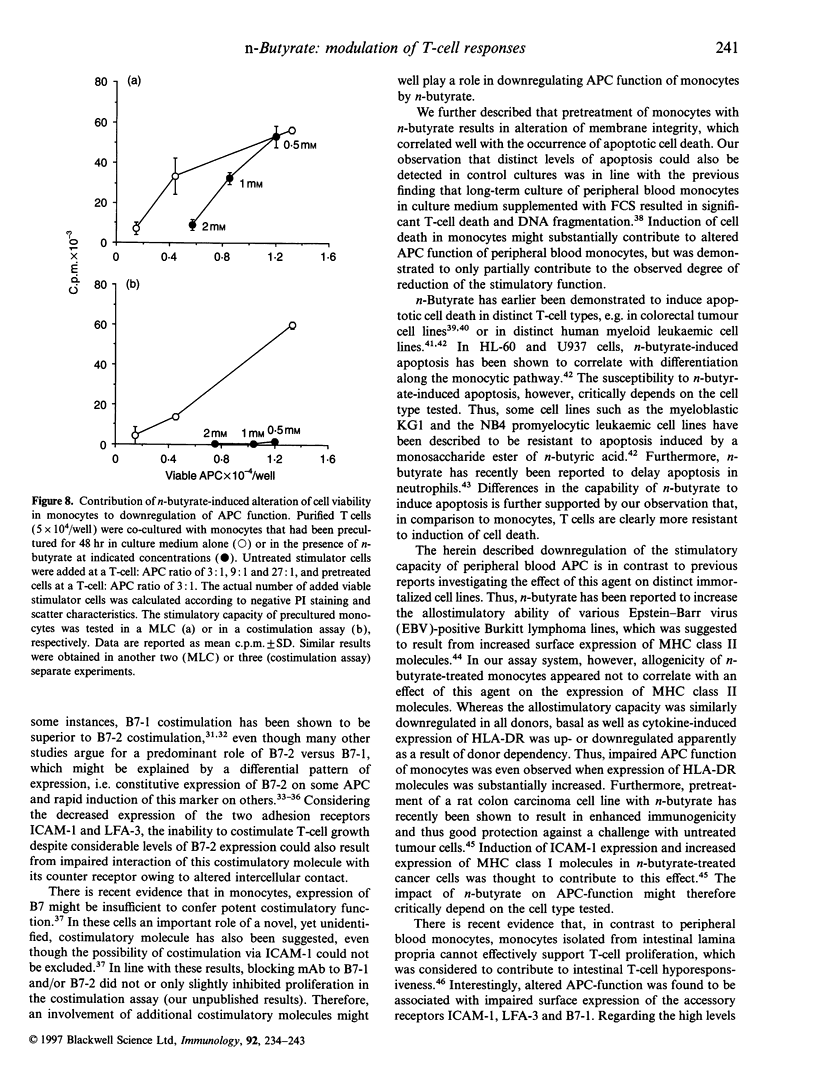


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Peterson A., Gorga J. C., Herrmann S. H., Burakoff S. J. Synergistic T cell activation via the physiological ligands for CD2 and the T cell receptor. J Exp Med. 1988 Sep 1;168(3):1145–1156. doi: 10.1084/jem.168.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhmig G. A., Csmarits B., Cerwenka A., Alaei P., Kovarik J., Zlabinger G. J. Induction of alloantigen-specific hyporesponsiveness in vitro by the short-chain fatty acid N-butyrate. Transplantation. 1995 May 27;59(10):1500–1503. doi: 10.1097/00007890-199505270-00029. [DOI] [PubMed] [Google Scholar]
- Böhmig G. A., Kovarik J., Holter W., Pohanka E., Zlabinger G. J. Specific down-regulation of proliferative T cell alloresponsiveness by interference with CD2/LFA-3 and LFA-1/ICAM-1 in vitro. J Immunol. 1994 Apr 15;152(8):3720–3728. [PubMed] [Google Scholar]
- Böhmig G. A., Wekerle T., Säemann M. D., Kovarik J., Zlabinger G. J. Induction of alloantigen-specific hyporesponsiveness in vitro by n-butyrate: antagonistic effect of cyclosporin A. Transpl Int. 1996;9 (Suppl 1):S318–S322. doi: 10.1007/978-3-662-00818-8_79. [DOI] [PubMed] [Google Scholar]
- Calabresse C., Venturini L., Ronco G., Villa P., Degos L., Belpomme D., Chomienne C. Selective induction of apoptosis in myeloid leukemic cell lines by monoacetone glucose-3 butyrate. Biochem Biophys Res Commun. 1994 May 30;201(1):266–283. doi: 10.1006/bbrc.1994.1698. [DOI] [PubMed] [Google Scholar]
- Caux C., Vanbervliet B., Massacrier C., Azuma M., Okumura K., Lanier L. L., Banchereau J. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J Exp Med. 1994 Nov 1;180(5):1841–1847. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Linsley P. S., Aruffo A., Ledbetter J. A. Differential regulatory effects of intercellular adhesion molecule-1 on costimulation by the CD28 counter-receptor B7. J Immunol. 1992 Oct 15;149(8):2541–2548. [PubMed] [Google Scholar]
- DeSilva D. R., Urdahl K. B., Jenkins M. K. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J Immunol. 1991 Nov 15;147(10):3261–3267. [PubMed] [Google Scholar]
- Di Renzo L., Avila-Cariño J., Klein E. Induction of the lytic viral cycle in Epstein Barr virus carrying Burkitt lymphoma lines is accompanied by increased expression of major histocompatibility complex molecules. Immunol Lett. 1993 Nov;38(3):207–214. doi: 10.1016/0165-2478(93)90008-p. [DOI] [PubMed] [Google Scholar]
- Dubey C., Croft M., Swain S. L. Costimulatory requirements of naive CD4+ T cells. ICAM-1 or B7-1 can costimulate naive CD4 T cell activation but both are required for optimum response. J Immunol. 1995 Jul 1;155(1):45–57. [PubMed] [Google Scholar]
- Freedman A. S., Freeman G. J., Rhynhart K., Nadler L. M. Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991 Oct 15;137(2):429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F. B7-1 but not B7-2 efficiently costimulates CD8+ T lymphocytes in the P815 tumor system in vitro. J Immunol. 1996 Jan 15;156(2):465–472. [PubMed] [Google Scholar]
- Gilbert K. M., Hoang K. D., Weigle W. O. Th1 and Th2 clones differ in their response to a tolerogenic signal. J Immunol. 1990 Mar 15;144(6):2063–2071. [PubMed] [Google Scholar]
- Gilbert K. M. T cell clonal anergy. Chem Immunol. 1994;58:92–116. [PubMed] [Google Scholar]
- Gilbert K. M., Weigle W. O. Th1 cell anergy and blockade in G1a phase of the cell cycle. J Immunol. 1993 Aug 1;151(3):1245–1254. [PubMed] [Google Scholar]
- Hague A., Elder D. J., Hicks D. J., Paraskeva C. Apoptosis in colorectal tumour cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int J Cancer. 1995 Jan 27;60(3):400–406. doi: 10.1002/ijc.2910600322. [DOI] [PubMed] [Google Scholar]
- Hargreaves R., Logiou V., Lechler R. The primary alloresponse of human CD4+ T cells is dependent on B7 (CD80), augmented by CD58, but relatively uninfluenced by CD54 expression. Int Immunol. 1995 Sep;7(9):1505–1513. doi: 10.1093/intimm/7.9.1505. [DOI] [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Pucillo C., Linsley P., Hodes R. J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994 Aug 1;180(2):631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerdt B. G., Houston M. A., Augenlicht L. H. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res. 1994 Jun 15;54(12):3288–3293. [PubMed] [Google Scholar]
- Jenkins M. K., Chen C. A., Jung G., Mueller D. L., Schwartz R. H. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990 Jan 1;144(1):16–22. [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987 Feb 1;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K. The role of cell division in the induction of clonal anergy. Immunol Today. 1992 Feb;13(2):69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- Johnson J. G., Jenkins M. K. Monocytes provide a novel costimulatory signal to T cells that is not mediated by the CD28/B7 interaction. J Immunol. 1994 Jan 15;152(2):429–437. [PubMed] [Google Scholar]
- Kyner D., Zabos P., Christman J., Acs G. Effect of sodium butyrate on lymphocyte activation. J Exp Med. 1976 Dec 1;144(6):1674–1678. doi: 10.1084/jem.144.6.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jones B., Aruffo A., Sullivan K. M., Linsley P. S., Janeway C. A., Jr Heat-stable antigen is a costimulatory molecule for CD4 T cell growth. J Exp Med. 1992 Feb 1;175(2):437–445. doi: 10.1084/jem.175.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Welch G. R., Wahl S. M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991 Mar 1;146(5):1541–1546. [PubMed] [Google Scholar]
- Matulonis U., Dosiou C., Freeman G., Lamont C., Mauch P., Nadler L. M., Griffin J. D. B7-1 is superior to B7-2 costimulation in the induction and maintenance of T cell-mediated antileukemia immunity. Further evidence that B7-1 and B7-2 are functionally distinct. J Immunol. 1996 Feb 1;156(3):1126–1131. [PubMed] [Google Scholar]
- Mondino A., Jenkins M. K. Surface proteins involved in T cell costimulation. J Leukoc Biol. 1994 Jun;55(6):805–815. doi: 10.1002/jlb.55.6.805. [DOI] [PubMed] [Google Scholar]
- Newmark H. L., Lupton J. R., Young C. W. Butyrate as a differentiating agent: pharmacokinetics, analogues and current status. Cancer Lett. 1994 Apr 1;78(1-3):1–5. doi: 10.1016/0304-3835(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Rubin A. L., Stenzel K. H. A new class of inhibitors of lymphocyte mitogenesis: agents that induce erythroid differentiation in Friend leukemia cells. J Immunol. 1980 Apr;124(4):1892–1897. [PubMed] [Google Scholar]
- Parra E., Wingren A. G., Hedlund G., Björklund M., Sjögren H. O., Kalland T., Sansom D., Dohlsten M. Costimulation of human CD4+ T lymphocytes with B7 and lymphocyte function-associated antigen-3 results in distinct cell activation profiles. J Immunol. 1994 Sep 15;153(6):2479–2487. [PubMed] [Google Scholar]
- Patry Y., Douillard J. Y., Meflah K., Le Pendu J. Immunization against a rat colon carcinoma by sodium butyrate-treated cells but not by interleukin 2-secreting cells. Gastroenterology. 1995 Nov;109(5):1555–1565. doi: 10.1016/0016-5085(95)90644-4. [DOI] [PubMed] [Google Scholar]
- Qiao L., Braunstein J., Golling M., Schürmann G., Autschbach F., Möller P., Meuer S. Differential regulation of human T cell responsiveness by mucosal versus blood monocytes. Eur J Immunol. 1996 Apr;26(4):922–927. doi: 10.1002/eji.1830260430. [DOI] [PubMed] [Google Scholar]
- Quill H., Schwartz R. H. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987 Jun 1;138(11):3704–3712. [PubMed] [Google Scholar]
- Rattis F. M., Péguet-Navarro J., Staquet M. J., Dezutter-Dambuyant C., Courtellemont P., Redziniak G., Schmitt D. Expression and function of B7-1 (CD80) and B7-2 (CD86) on human epidermal Langerhans cells. Eur J Immunol. 1996 Feb;26(2):449–453. doi: 10.1002/eji.1830260227. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Solary E., Bertrand R., Pommier Y. Apoptosis of human leukemic HL-60 cells induced to differentiate by phorbol ester treatment. Leukemia. 1994 May;8(5):792–797. [PubMed] [Google Scholar]
- Stenzel K. H., Schwartz R., Rubin A. L., Novogrodsky A. Chemical inducers of differentiation in Friend leukaemia cells inhibit lymphocyte mitogenesis. Nature. 1980 May 8;285(5760):106–108. doi: 10.1038/285106a0. [DOI] [PubMed] [Google Scholar]
- Stringer R. E., Hart C. A., Edwards S. W. Sodium butyrate delays neutrophil apoptosis: role of protein biosynthesis in neutrophil survival. Br J Haematol. 1996 Jan;92(1):169–175. doi: 10.1046/j.1365-2141.1996.00307.x. [DOI] [PubMed] [Google Scholar]
- Suthanthiran M., Rubin A. L., Novogrodsky A., Stenzel K. H. Immunosuppressive properties of polar organic compounds that induce cellular differentiation in Friend erythroleukemia cells. Transplantation. 1982 May;33(5):534–540. doi: 10.1097/00007890-198205000-00014. [DOI] [PubMed] [Google Scholar]
- Tan P., Anasetti C., Hansen J. A., Melrose J., Brunvand M., Bradshaw J., Ledbetter J. A., Linsley P. S. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993 Jan 1;177(1):165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford W. G., King L. E., Fraker P. J. Evaluation of glucocorticoid-induced DNA fragmentation in mouse thymocytes by flow cytometry. Cell Prolif. 1991 Sep;24(5):447–459. doi: 10.1111/j.1365-2184.1991.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- Young J. W., Koulova L., Soergel S. A., Clark E. A., Steinman R. M., Dupont B. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+ T lymphocytes by human blood dendritic cells in vitro. J Clin Invest. 1992 Jul;90(1):229–237. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]



