Abstract
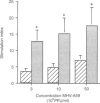
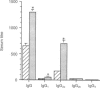
Owing to their scavenging and phagocytic functions, spleen macrophages are regarded to be important in the induction and maintenance of both innate and acquired immune defence mechanisms. In this study, we investigated the role of spleen macrophages in immunity against mouse hepatitis virus strain A59 (MHV-A59). Previous studies showed that spleen and liver macrophages are the first target cells for infection by MHV-A59 in vivo, suggesting that they could be involved in the induction of immune responses against MHV-A59. We used a macrophage depletion technique to deplete macrophages in vivo and studied the induction of virus-specific antibody and cytotoxic T-cell (CTL) responses and non-immune resistance against MHV-A59 in normal and macrophage-depleted mice. Virus titres in spleen and liver increased rapidly in macrophage-depleted mice, resulting in death of mice within 4 days after infection. Elimination of macrophages before immunization with MHV-A59 resulted in increased virus-specific humoral and T-cell proliferative responses. However, virus-specific CTL responses were not altered in macrophage-depleted mice. Our results show that spleen macrophages are of major importance as scavenger cells during MHV-A59 infection and are involved in clearance of virus from the host. In addition, macrophages may be involved in the regulation of acquired immune responses. In the absence of macrophages, increased virus-specific T-cell and antibody responses are detectable, suggesting that macrophages suppress MHV-A59-specific T- and B-cell responses and that other cells serve as antigen-presenting cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Ben-Nathan D., Huitinga I., Lustig S., van Rooijen N., Kobiler D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch Virol. 1996;141(3-4):459–469. doi: 10.1007/BF01718310. [DOI] [PubMed] [Google Scholar]
- Biron C. A. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol. 1994 Aug;6(4):530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Boorman G. A., Luster M. I., Dean J. H., Campbell M. L., Lauer L. A., Talley F. A., Wilson R. E., Collins M. J. Peritoneal macrophage alterations caused by naturally occurring mouse hepatitis virus. Am J Pathol. 1982 Jan;106(1):110–117. [PMC free article] [PubMed] [Google Scholar]
- Casebolt D. B., Spalding D. M., Schoeb T. R., Lindsey J. R. Suppression of immune response induction in Peyer's patch lymphoid cells from mice infected with mouse hepatitis virus. Cell Immunol. 1987 Oct 1;109(1):97–103. doi: 10.1016/0008-8749(87)90295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutelier J. P., Godfraind C., Dveksler G. S., Wysocka M., Cardellichio C. B., Noël H., Holmes K. V. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur J Immunol. 1994 Jun;24(6):1383–1390. doi: 10.1002/eji.1830240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrick J. E., Campbell P. A., Staerz U. D. Macrophages as accessory cells for class I MHC-restricted immune responses. J Immunol. 1991 Nov 1;147(9):2846–2851. [PubMed] [Google Scholar]
- Delemarre F. G., Kors N., van Rooijen N. Elimination of spleen and of lymph node macrophages and its difference in the effect on the immune response to particulate antigens. Immunobiology. 1990 Dec;182(1):70–78. doi: 10.1016/S0171-2985(11)80584-X. [DOI] [PubMed] [Google Scholar]
- Dempsey W. L., Smith A. L., Morahan P. S. Effect of inapparent murine hepatitis virus infections on macrophages and host resistance. J Leukoc Biol. 1986 May;39(5):559–565. doi: 10.1002/jlb.39.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk M. H., Schoemaker H. M., Spaan W. J., Boog C. J. Predominance of MHC class II-restricted CD4+ cytotoxic T cells against mouse hepatitis virus A59. Immunology. 1995 Apr;84(4):521–527. [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Lamontagne L. Impaired T and B cell subpopulations involved in a chronic disease induced by mouse hepatitis virus type 3. J Immunol. 1994 Aug 1;153(3):1318–1317. [PubMed] [Google Scholar]
- Karp C. L., Wysocka M., Wahl L. M., Ahearn J. M., Cuomo P. J., Sherry B., Trinchieri G., Griffin D. E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996 Jul 12;273(5272):228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- Karupiah G., Buller R. M., Van Rooijen N., Duarte C. J., Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996 Dec;70(12):8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Koolen M. J., Osterhaus A. D., Van Steenis G., Horzinek M. C., Van der Zeijst B. A. Temperature-sensitive mutants of mouse hepatitis virus strain A59: isolation, characterization and neuropathogenic properties. Virology. 1983 Mar;125(2):393–402. doi: 10.1016/0042-6822(83)90211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahmy C., Virelizier J. L. Prostaglandins as probable mediators of the suppression of antibody production by mouse hepatitis virus infection. Ann Immunol (Paris) 1981 Jan-Feb;132C(1):101–105. doi: 10.1016/0769-2625(81)90010-6. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Qian Q., Jutila M. A., Van Rooijen N., Cutler J. E. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol. 1994 May 15;152(10):5000–5008. [PubMed] [Google Scholar]
- Smith A. L., Bottomly K., Winograd D. F. Altered splenic T cell function of BALB/cByJ mice infected with mouse hepatitis virus or Sendai virus. J Immunol. 1987 May 15;138(10):3426–3430. [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma). Curr Opin Immunol. 1997 Feb;9(1):17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994 Sep 14;174(1-2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Chan E. L., Allison A. C. Immunosuppressive effects of lymphocyte (type II) and leucocyte (type I) interferon on primary antibody responses in vivo and in vitro. Clin Exp Immunol. 1977 Nov;30(2):299–304. [PMC free article] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Wijburg O. L., Heemskerk M. H., Sanders A., Boog C. J., Van Rooijen N. Role of virus-specific CD4+ cytotoxic T cells in recovery from mouse hepatitis virus infection. Immunology. 1996 Jan;87(1):34–41. [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Rouse B. T., Huang L. Induction of cytotoxic T lymphocytes in vivo with protein antigen entrapped in membranous vehicles. J Immunol. 1992 Sep 1;149(5):1599–1604. [PubMed] [Google Scholar]
- Zisman B., Wheelock E. F., Allison A. C. Role of macrophages and antibody in resistance of mice against yellow fever virus. J Immunol. 1971 Jul;107(1):236–243. [PubMed] [Google Scholar]
- de Souza M. S., Smith A. L., Bottomly K. Infection of BALB/cByJ mice with the JHM strain of mouse hepatitis virus alters in vitro splenic T cell proliferation and cytokine production. Lab Anim Sci. 1991 Apr;41(2):99–105. [PubMed] [Google Scholar]
- de Souza M. S., Smith A. L. Characterization of accessory cell function during acute infection of BALB/cByJ mice with mouse hepatitis virus (MHV), strain JHM. Lab Anim Sci. 1991 Apr;41(2):112–118. [PubMed] [Google Scholar]
- van Rooijen N., Kors N., Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989 Feb;45(2):97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]
- van Rooijen N. Macrophages as accessory cells in the in vivo humoral immune response: from processing of particulate antigens to regulation by suppression. Semin Immunol. 1992 Aug;4(4):237–245. [PubMed] [Google Scholar]
- van Rooijen N., Wijburg O. L., van den Dobbelsteen G. P., Sanders A. Macrophages in host defense mechanisms. Curr Top Microbiol Immunol. 1996;210:159–165. doi: 10.1007/978-3-642-85226-8_16. [DOI] [PubMed] [Google Scholar]