Abstract
High rates of genetic variation ensure the survival of RNA viruses. Although this variation is thought to result from error-prone replication, RNA viruses must also maintain highly conserved genomic segments. A balance between conserved and variable viral elements is especially important in order for viruses to avoid “error catastrophe.” Ribavirin has been shown to induce error catastrophe in other RNA viruses. We therefore used a novel hepatitis C virus (HCV) replication system to determine relative mutation frequencies in variable and conserved regions of the HCV genome, and we further evaluated these frequencies in response to ribavirin. We sequenced the 5′ untranslated region (5′ UTR) and the core, E2 HVR-1, NS5A, and NS5B regions of replicating HCV RNA isolated from cells transfected with a T7 polymerase-driven full-length HCV cDNA plasmid containing a cis-acting hepatitis delta virus ribozyme to control 3′ cleavage. We found quasispecies in the E2 HVR-1 and NS5B regions of untreated replicating viral RNAs but not in conserved 5′ UTR, core, or NS5A regions, demonstrating that important cis elements regulate mutation rates within specific viral segments. Neither T7-driven replication nor sequencing artifacts produced these nucleotide substitutions in control experiments. Ribavirin broadly increased error generation, especially in otherwise invariant regions, indicating that it acts as an HCV RNA mutagen in vivo. Similar results were obtained in hepatocyte-derived cell lines. These results demonstrate the potential utility of our system for the study of intrinsic factors regulating genetic variation in HCV. Our results further suggest that ribavirin acts clinically by promoting nonviable HCV RNA mutation rates. Finally, the latter result suggests that our replication model may be useful for identifying agents capable of driving replicating virus into error catastrophe.
Genetic variation provides a selective advantage for RNA virus populations, promoting escape from immune selection and rapid adaptation to novel environments (8). The absence of proofreading-repair mechanisms in RNA replicases and transcriptases is thought to contribute to mutation rates in the range of 10−3 to 10−5 substitutions per nucleotide per round of RNA replication (32). However, two factors exert a counterbalancing pressure against excessive genetic variation. A high degree of conservation of viral genomic sequences must be maintained for interactions with specific cellular proteins, as in the case of internal ribosomal entry. Excessive error rates can also contribute to net loss of fitness by leading to “error catastrophes” that threaten the viability of populations of quasispecies present in viral swarms. While the RNA genome of hepatitis C virus (HCV) contains hypervariable regions that are thought to contribute to immune escape, little is known about their intrinsic origins or their control in the absence of immune or drug selection.
The extraordinary genetic diversity of HCV is reflected in its in vivo generation of quasispecies, which occur throughout the coding regions of its genome. In contrast, the 5′ and 3′ untranslated regions (5′ and 3′ UTRs) display exceptional sequence conservation, likely a consequence of the critical roles these regions play in translation and RNA replication initiation (12). The most-variable regions of the genome are situated in regions associated with B-cell epitopes (3, 9), strongly suggesting that immune selection pressure plays a significant role in quasispecies diversity. In general, the outcome of acute HCV infection may be controlled by the results of a race between the evolution of viral sequences in the E2 hypervariable region and the expansion of cytotoxic T-cell responses mounted by infected persons (10, 18). Nevertheless, the absence of in vitro HCV replication systems has precluded analysis of intrinsic mutation rates that might explain some of the clinical variability found in this region (22, 29, 31).
Genetic variation in any specific viral segment results from a combination of the general infidelity of the RNA replicase, local nucleotide signals, and two major constraints: first, constraints on viral proteins related to both structure and function, and second, constraints on the RNA sequence itself. Sequence constraints might appear in regulatory regions such as the 5′ and 3′ UTRs but could also exist in coding regions. While both drugs and the immune system have been studied extensively as selective factors, little attention has been given to potential intrinsic control mechanisms that might regulate the variability of regions of HCV. The error rate of the HCV RNA-dependent RNA polymerase (RdRp) is largely unknown (26). Although local nucleotide structures including RNA stem-loops, polyadenosine stretches, and open regions with little intramolecular base pairing favor localized mutations in human immunodeficiency virus (7, 14, 16, 17), none of these structures have been evaluated in the HCV genome. Conservation of both positive charges and conformation has been found in E2 hypervariable region protein sequences, which argues for a convergence of sequence variability on a limited number of structures compatible with the postulated role of the E2 protein in cell attachment (28). Examination of all of these factors is required for better understanding of the clinical variations found in patients.
Viral genetic variation would be of special interest if viral mutation rates could be therapeutically increased to a range where error catastrophe broke the infectivity cycle of RNA viral populations (5). One postulated mechanism for the otherwise unknown efficacy of ribavirin (RBV) in HCV infection is its ability to act as an RNA virus mutagen and contribute to this kind of error catastrophe (5, 6). RBV has been shown to induce error-prone replication of the related GB virus (GBV) (15). Although HCV replicon systems demonstrate genetic variation after model infections, their mutations are likely to result from very specialized selection for the antibiotic resistance markers used to maintain the replicons (2, 19). We have recently demonstrated successful HCV RNA replication in a novel binary full-length expression system (4). This system also generates quasispecies corresponding to E2 hypervariable region 1 (HVR-1). Our culture system is uniquely capable of revealing sequence variability during early HCV replication because it starts with a single introduced viral sequence, and mutant viral genomes can be detected within hours to days. We therefore used this culture system to determine whether we could detect local variation in mutation rates throughout the HCV genome and to directly test the possible role of RBV in further driving error-prone replication of HCV.
MATERIALS AND METHODS
Cell lines.
CV-1 and HepG2 cells (American Type Culture Collection, Manassas, Va.) were maintained in Dulbecco's modified Eagle medium containing 10% (vol/vol) fetal bovine serum.
Drugs.
RBV and alpha interferon 2b (IFN-α2b) were both obtained from Schering Plough (Kenilworth, N.J.)
Plasmids and transfection-infection.
Our binary replication system has been described previously and is capable of successful HCV protein, negative-strand RNA, and positive-strand RNA synthesis (4). Briefly, a plasmid containing the infectious full-length genotype 1a H77 cDNA sequence (35) was adapted at its 5′ and 3′ termini with the T7 promoter and a hepatitis delta virus ribozyme sequence, respectively, to yield pT7flHCV-Rz, referred to below as H77. As a negative control, we removed a BglII-BglII fragment from pT7-flHCV-Rz to create an in-frame deletion mutant, pT7-HCVΔBglII-Rz (referred to below as ΔBglII), lacking the critical NS5B RdRp. H77 and ΔBglII were used to transfect subconfluent CV-1 cells. At 24 h following transfection, T7 polymerase was delivered by using a recombinant vaccinia virus vector, vv-T7 (referred to below as T7) (11).
RBV (50 or 400 μM) or IFN-α2b (100,000 IU) was added to selected transfected-infected CV-1 cells 20 h prior to the introduction of vv-T7. Twenty-four hours post-vv-T7 introduction, cells were lysed, and RNA was extracted by use of TRIzol (GIBCO/BRL, Rockville, Md.), DNase I treated (Roche Molecular Biochemicals, Indianapolis, Ind.), and phenol-chloroform extracted.
RT-PCR.
Reverse transcription (RT)-PCR was performed for the 5′ UTR, core, E2 HVR-1, NS5A, and NS5B regions by using region-specific primers derived from the parent H77 sequence. For the 5′ UTR, the sense primer was nt 41 to 60, 5′-CCCCTGTGAGGAACTACTGT-3′, and the antisense primer was nt 360 to 341, 5′-GGTGCACGGTCTACGAGACC-3′; for the core, the sense primer was nt 266 to 297, 5′-GGGTCGCGAAAGGCCTTGTGGTACTGCCTGAT-3′, and the antisense primer was nt 838 to 809, 5′-GTTGCATAGTTCACGCCGTCTTCCAGAACC-3′; for E1/E2, the sense primer was nt 802 to 841, 5′-GCGTCCGGGTTCTGGAAGACGGCGTGAACTATGCAACAGG-3′, and the antisense primer was nt 1639 to 1600, 5′-AGGCTTTCATTGCAGTTCAAGGCCGT GCTATTGATGTGCC-3′; for NS5A, the sense primer was nt 6853 to 6872, 5′-TGACGTCCATGCTCACTGAT-3′, and the antisense primer was nt 7176 to 7157, 5′-GAGACTTCCGCAGGATTTCT; and for NS5B, the sense primer was nt 8245 to 8275, 5′-TGGGGATCCCGTATGATACCCGCTGCTTTGA-3′, and the antisense primer was nt 8645 to 8616, 5′-GGCGGAATTCCTGGTCATAGCCTCCGTGAA-3′ (13, 33). RT was carried out on extracted RNA by using avian myeloblastosis virus reverse transcriptase (Perkin-Elmer, Branchburg, N.J.) and standard conditions. Thirty cycles of PCR were carried out using 25 pmol each of the relevant sense and antisense primers, 0.5 μM each deoxynucleoside triphosphate, 1.5 mM MgCl2, and 0.5 U of Taq polymerase (Perkin-Elmer) under the following cycling conditions: 95°C for 30 s, 45°C for 20 s, and 72°C for 60 s.
RNase protection assay.
Negative-strand HCV RNA was detected as described by Chung et al. (4). Detection of β-galactosidase RNA transcripts from the T7-dependent reporter plasmid OS8 was carried out as described by Chung et al. (4).
Immunoblotting.
Western blot analyses of HCV core protein and β-galactosidase were carried out as described by Chung et al. (4).
HCV RNA quasispecies analysis.
Amplicons were cloned by using the TOPO-PCR system (Invitrogen, Carlsbad, Calif.). Eleven to 20 individual transformants corresponding to each region were sequenced bidirectionally by the ABI Prism automated sequencer using primers flanking the insert. Synonymous and nonsynonymous substitutions at variance with the parental H77 and ΔBglII sequences were determined, and error generation rates were calculated as the number of nucleotide substitutions divided by the total number of nucleotides sequenced. Sequences were aligned, and quasispecies diversity rates were compared between groups by using the SPSS 9.0 statistical package.
RESULTS
HCV quasispecies generation is template dependent.
We used our binary HCV expression system previously to demonstrate the successful synthesis of HCV positive- and negative-strand RNA and protein in the presence of T7 and full-length infectious H77, whereas the presence of T7 and the mutant construct ΔBglII, lacking critical RNA polymerase sequences, was associated with HCV protein and positive-strand, but not negative-strand, synthesis (4). In addition, we observed the generation of quasispecies corresponding to E1/E2 HVR-1 in cells treated with wild-type T7/H77 but not in cells treated with the mutant T7/ΔBglII. These data provided strong evidence for the action of the low-fidelity NS5B RdRp on the viral RNA template. We therefore asked whether the generation of quasispecies extended to other portions of the HCV genomic template.
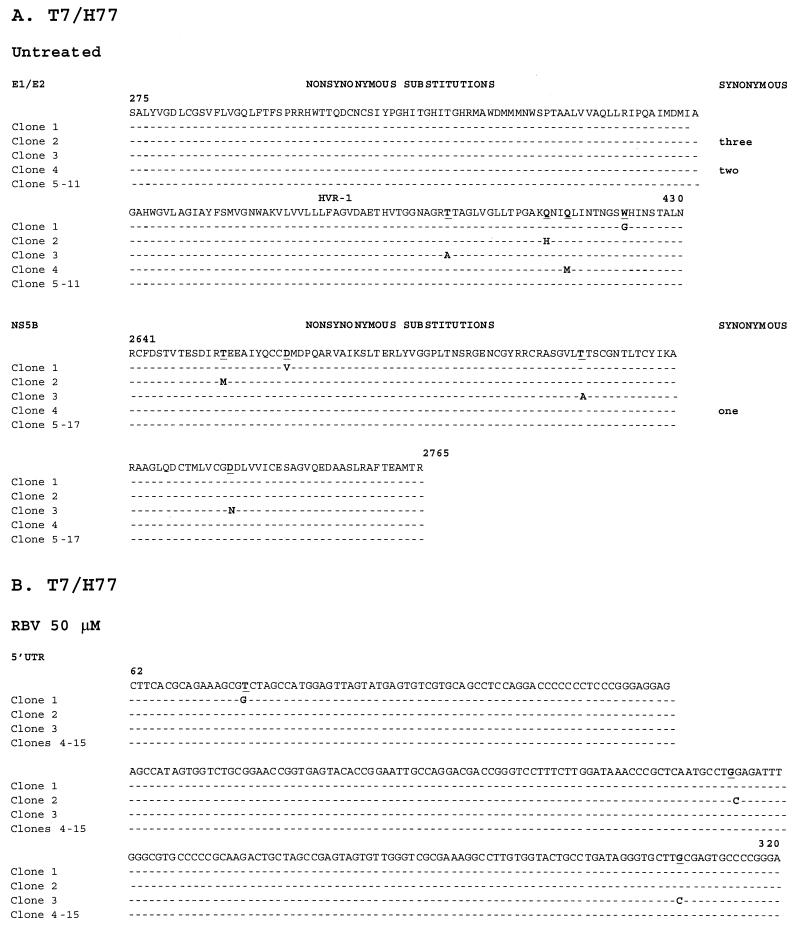
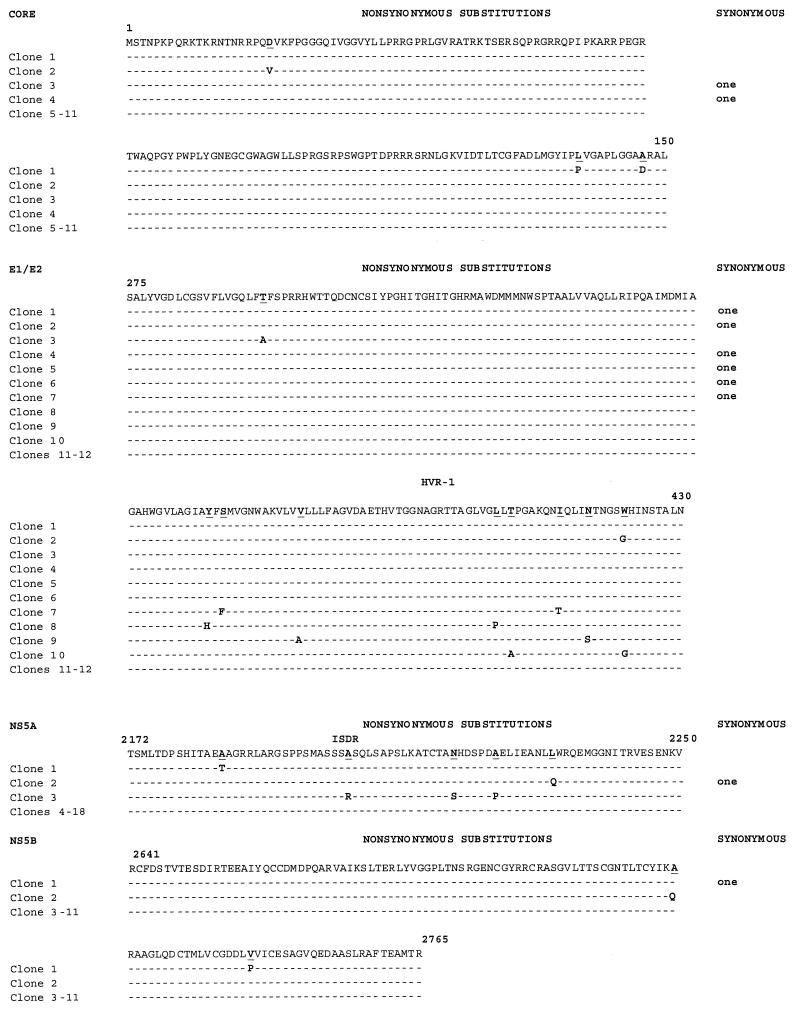
In addition to E1/E2 HVR-1, we examined sequences from the 5′ UTR and from the core, NS5A, and NS5B regions. Table 1 and Fig. 1 demonstrate the locations and frequencies of nucleotide substitutions corresponding to these regions. While we observed nine nucleotide substitutions (four nonsynonymous) in four independent clones for E1/E2 HVR-1 and five substitutions (four nonsynonymous) in NS5B, we found no substitutions in the 5′ UTR, the core region, or NS5A. In contrast, in the control samples, we observed no substitutions corresponding to the T7/ΔBglII-infected-transfected cells in the 5′ UTR, core region, or E1/E2 HVR-1, and two substitutions in NS5B, one in the core region, and one in E1/E2 HVR-1 corresponding to amplicons generated directly from the DNA template H77. The DNA template was used as a control for Taq and sequencing errors for the nonstructural regions (NS5A and NS5B) not amplifiable from the deletion mutant ΔBglII. Thus, quasispecies generation is dependent on the template region selected. Furthermore, quasispecies generation is not confined to E1/E2 HVR-1, although this region displayed the greatest variability. Specifically, the error generation rate (number of substitutions per total number of nucleotides sequenced) was greatest for E1/E2 HVR1 (1.7 × 10−3 [P < 0.001 relative to T7/ΔBglII and H77 by Fisher's exact test]), followed by NS5B (0.7 × 10−3 [not significantly different from that for H77]).
TABLE 1.
Comparison of mutation rates and synonymous and nonsynonymous nucleotide substitutions in five genomic regions between replicating HCV RNAs, transfected DNA only, and nonreplicating HCV RNAs
| Region and conditiona | No. of clones | No. (%) of mutant clones | Nonsynonymous/synonymous substitutions | Error generation rate (10−3)b |
|---|---|---|---|---|
| 5′ UTR | ||||
| T7/H77 | 11 | 0 | 0 | 0 |
| H77 | 11 | 0 | 0 | 0 |
| T7/ΔBglII | 14 | 0 | 0 | 0 |
| Core | ||||
| T7/H77 | 20 | 0 | 0 | 0 |
| H77 | 11 | 1 | 1/0 | 0.1 |
| T7/ΔBglII | 11 | 0 | 0 | 0 |
| E1/E2 | ||||
| T7/H77 | 11 | 4 (36) | 4/5 | 1.7∗ |
| H77 | 11 | 1 | 1/0 | 0.1 |
| T7/ΔBglII | 11 | 0 | 0 | 0 |
| NS5A | ||||
| T7/H77 | 14 | 0 | 0 | 0 |
| H77 | 12 | 0 | 0 | 0 |
| NS5B | ||||
| T7/H77 | 17 | 4 (17) | 4/1 | 0.7 |
| H77 | 12 | 2 | 2/0 | 0.4 |
T7/H77, replicating HCV RNAs; H77, transfected DNA only; T7/ΔBglII, nonreplicating HCV RNAs.
∗, P < 0.001 for comparison with T7/ΔBglII and with H77 by the χ2 test.
FIG. 1.
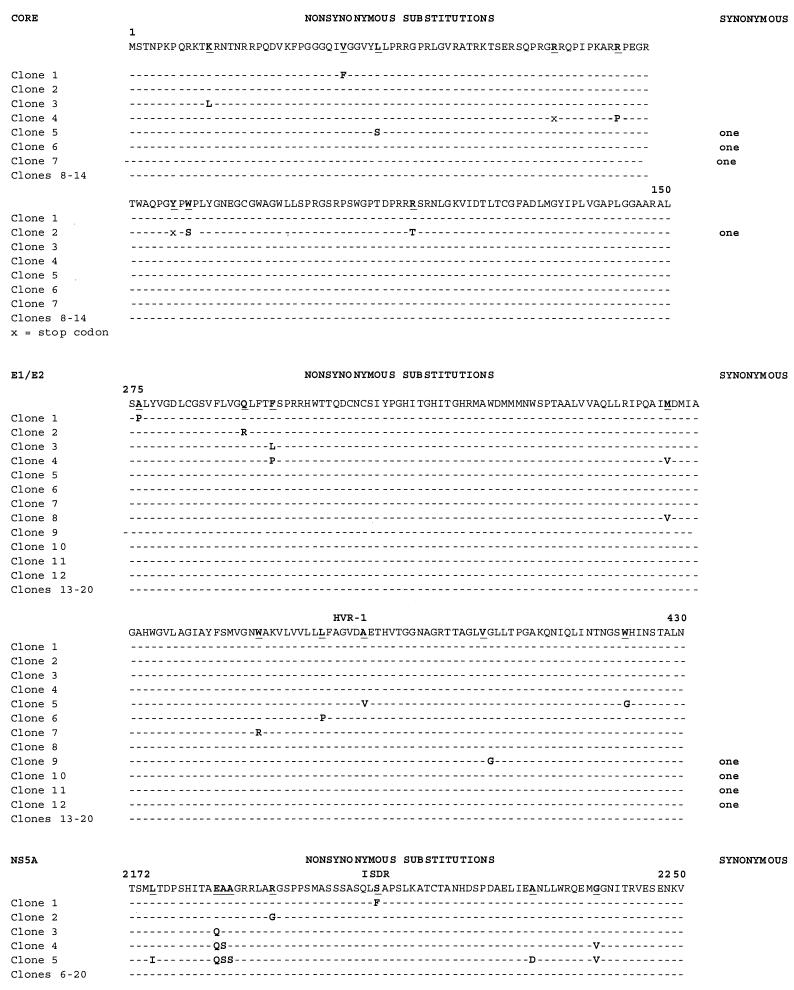
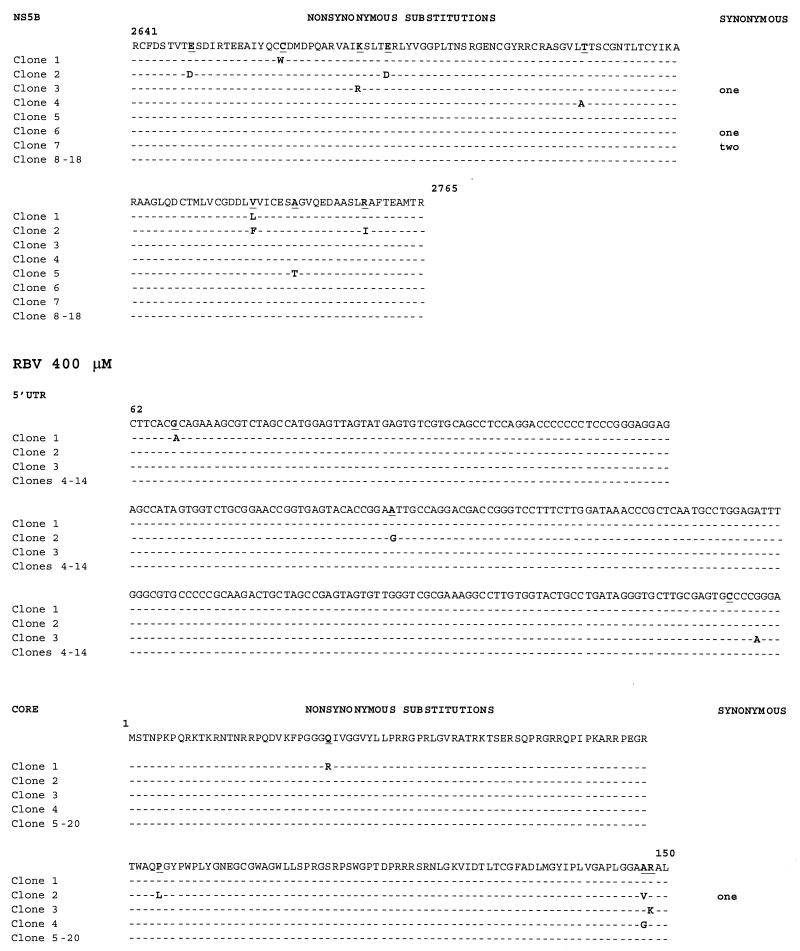
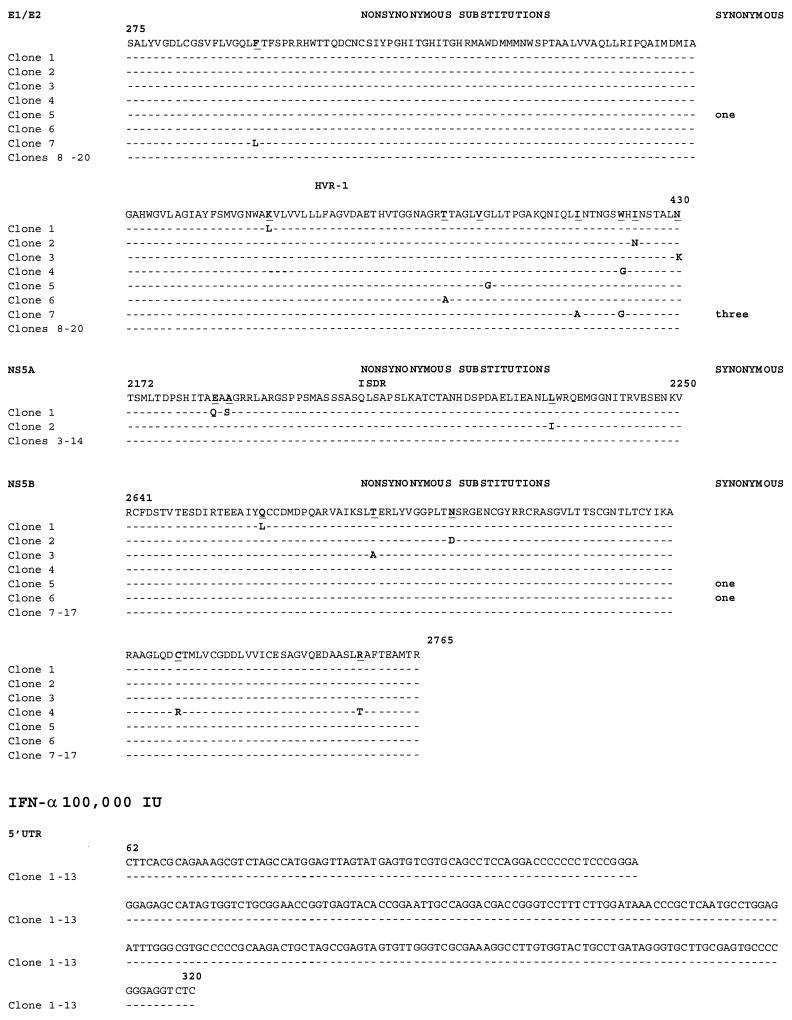
Sequence alignments for replicating RNA sequences corresponding to selected HCV genomic regions under untreated and treated conditions. RNAs were harvested, reverse transcribed, cloned, and independently sequenced. (A) Alignment of cDNA sequences of E1/E2 HVR-1 and NS5B (the only variant regions) following T7/full-length H77 transfection under untreated conditions. (B) Alignment of 5′ UTR, core, E1/E2 HVR-1, NS5A, and NS5B cDNA sequences under treatment with RBV at 50 or 400 μM or with IFN-α at 100,000 IU. Alignments were performed and compared with the parent H77 sequence. Nonsynonymous and synonymous nucleotide substitutions are indicated. (C and D) Alignment of 5′ UTR, core, and E1/E2 HVR-1 sequences following T7/ΔBglII transfection under untreated conditions (C) or under treatment with 50 μM RBV (D). Alignments were performed and compared with the parent H77 sequence.
RBV increases the HCV RNA mutation rate in vivo.
We examined the effects of RBV at a clinically relevant dose and an additional, higher dose to determine whether this agent acts as an RNA mutagen. Both doses inhibited herpes simplex virus type 1 (HSV-1) plaque formation in CV-1 cells (4).
We found that RBV induces mutations across all regions of the HCV genome tested (Table 2; Fig. 1A and B). At the lower dose (50 μM), we found 15 nucleotide substitutions, 1 insertion, and 1 deletion in HVR-1 in 12 of 20 clones examined (error generation rate, 1.5 × 10−3). Of greater interest, we found significant or near-significant increases in error generation in the 5′ UTR and the core, NS5A, and NS5B regions (Table 2; Fig. 1) over that in untreated T7/H77 HCV-replicating cells. For instance, in the core region, we found 12 substitutions (leading to 2 stop codons) and 4 insertions (leading to 2 stop codons) in 7 of 14 clones sequenced, whereas no errors were seen in untreated T7/H77 cells (P < 0.0006). The most-significant differences were seen in regions that had previously demonstrated no sequence variation (5′ UTR, core, and NS5A). These differences were even more prominent when compared to a composite nonreplicating negative control consisting of RT-PCR amplicons from T7/ΔBglII (to encompass the 5′ UTR and structural regions of the template) and PCR amplicons from the control plasmid H77 itself (to encompass nonstructural regions of the template not transcribed by T7/ΔBglII) (data not shown).
TABLE 2.
Comparison of mutation rates in five genomic regions between untreated replicating HCV RNAsa and replicating RNAs treated with RBV or IFN
| Region and condition | No. of clones | No. (%) of mutant clones | Nonsynonymous/synonymous substitutions | Error generation rate (10−3) |
|---|---|---|---|---|
| 5′ UTR | ||||
| T7/H77 | 11 | 0 | 0 | 0 |
| + RBV (50 μM) | 15 | 3 (20) | 0/3 | 0.7 |
| + RBV (400 μM) | 14 | 3 (21) | 0/3 | 0.7 |
| + IFN (100,000 IU) | 13 | 0 | 0 | 0 |
| Core | ||||
| T7/H77 | 20 | 0 | 0 | 0 |
| + RBV (50 μM) | 14 | 7 (50) | 8/4 | 1.9b |
| + RBV (400 μM) | 20 | 4 (20) | 5/1 | 0.6c |
| + IFN (100,000 IU) | 11 | 4 (36) | 3/2 | 1.0d |
| E1/E2 | ||||
| T7/H77 | 11 | 4 (36) | 4/5 | 1.7e |
| + RBV (50 μM) | 20 | 12 (60) | 11/4 | 1.5f |
| + RBV (400 μM) | 20 | 7 (35) | 9/4 | 1.2 |
| + IFN (100,000 IU) | 12 | 10 (83) | 10/6 | 2.8g |
| NS5A | ||||
| T7/H77 | 14 | 0 | 0 | 0 |
| + RBV (50 μM) | 20 | 5 (20) | 12/0 | 2.5h |
| + RBV (400 μM) | 14 | 2 (14) | 3/0 | 0.9 |
| + IFN (100,000 IU) | 18 | 3 (16) | 5/1 | 1.4 |
| NS5B | ||||
| T7/H77 | 17 | 4 (17) | 4/1 | 0.7 |
| + RBV (50 μM) | 18 | 7 (39) | 9/4 | 1.7 |
| + RBV (400 μM) | 17 | 6 (35) | 5/2 | 1 |
| + IFN (100,000 IU) | 11 | 2 (18) | 2/1 | 0.7 |
T7/H77.
Four nucleotide insertions (2 stop codons). P < 0.00006 relative to untreated T7/H77.
P < 0.05 relative to untreated T7/H77.
P < 0.01 relative to untreated T7/H77.
P < 0.04 relative to untreated T7/H77.
One nucleotide insertion (one stop codon); one nucleotide deletion.
Four nucleotide insertions. P < 0.03 relative to untreated T7/H77.
P < 0.06 relative to untreated T7/H77.
At higher doses of RBV (400 μM), we continued to observe error generation throughout the viral genome, but to a lesser degree than at the lower dose (Table 2). The degree of variability was evenly balanced across all regions (error generation rate, 0.6 × 10−3 to 1.2 × 10−3). Again, at this RBV dose, the most significant changes relative to untreated replicating HCV RNA were observed in the core region (P < 0.05).
To confirm that the mutations observed in the presence of RBV were dependent on HCV replication, we examined region-specific mutation rates in viral RNA produced by the ΔBglII deletion mutant. At mutagenic doses of RBV, only 2 mutations, encompassing the 5′ UTR, core, and E1/E2, were observed among 28 clones (Fig. 1C and D; Table 3). This did not differ significantly from the RNA sequences characterized for untreated T7/ΔBglII, indicating that the RBV-associated mutations observed were dependent on HCV RNA replication.
TABLE 3.
RBV does not induce mutations in nonreplicating HCV RNA
| Condition | Total no. of clones | No. (%) of mutant clones | Total no. of mutations (all regions) | Nonsynonymous/synonymous substitutions (ratio) | Error generation rate (10−3) (all regions) |
|---|---|---|---|---|---|
| Untreated T7/ΔBglII | 42 | 0 (0) | 0 | 0/0 | 0 |
| + RBV (50 μM) | 28 | 2 (6) | 2 | 1/1 (1.0) | 0.16a |
Not significantly different from the rate for untreated T7/ΔBglII.
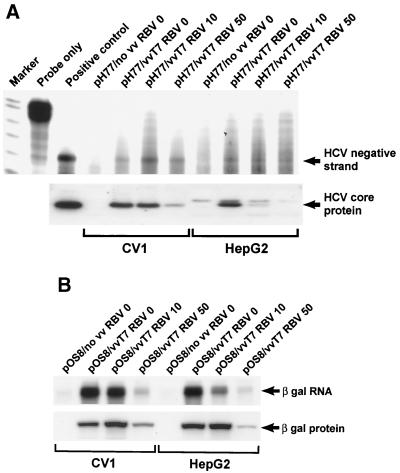
To address the effects of RBV on vaccinia virus-mediated T7 delivery, we compared T7-initiated replicating HCV to expression of a T7-dependent control β-galactosidase reporter (Fig. 2). Interestingly, we saw no RBV effect on HCV negative-strand synthesis at doses up to 50 μM (Fig. 2A). At 400 μM we found modest inhibition of HCV negative-strand RNA synthesis (data not shown). Importantly, we observed a significant reduction in T7-dependent β-galactosidase transcription and protein synthesis at 50 μM RBV, implying that RBV inhibits the vaccinia virus delivery system (Fig. 2B). Thus, although RBV significantly decreased T7 delivery, HCV negative-strand synthesis was preserved, suggesting strongly that HCV RNA replication is autonomously maintained in our system. We also found that HCV core expression was decreased by RBV, suggesting that RBV may specifically inhibit HCV core translation rates without disturbing viral replication.
FIG. 2.
(A) RNase protection assay and Western blot analysis of RBV dose effects (in micromolar concentrations) on HCV negative-strand (top panel) and core protein (bottom panel) synthesis under conditions of HCV RNA replication in CV-1 and HepG2 cells. RBV at 400 μM produced moderate inhibition of HCV negative-strand RNA synthesis in both cell types (data not shown). (B) RNase protection assay and Western blot analysis of RBV dose effects (in micromolar concentrations) on T7-dependent β-galactosidase RNA (top panel) and protein (bottom panel) synthesis under conditions of HCV RNA replication in CV-1 and HepG2 cells. pOS8, β-galactosidase expression plasmid.
To address whether these findings were applicable to hepatocyte-derived cell lines, we performed similar analyses with HepG2 cells. RBV exhibited similar effects on T7-dependent β-galactosidase transcription and protein synthesis as well as HCV negative-strand RNA synthesis in HepG2 and CV-1 cells (Fig. 2). For quasispecies analysis, we examined sequences from three regions: core, E1/E2, and NS5B (Table 4). Under untreated conditions, the T7/H77 construct displayed variability only in the E1/E2 region, at a rate lower than that observed in CV-1 cells. However, in the presence of RBV at doses mutagenic in CV-1 cells, a significant and broad increase in the mutation rate was observed across all regions. Thus, the effects of RBV are observed in cells of both hepatocyte and nonhepatocyte origin.
TABLE 4.
RBV induces mutations in replicating HCV RNA in HepG2 cells
| Region and condition | Total no. of clones | No. (%) of mutant clones | Total no. of mutations | Nonsynonymous/synonymous substitutions (ratio) | Error generation rate (10−3) |
|---|---|---|---|---|---|
| Core | |||||
| T7/H77 | 14 | 0 | 0 | 0 | 0 |
| + RBV (50 μM) | 11 | 4 (36) | 9 | 7/2 (3.50) | 1.8a |
| E1/E2 | |||||
| T7/H77 | 12 | 2 (17) | 2 | 1/1 (1.00) | 0.33 |
| + RBV (50 μM) | 14 | 7 (50) | 15 | 10/5 (2.00) | 2.1b |
| NS5B | |||||
| T7/H77 | 10 | 0 | 0 | 0 | 0 |
| + RBV (50 μM) | 12 | 3 (25) | 4 | 2/2 (1.00) | 0.79c |
| All regions | |||||
| T7/H77 | 36 | 2 (5.5) | 2 | 1/1 (1.00) | 0.1 |
| + RBV (50 μM) | 37 | 14 (38) | 28d | 19/9 (2.11) | 1.6 |
One nucleotide deletion (one stop codon). P < 0.001.
P < 0.01.
Two nucleotide deletions (one stop codon).
P < 0.0001 relative to untreated T7/H77 by the χ2 test with Yates' correction.
IFN-α increases the HCV RNA error generation rate.
We examined the effects of IFN-α on replicative HCV RNA sequences generated by our system. When a dose of IFN (100,000 IU) sufficient to inhibit HCV RNA synthesis (4) was administered to cells in the presence of T7 and H77, we observed an increase in error generation in four of the five regions sequenced (Table 2; Fig. 1). This dose, while in excess of doses used clinically, was selected because it overcomes the known inhibitory effects of vaccinia virus on downstream IFN effectors. The HCV core region, E1/E2 HVR-1, NS5A, and NS5B all showed increased rates of mutation. For the core region and E1/E2 HVR-1, this difference was statistically significant relative to mutation rates in untreated cells expressing wild-type full-length HCV sequences. Only 5′ UTR sequences remained invariant in the presence of IFN. Overall, the mutation rates attributable to RBV and IFN treatment were significantly different from those in untreated T7/H77 transfected-infected CV-1 cells (by the χ2 test with Yates' correction). Similarly, the proportions of mutant clones from each treated group were similar (about 30%), but the number of variant nucleotides per clone differed. When the error generation rate was averaged across all regions examined, we found an overall mutation rate of 0 to 2.8 × 10−3/site.
Synonymous- to nonsynonymous-mutation ratio and G→A or C→U transition mutations.
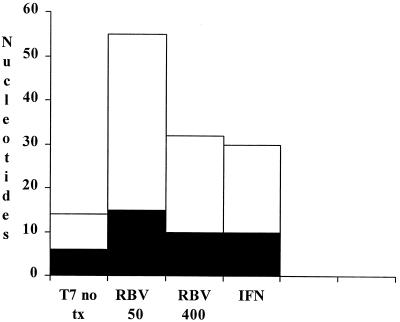
Under untreated replicative conditions (T7/H77), we observed a dn/ds (nonsynonymous/synonymous mutation ratio) of 1.33 (8:6) for all regions tested (Table 5; Fig. 1A and B). This ratio increased modestly to 2.67 with RBV at 50 μM, to 2.2 with RBV at 400 μM, and to 2.0 with IFN-α (Table 5; Fig. 3). We observed a large number of transition mutations in the presence of RBV at both doses (Table 6), including G-to-A and C-to-U transitions, which would be predicted by in vitro RBV incorporation experiments (6). However, C-to-U transitions were also observed in the absence of treatment in E1/E2 HVR-1. We also observed a number of transition mutations with IFN treatment, although there were fewer absolute and relative G-to-A and C-to-U transitions under these conditions. These differences were not statistically significant. Characterization of the direct effects of the observed substitutions, insertions, and deletions revealed that at least five mutations (three insertions [Table 2] and two substitutions [Fig. 1]) led to premature stop codons within the directly sequenced regions. These data directly demonstrate that a large proportion of these mutations would be predicted to result in nonviable proteins.
TABLE 5.
Comparison of mutation rates across all regions tested for untreated, RBV-treated, and IFN-treated replicating HCV RNA in CV-1 cells
| Condition | Total no. of clones | No. (%) of mutant clones | Total mutations (all regions) | Nonsynonymous/synonymous substitutions (ratio) | Error generation rate (10−3) (all regions) | Pa |
|---|---|---|---|---|---|---|
| T7/H77 (untreated) | 73 | 8 (11) | 14 | 8/6 (1.33) | 0.09 | |
| + RBV (50 μM) | 87 | 29 (33) | 55 | 40/15 (2.67) | 0.33 | <0.001 |
| + RBV (400 μM) | 85 | 25 (30) | 32 | 22/10 (2.2) | 0.20 | <0.008 |
| + IFN | 65 | 19 (30) | 30 | 20/10 (2.0) | 0.23 | <0.01 |
Significantly different from untreated T7/H77 by the χ2 test with Yates' correction.
FIG. 3.
Numbers of mutations from all tested regions of the HCV genome (5′ UTR, core, E1/E2, NS5A, and NS5B) for T7/H77 RNAs that were either left untreated (T7 no tx) or treated either with RBV at 50 or 400 μM or with IFN. Numbers of synonymous (solid) and nonsynonymous (open) nucleotide substitutions are shown for each condition.
TABLE 6.
Transition mutations recorded for all genomic regions for untreated, RBV-treated, and IFN-treated replicating HCV RNA
| Condition | No. of transition mutations
|
|||
|---|---|---|---|---|
| G→A | A→G | U→C | C→U | |
| T7/H77 (untreated) | 0 | 3 | 1 | 6 |
| + RBV (50 μM) | 5 | 7 | 10 | 5 |
| + RBV (400 μM) | 4 | 7 | 4 | 4 |
| + IFN | 2 | 5 | 12 | 2 |
DISCUSSION
Using a novel HCV RNA replication system capable of recapitulating the early steps in the HCV life cycle, we found that RNA quasispecies generation is observed in regions of the HCV genome in addition to HVR-1 but that mutation generation rates are highly variable. Specifically, significant error generation above that observed with the control plasmid lacking critical nonstructural region sequences necessary for RNA replication was most frequently observed in HVR-1 but was also seen in the NS5B region. However, no mutations were observed in three other, more conserved regions: the 5′ UTR, core, and NS5A. These findings, in the complete absence of immune selection pressure, have a number of implications for the HCV NS5B RdRp.
First, the finding of variable error generation suggests that the low fidelity attributable to the NS5B enzyme is template dependent. The fact that the highest error frequency occurred in a region (E1/E2 HVR-1) exhibiting the greatest quasispecies diversity from in vivo isolates suggests that, in addition to immune selection pressure, there is intrinsic quasispecies generation as a result of impaired template copying. This miscopying is likely to be a result of a complex RNA template secondary structure, such as that observed in E1/E2 HVR-1. Hence, additional cis-acting mechanisms appear to underlie the observed hypervariability of this region. It is also noteworthy that the accumulation of mutations in this region in naturally occurring HCV infection does not appear to be random (28), suggesting that there are specific cis-acting determinants of error generation. We also found variability in the NS5B region; in this regard, it is notable that modeling and physical analyses have demonstrated significant RNA secondary structure in this region as well (34; J. Wood, A. Tuplin, A. H. Patel, and P. Simmonds, presented at the 8th International HCV Conference, 2001; A. Branch, personal communication). Our data raise the intriguing possibility that the mutations observed in NS5B may be adaptive for replication. Further functional study of these sequences is warranted.
Second, the finding of quasispecies in a short-term expression system suggests that the regional accumulation of mutations is likely to be very high. Hence, it is very likely that a significant fraction of the observed substitutions, insertions, and deletions lead to nonviable polyprotein. Thus, the mutation rates reported here can offer estimates of the NS5B error generation rate but not of the true viable quasispecies generation rate.
Using our replication system, we found that RBV increases error generation, especially in otherwise invariant regions, suggesting that it acts as an RNA mutagen in vivo. Our findings confirm in principle those of Crotty et al. that RBV acts as an RNA mutagen in poliovirus (6). Our findings differ in several respects, however. First, the RNA mutation rate observed did not approach those described by Crotty et al. (6). Second, we found a decrease in HCV negative-strand RNA synthesis attributable to the use of RBV only at the highest dose tested, despite observation of its activity against HSV-1 at all doses in the same cell line (4). Third, there was no demonstrable dose-responsiveness of mutation rates. We speculate that RBV increases the HCV RNA mutagenesis rate, perhaps just to the brink of error catastrophe described for poliovirus and thus with minimal inhibitory effects on actual viral RNA synthesis in our short-term assay. RBV seems to function against HCV in a manner more comparable to its action against the related flavivirus GBV-B. GBV-B virions from cultured primary hepatocytes treated with RBV have reduced infectivity, but RBV does not directly inhibit GBV replication in vivo (15). It is also possible that demonstration of direct inhibition of HCV RNA synthesis by RBV will require long-term studies. We speculate that the failure to observe a linear RBV dose-response effect on HCV RNA mutation rates may reflect threshold effects obtained at the lower dose used.
The activity of RBV on replicating HCV RNA was also seen in hepatic cells. The lower quasispecies variability seen in replicating HCV RNA in HepG2 cells may reflect the presence of host cell factors in the replication complex that are less permissive for nucleotide misincorporation. We speculate that these factors may help account for observations of quasispecies compartmentalization in infected persons (24). The finding that RBV appears to increase mutation rates of HCV RNA in multiple cell types supports its direct interaction with the HCV replication complex.
We also observed an increase in quasispecies generation across all coding regions by treatment of our system with IFN. We found this of interest, since we and others have previously demonstrated a direct inhibitory effect of IFN on HCV RNA and protein synthesis (2, 4, 19). The finding of decreased viral RNA synthesis at doses that generate mutations raises the possibility that in addition to its well-described effects on RNA synthesis, IFN may also increase the generation of nonviable RNA species. This might occur not through RNA mutagenesis per se, but rather indirectly, via alteration of the availability of template and host factors critical to successful HCV RNA polymerization. These factors may be directly sensitive to the downstream effectors of IFN signaling, which are activated in CV-1 cells and other cell types (23). The observation that IFN increases viral heterogeneity in persons who fail to respond to therapy may be explained in part by this observation. These findings also raise the possibilities that IFN and RBV act synergistically to drive error-prone replication toward catastrophe and that in patients who fail to respond to IFN and RBV, error generation is increased but not to a level deleterious to virus survival.
It has been hypothesized that nonsynonymous mutations are a reflection of exogenously applied selection pressure rather than the result of enzymatic activity (30). However, in our system we consistently observed a moderate excess of nonsynonymous over synonymous mutations. This finding would not be predicted to occur by chance, given the increased likelihood that a random mutation would be predicted to produce synonymous substitutions. One possible explanation for these findings is that there are structural constraints imposed by the existence of cryptic alternate open reading frames, such as that described within the core region (34). However, a more plausible explanation for the excess of nonsynonymous mutations is the lack of conservative constraints imposed by our replication system generally. Indeed, the finding of similar dn/ds ratios across the genome (Fig. 1) argues that no single region is subjected to positive selection pressure. It should also be noted that not all of the RNA species generated by our system are destined to become viable quasispecies. Indeed, those producing true nonsynonymous substitutions have a significantly higher likelihood of being nonviable (some directly encode stop codons) and are thus never incorporated into mature virions. The RNA species identified in our system do not distinguish between viable and nonviable sequences and thus do not necessarily reflect the balance of circulating quasispecies in vivo.
Because our system relies exclusively on cultured epithelial cell lines and thus does not introduce immune selection pressure, our findings may reflect the in vivo fidelity and species generation of wild-type NS5B RdRp. They suggest not only that the RNA template may be susceptible to miscopying but also that this susceptibility is enhanced in regions where miscopying is likely to lead to amino acid substitutions. These findings could reflect an evolutionary template strategy for further generation of sequence diversity, even at the cost of increased generation of nonviable species. The fragile balance between viral strategies to generate increased sequence diversity and viral strategies to maintain functions critical for the viral life cycle can thus be upset by RNA mutagenic agents. It is noteworthy that the mutations observed could also reflect viral protein constraints related to interactions with cellular factors (e.g., enzymes or RNAs) that may induce region-specific mutation by trans mechanisms, thus resulting in selection for viral mutants. These trans-acting factors may be cell line specific, so that qualitative differences may exist between infected hepatocytes in vivo and the transfected CV-1 cells used in these studies.
A number of studies have examined the longitudinal mutation rate of HCV sequences from infected humans and chimpanzees. Estimates have ranged from 0.4 × 10−3 to 1.9 × 10−3 bases per site per year (1, 20, 25, 27). The findings in our study suggest that the mutation rate attributable to NS5B miscopying is substantially higher, leading to generation of quasispecies in some regions over a short term. It is thus likely that the combination of nonviability and host immune clearance narrows the range of circulating species to the more modest numbers observed over time in infected hosts.
Because it can detect viral RNA mutations, our HCV replication system offers an assay for the study of intrinsic factors that regulate the origins of genetic variation in HCV. It will permit identification of cis-acting sequences responsible for driving regional RNA and amino acid mutation rates. In addition, it will facilitate identification of trans-acting factors contributing to error generation. Among these factors is not only the NS5B polymerase but also other viral and cellular proteins likely to interact with NS5B in the multiprotein RNA replication complex. Finally, the finding of increased error generation induced by RBV suggests that our replication model may be of special interest for identifying other inhibitors of HCV capable of driving replicating virus into error catastrophe. This will be especially true of compounds with affinity for the NS5B RdRp, including agents of the nucleoside analogue class. The recent finding that RBV exerts its effects on HCV replication through the NS5B polymerase provides further support for the validity of this approach (21).
Acknowledgments
A. M. Contreras and Y. Hiasa contributed equally to this work.
We thank Kevin Korenblat for helpful comments.
A.M.C. is the recipient of a scholarship from the Mexican Institute of Social Security. R.T.C. was supported by NIH grant R01 DK 57857, the Hepatitis Foundation International, and the Christopher Tripoli Hepatitis C Research Fund. E.V.S. was supported by NIH grant R01 AI43478.
REFERENCES
- 1.Abe, K., G. Inchauspe, and K. Fujisawa. 1992. Genomic characterization and mutation rate of hepatitis C virus isolated from a patient who contracted hepatitis during an epidemic of non-A, non-B hepatitis in Japan. J. Gen. Virol. 73:2725-2729. [DOI] [PubMed] [Google Scholar]
- 2.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1975. [DOI] [PubMed] [Google Scholar]
- 3.Bukh, J., R. H. Miller, and R. H. Purcell. 1995. Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes. Semin. Liver Dis. 15:41-63. [DOI] [PubMed] [Google Scholar]
- 4.Chung, R. T., W. He, A. Saquib, A. M. Contreras, R. J. Xavier, A. Chawla, T. C. Wang, and E. V. Schmidt. 2001. Hepatitis C virus replication is directly inhibited by IFN-alpha in a full-length binary expression system. Proc. Natl. Acad. Sci. USA 98:9847-9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crotty, S., C. E. Cameron, and R. Andino. 2001. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc. Natl. Acad. Sci. USA 98:6895-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty, S., D. Maag, J. J. Arnold, W. Zhong, J. Y. Lau, Z. Hong, R. Andino, and C. E. Cameron. 2000. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 6:1375-1379. [DOI] [PubMed] [Google Scholar]
- 7.Doi, H. 1991. Importance of purine and pyrimidine content of local nucleotide sequences (six bases long) for evolution of the human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 88:9282-9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domingo, E., and J. J. Holland. 1997. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 51:151-178. [DOI] [PubMed] [Google Scholar]
- 9.Farci, P., and R. H. Purcell. 2000. Clinical significance of hepatitis C virus genotypes and quasispecies. Semin. Liver Dis. 20:103-126. [PubMed] [Google Scholar]
- 10.Farci, P., A. Shimoda, A. Coiana, G. Diaz, G. Peddis, J. C. Melpolder, A. Strazzera, D. Y. Chien, S. J. Munoz, A. Balestrieri, R. H. Purcell, and H. J. Alter. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339-344. [DOI] [PubMed] [Google Scholar]
- 11.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellen, C. U., and T. V. Pestova. 1999. Translation of hepatitis C virus RNA. J. Viral Hepat. 6:79-87. [DOI] [PubMed] [Google Scholar]
- 13.Hofgartner, W. T., S. J. Polyak, D. G. Sullivan, R. L. Carithers, Jr., and D. R. Gretch. 1997. Mutations in the NS5A gene of hepatitis C virus in North American patients infected with HCV genotype 1a or 1b. J. Med. Virol. 53:118-126. [DOI] [PubMed] [Google Scholar]
- 14.Ji, J., J. S. Hoffmann, and L. Loeb. 1994. Mutagenicity and pausing of HIV reverse transcriptase during HIV plus-strand DNA synthesis. Nucleic Acids Res. 22:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanford, R. E., D. Chavez, B. Guerra, J. Y. Lau, Z. Hong, K. M. Brasky, and B. Beames. 2001. Ribavirin induces error-prone replication of GB virus B in primary tamarin hepatocytes. J. Virol. 75:8074-8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le, S. Y., J. H. Chen, M. J. Braun, M. A. Gonda, and J. V. Maizel. 1988. Stability of RNA stem-loop structure and distribution of non-random structure in the human immunodeficiency virus (HIV-I). Nucleic Acids Res. 16:5153-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le, S. Y., J. H. Chen, D. Chatterjee, and J. V. Maizel. 1989. Sequence divergence and open regions of RNA secondary structures in the envelope regions of the 17 human immunodeficiency virus isolates. Nucleic Acids Res. 17:3275-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 20.Lu, L., T. Nakano, E. Orito, M. Mizokami, and B. H. Robertson. 2001. Evaluation of accumulation of hepatitis C virus mutations in a chronically infected chimpanzee: comparison of the core, E1, HVR1, and NS5B regions. J. Virol. 75:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maag, D., C. Castro, Z. Hong, and C. E. Cameron. 2001. Hepatitis C virus RNA-dependent RNA polymerase (NS5B) as a mediator of the antiviral activity of ribavirin. J. Biol. Chem. 276:46094-46098. [DOI] [PubMed] [Google Scholar]
- 22.Mao, Q., S. C. Ray, O. Laeyendecker, J. R. Ticehurst, S. A. Strathdee, D. Vlahov, and D. L. Thomas. 2001. Human immunodeficiency virus seroconversion and evolution of the hepatitis C virus quasispecies. J. Virol. 75:3259-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa, Y., E. F. Petricoin III, H. Akai, P. M. Grimley, B. Rupp, and A. C. Larner. 1992. Interferon-alpha-induced gene expression: evidence for a selective effect of ouabain on activation of the ISGF3 transcription complex. Virology 190:210-220. [DOI] [PubMed] [Google Scholar]
- 24.Navas, S., J. Martin, J. A. Quiroga, I. Castillo, and V. Carreno. 1998. Genetic diversity and tissue compartmentalization of the hepatitis C virus genome in blood mononuclear cells, liver, and serum from chronic hepatitis C patients. J. Virol. 72:1640-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogata, N., H. J. Alter, R. H. Miller, and R. H. Purcell. 1991. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc. Natl. Acad. Sci. USA 88:3392-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh, J. W., T. Ito, and M. M. Lai. 1999. A recombinant hepatitis C virus RNA-dependent RNA polymerase capable of copying the full-length viral RNA. J. Virol. 73:7694-7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto, H., M. Kojima, S. Okada, H. Yoshizawa, H. Iizuka, T. Tanaka, E. E. Muchmore, D. A. Peterson, Y. Ito, and S. Mishiro. 1992. Genetic drift of hepatitis C virus during an 8.2-year infection in a chimpanzee: variability and stability. Virology 190:894-899. [DOI] [PubMed] [Google Scholar]
- 28.Penin, F., C. Combet, G. Germanidis, P. O. Frainais, G. Deleage, and J. M. Pawlotsky. 2001. Conservation of the conformation and positive charges of hepatitis C virus E2 envelope glycoprotein hypervariable region 1 points to a role in cell attachment. J. Virol. 75:5703-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polyak, S. J., S. McArdle, S. L. Liu, D. G. Sullivan, M. Chung, W. T. Hofgartner, R. L. Carithers, Jr., B. J. McMahon, J. I. Mullins, L. Corey, and D. R. Gretch. 1998. Evolution of hepatitis C virus quasispecies in hypervariable region 1 and the putative interferon sensitivity-determining region during interferon therapy and natural infection. J. Virol. 72:4288-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray, S. C., Q. Mao, R. E. Lanford, S. Bassett, O. Laeyendecker, Y. M. Wang, and D. L. Thomas. 2000. Hypervariable region 1 sequence stability during hepatitis C virus replication in chimpanzees. J. Virol. 74:3058-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandres, K., M. Dubois, C. Pasquier, J. L. Payen, L. Alric, M. Duffaut, J. P. Vinel, J. P. Pascal, J. Puel, and J. Izopet. 2000. Genetic heterogeneity of hypervariable region 1 of the hepatitis C virus (HCV) genome and sensitivity of HCV to alpha interferon therapy. J. Virol. 74:661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinhauer, D. A., E. Domingo, and J. J. Holland. 1992. Lack of evidence for proofreading mechanisms associated with an RNA virus polymerase. Gene 122:281-288. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan, D. G., J. J. Wilson, R. L. Carithers, Jr., J. D. Perkins, and D. R. Gretch. 1998. Multigene tracking of hepatitis C virus quasispecies after liver transplantation: correlation of genetic diversification in the envelope region with asymptomatic or mild disease patterns. J. Virol. 72:10036-10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walewski, J. L., T. R. Keller, D. D. Stump, and A. D. Branch. 2001. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA 7:710-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanagi, M., R. H. Purcell, S. U. Emerson, and J. Bukh. 1997. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc. Natl. Acad. Sci. USA 94:8738-8743. [DOI] [PMC free article] [PubMed] [Google Scholar]