Abstract
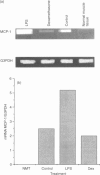
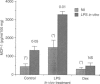
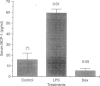
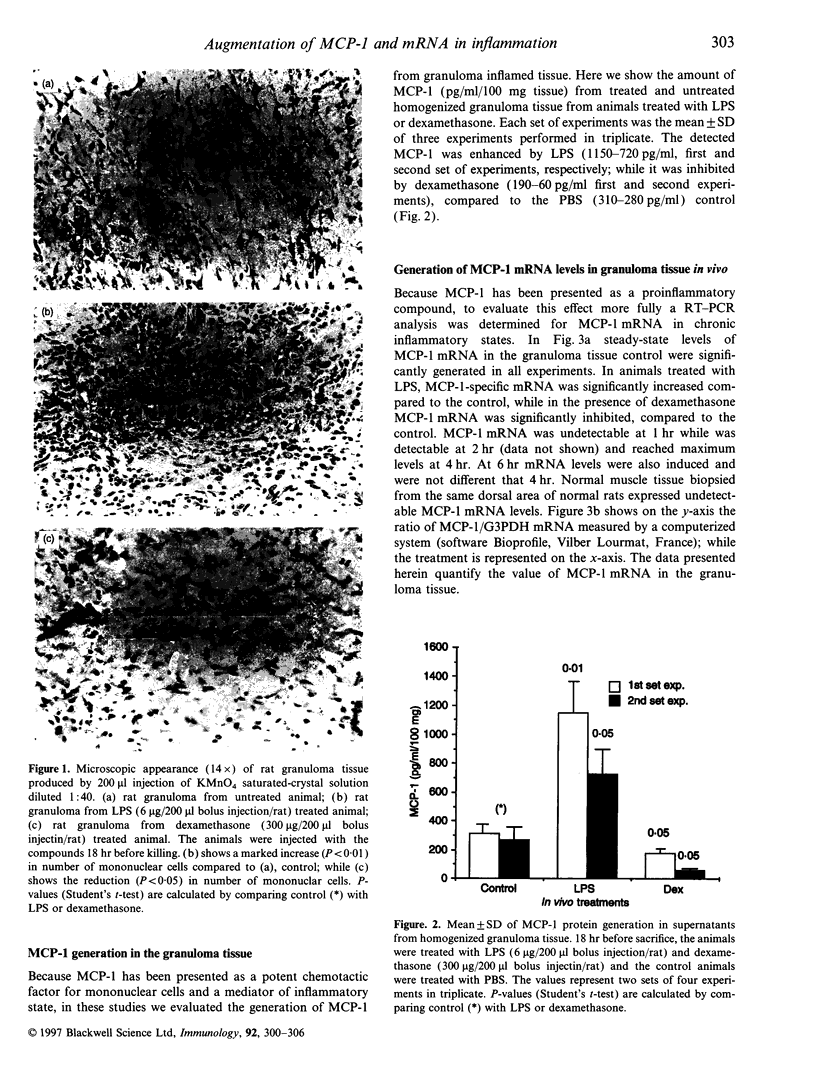
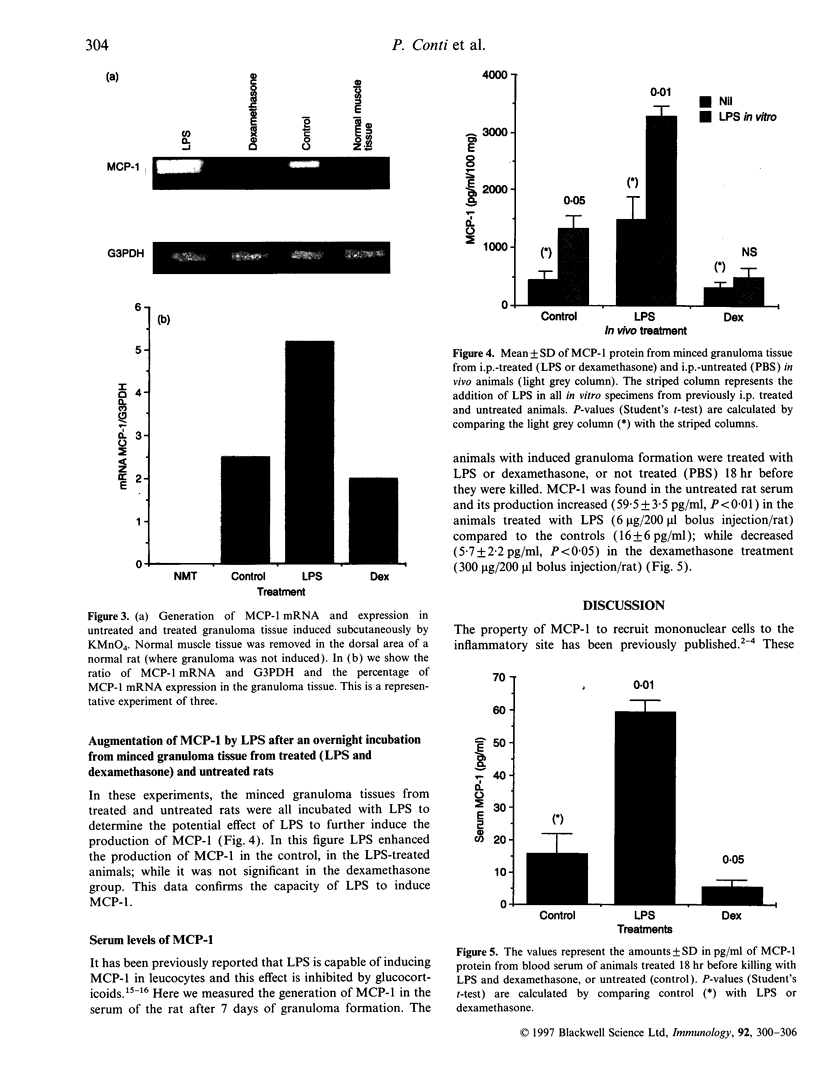
Monocyte chemotactic protein-1 (MCP-1) is a proinflammatory cytokine that attracts and activates specific types of leucocytes. The purpose of this work was to analyse the generation of MCP-1 and mRNA transcript in a model of chronic inflammation using a granulomatous tissue induced by potassium permanganate (KMnO4; water soluble crystals). The data presented here shows that MCP-1 is generated in granuloma tissue and its level was strongly increased by i.p. injections of lipopolysaccharide (LPS) and inhibited in rats treated with injections of dexamethasone, 18 hr before the animals were killed. In histological studies LPS and dexamethasone increased and decreased, respectively, the recruitment of mononuclear cells in the granuloma tissue compared with the control granulomas from phosphate-buffered saline (PBS)-treated animals. Reverse transcriptase-polymerase chain reaction (RT-PCR) was used for mRNA extraction and cDNA synthesis. mRNA MCP-1 was significantly produced in the granuloma tissue of untreated animals, an effect increased by LPS and inhibited by dexamethasone, compared with the controls. Moreover, MCP-1 protein was found in the supernatant from homogenized granuloma tissues and the levels of MCP-1 were higher in the LPS-treated animals, while they were lower in the dexamethasone group, compared with the granulomas from the PBS-treated groups (control). The generation of MCP-1 was also found in minced granuloma tissue incubated for 18 hr (overnight) from treated (LPS or dexamethasone) and untreated (PBS) rats. When LPS was added in vitro for 18 hr to the controls and treated animals the production of MCP-1 was further increased except in the dexamethasone group (P > 0.05). Analysing blood serum from LPS, dexamethasone or PBS-treated rats, we found that MCP-1 was also present. The level was higher in the LPS group and lower in the dexamethasone group, compared with the control (PBS). In these studies we show for the first time that MCP-1 transcript and translation is generated in chronic experimental inflammatory tissue, an effect inhibited by dexamethasone.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baggiolini M., Dahinden C. A. CC chemokines in allergic inflammation. Immunol Today. 1994 Mar;15(3):127–133. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Baumgartner J. D., Heumann D., Gerain J., Weinbreck P., Grau G. E., Glauser M. P. Association between protective efficacy of anti-lipopolysaccharide (LPS) antibodies and suppression of LPS-induced tumor necrosis factor alpha and interleukin 6. Comparison of O side chain-specific antibodies with core LPS antibodies. J Exp Med. 1990 Mar 1;171(3):889–896. doi: 10.1084/jem.171.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M. W., Roth S. J., Luther E., Rose S. S., Springer T. A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Boucher W., Letourneau R., Feliciani C., Reale M., Barbacane R. C., Vlagopoulos P., Bruneau G., Thibault J., Theoharides T. C. Monocyte chemotactic protein-1 provokes mast cell aggregation and [3H]5HT release. Immunology. 1995 Nov;86(3):434–440. [PMC free article] [PubMed] [Google Scholar]
- Conti P., Continenza A. Decreased ulcerogenic effect of indomethacin in the rat when given in association with diflunisal. J Pathol. 1980 Aug;131(4):357–361. doi: 10.1002/path.1711310407. [DOI] [PubMed] [Google Scholar]
- Conti P., Dempsey R. A., Reale M., Barbacane R. C., Panara M. R., Bongrazio M., Mier J. W. Activation of human natural killer cells by lipopolysaccharide and generation of interleukin-1 alpha, beta, tumour necrosis factor and interleukin-6. Effect of IL-1 receptor antagonist. Immunology. 1991 Aug;73(4):450–456. [PMC free article] [PubMed] [Google Scholar]
- Conti P., Panara M. R., Barbacane R. C., Bongrazio M., Dempsey R. A., Reale M. Human recombinant IL-1 receptor antagonist (IL-1Ra) inhibits leukotriene B4 generation from human monocyte suspensions stimulated by lipopolysaccharide (LPS). Clin Exp Immunol. 1993 Mar;91(3):526–531. doi: 10.1111/j.1365-2249.1993.tb05936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Panara M. R., Fridas S., Barbacane R. C., Grilli A., Placido F. C., Reale M., Fiore S. Inhibition of granuloma formation induced by potassium permanganate in the mouse by a specific human recombinant receptor antagonist for interleukin-1 (hrIL-1ra). Cell Immunol. 1993 Apr 1;147(2):446–457. doi: 10.1006/cimm.1993.1083. [DOI] [PubMed] [Google Scholar]
- Conti P., Pang X., Boucher W., Letourneau R., Reale M., Barbacane R. C., Thibault J., Theoharides T. C. Impact of Rantes and MCP-1 chemokines on in vivo basophilic cell recruitment in rat skin injection model and their role in modifying the protein and mRNA levels for histidine decarboxylase. Blood. 1997 Jun 1;89(11):4120–4127. [PubMed] [Google Scholar]
- Conti P., Reale M., Panara M. R., Fridas S., Placido F. C., Barbacane R. C. Interleukin-1 receptor antagonist inhibits calcium accumulation in in vivo chronic granuloma induced by potassium permanganate. Calcif Tissue Int. 1993 Apr;52(4):300–304. doi: 10.1007/BF00296655. [DOI] [PubMed] [Google Scholar]
- Evanoff H. L., Burdick M. D., Moore S. A., Kunkel S. L., Strieter R. M. A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest. 1992 Feb;21(1):39–45. doi: 10.3109/08820139209069361. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Dohlsten M., Andersson U., Hedlund G., Ericsson P., Hansson J., Sjögren H. O. Production of TNF-alpha and TNF-beta by staphylococcal enterotoxin A activated human T cells. J Immunol. 1990 Jun 15;144(12):4663–4669. [PubMed] [Google Scholar]
- Frazier-Jessen M. R., Kovacs E. J. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. J Immunol. 1995 Feb 15;154(4):1838–1845. [PubMed] [Google Scholar]
- Ku G., Doherty N. S., Schmidt L. F., Jackson R. L., Dinerstein R. J. Ex vivo lipopolysaccharide-induced interleukin-1 secretion from murine peritoneal macrophages inhibited by probucol, a hypocholesterolemic agent with antioxidant properties. FASEB J. 1990 Apr 1;4(6):1645–1653. doi: 10.1096/fasebj.4.6.2318380. [DOI] [PubMed] [Google Scholar]
- Lise L. D., Audibert F. Immunoadjuvants and analogs of immunomodulatory bacterial structures. Curr Opin Immunol. 1989 Dec;2(2):269–274. doi: 10.1016/0952-7915(89)90199-4. [DOI] [PubMed] [Google Scholar]
- Locati M., Zhou D., Luini W., Evangelista V., Mantovani A., Sozzani S. Rapid induction of arachidonic acid release by monocyte chemotactic protein-1 and related chemokines. Role of Ca2+ influx, synergism with platelet-activating factor and significance for chemotaxis. J Biol Chem. 1994 Feb 18;269(7):4746–4753. [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurer R., Van Riper G., Feeney W., Cunningham P., Hora D., Jr, Springer M. S., MacIntyre D. E., Rosen H. Formation of eosinophilic and monocytic intradermal inflammatory sites in the dog by injection of human RANTES but not human monocyte chemoattractant protein 1, human macrophage inflammatory protein 1 alpha, or human interleukin 8. J Exp Med. 1993 Dec 1;178(6):1913–1921. doi: 10.1084/jem.178.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus R. P., Stamp G. W., Relf M. G., Burke F., Malik S. T., Bernasconi S., Allavena P., Sozzani S., Mantovani A., Balkwill F. R. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995 May;95(5):2391–2396. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Taub D. D., Proost P., Murphy W. J., Anver M., Longo D. L., van Damme J., Oppenheim J. J. Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J Clin Invest. 1995 Mar;95(3):1370–1376. doi: 10.1172/JCI117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub D., Dastych J., Inamura N., Upton J., Kelvin D., Metcalfe D., Oppenheim J. Bone marrow-derived murine mast cells migrate, but do not degranulate, in response to chemokines. J Immunol. 1995 Mar 1;154(5):2393–2402. [PubMed] [Google Scholar]
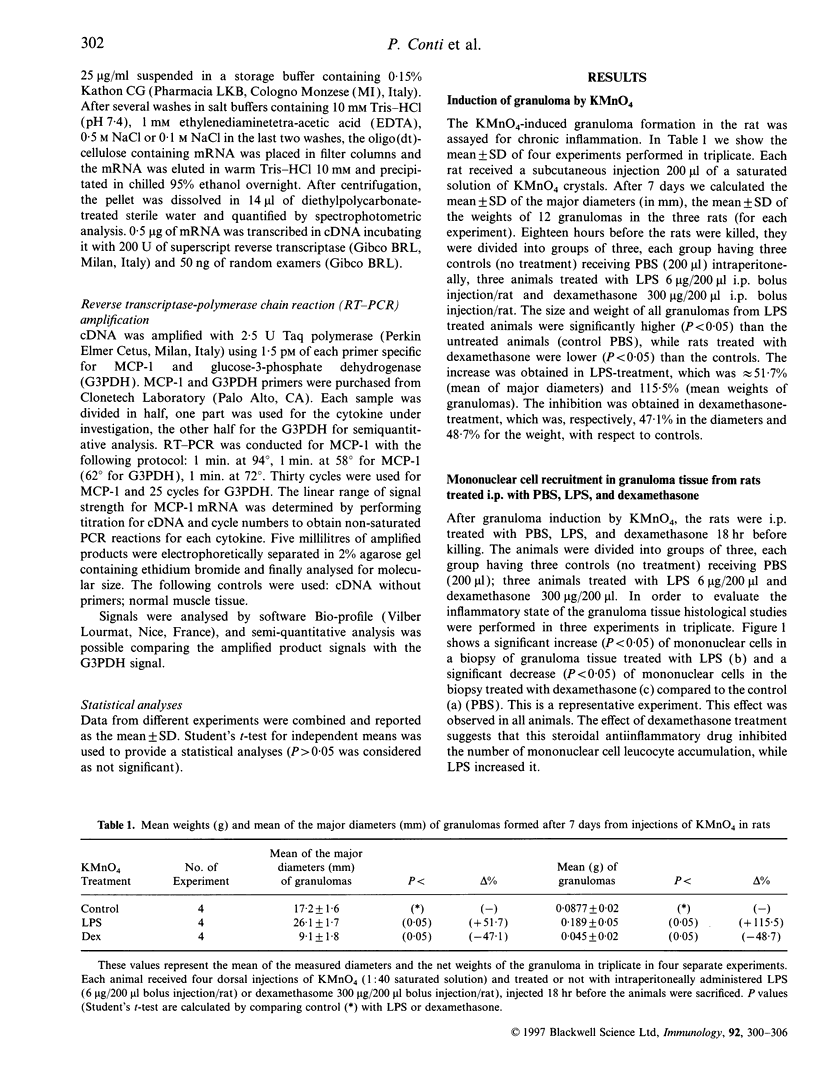
- Van Damme J., Proost P., Lenaerts J. P., Opdenakker G. Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J Exp Med. 1992 Jul 1;176(1):59–65. doi: 10.1084/jem.176.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]