Abstract
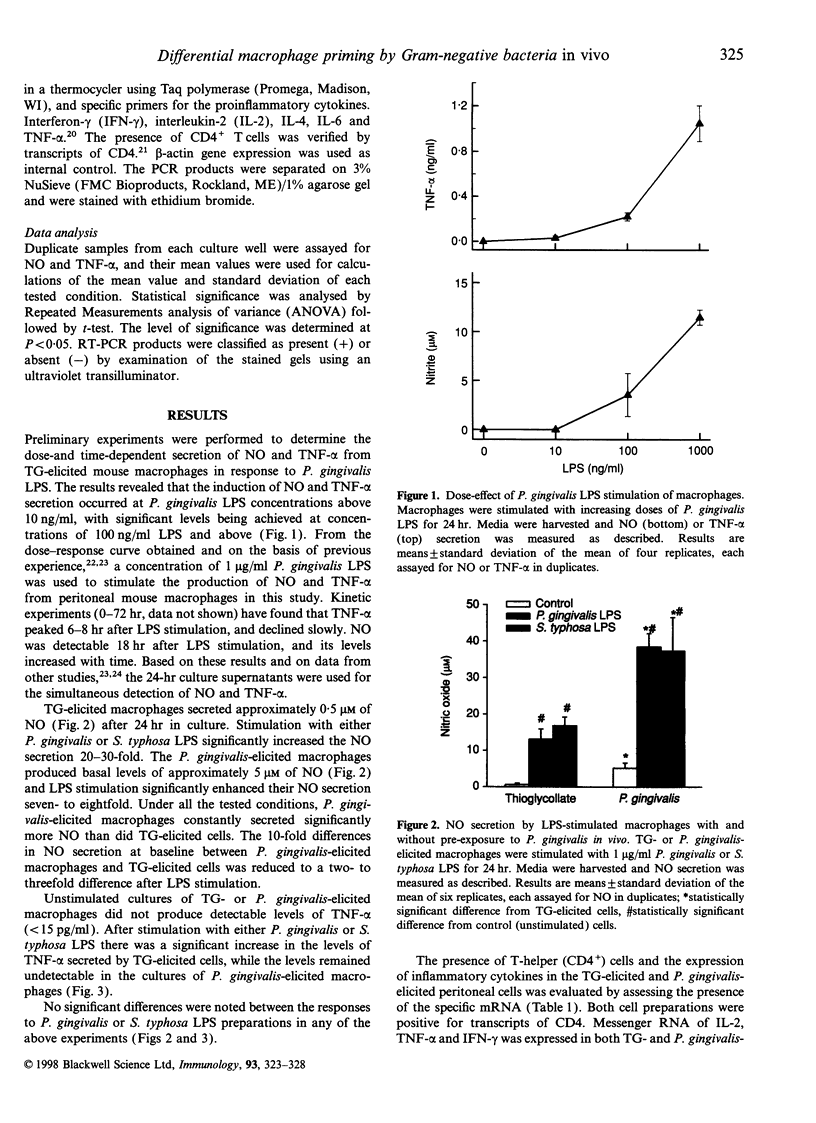
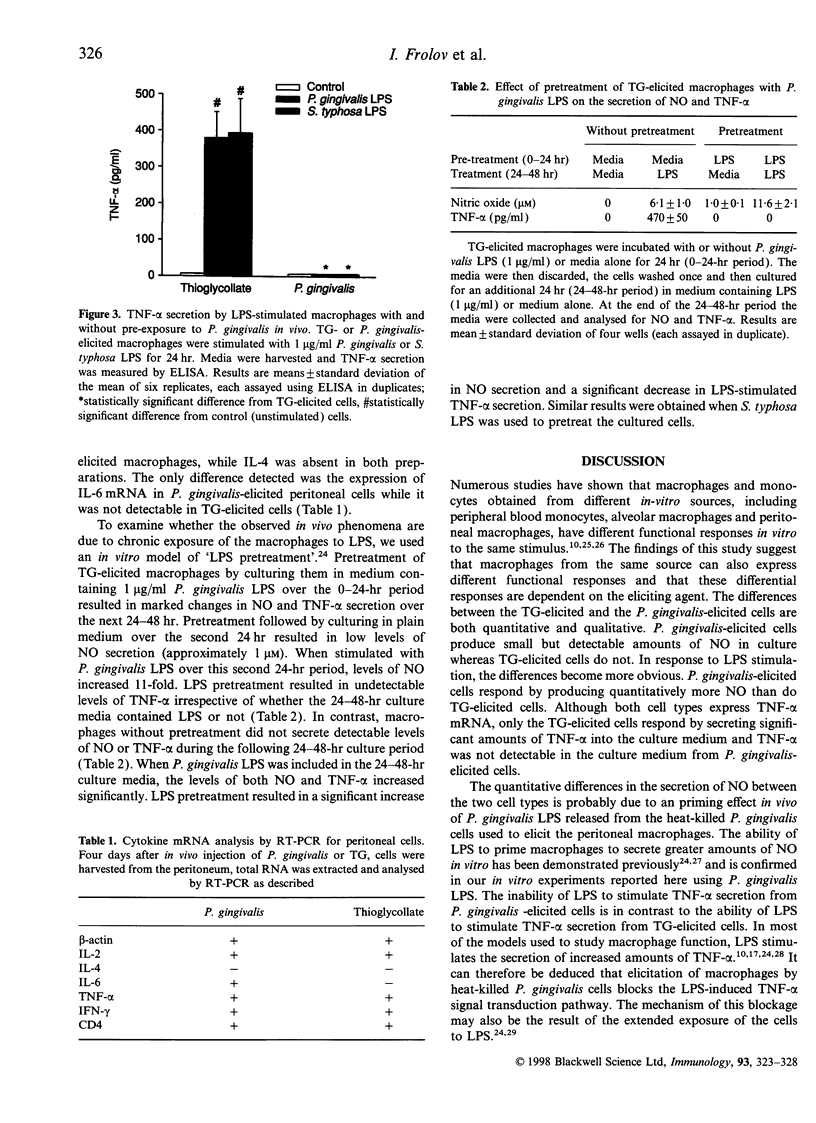
The present study was designed to test whether the functional response of mouse macrophages elicited by chronic exposure to bacteria will be different from that of cells elicited by a non-bacterial irritant. Macrophage elicitation was conducted by Porphyromonas gingivalis, a major periodontal pathogen, in comparison to a standard elicitation by thioglycollate (TG). We measured lipopolysaccharide (LPS)-induced nitric oxide (NO) and tumour necrosis factor-alpha (TNF-alpha) secretion by the elicited macrophages, and the expression of inflammatory cytokines in the whole elicited cell population. In addition, we tested the response of TG-elicited macrophages to pretreatment with P. gingivalis LPS in vitro. Mouse peritoneal macrophages were harvested 4 days after intraperitoneal injection of TG or heat-killed P. gingivalis. TG-elicited macrophages produced undetectable levels of TNF-alpha and approximately 0.5 microM of NO. The stimulation of the macrophages with LPS resulted in the secretion of NO and TNF-alpha in a dose-dependent manner. The P. gingivalis-elicited macrophages produced basal levels of approximately 5 microM NO, but TNF-alpha was not detectable. LPS stimulation of these cells further increased the secretion of NO eightfold while TNF-alpha remained undetectable. The NO secretion by P. gingivalis-elicited cells was significantly higher than that by TG-elicited cells. Examination of cytokine expression in the whole elicited cell population revealed that both P. gingivalis-elicited cells and TG-elicited cells expressed messenger RNA for interleukin-2 (IL-2), TNF-alpha and interferon-gamma (IFN-gamma), but not for IL-4. IL-6 was expressed in P. gingivalis-elicited cells only. Pretreatment of TG-elicited macrophages with P. gingivalis LPS for 24 hr prior to a second LPS challenge resulted in down-regulation of TNF-alpha secretion and up-regulation of NO secretion, a response similar to that seen in P. gingivalis-elicited peritoneal macrophages. The results suggest that the in vivo exposure of resident macrophages to P. gingivalis induces functional changes in peritoneal macrophages. These changes might be due to the effect of P. gingivalis LPS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Brenner T., Brocke S., Szafer F., Sobel R. A., Parkinson J. F., Perez D. H., Steinman L. Inhibition of nitric oxide synthase for treatment of experimental autoimmune encephalomyelitis. J Immunol. 1997 Mar 15;158(6):2940–2946. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Ehlers S., Mielke M. E., Blankenstein T., Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. The immediate early phase in innate resistance and acquired immunity. J Immunol. 1992 Nov 1;149(9):3016–3022. [PubMed] [Google Scholar]
- Fine D. H., Mendieta C., Barnett M. L., Furgang D., Naini A., Vincent J. W. Endotoxin levels in periodontally healthy and diseased sites: correlation with levels of gram-negative bacteria. J Periodontol. 1992 Nov;63(11):897–901. doi: 10.1902/jop.1992.63.11.897. [DOI] [PubMed] [Google Scholar]
- Gordon S., Fraser I., Nath D., Hughes D., Clarke S. Macrophages in tissues and in vitro. Curr Opin Immunol. 1992 Feb;4(1):25–32. doi: 10.1016/0952-7915(92)90119-y. [DOI] [PubMed] [Google Scholar]
- Kesavalu L., Ebersole J. L., Machen R. L., Holt S. C. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infect Immun. 1992 Apr;60(4):1455–1464. doi: 10.1128/iai.60.4.1455-1464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J. W., Steinman L. Competitive PCR quantification of CD4, CD8, ICAM-1, VCAM-1, and MHC class II mRNA in the central nervous system during development and resolution of experimental allergic encephalomyelitis. J Neuroimmunol. 1993 Nov-Dec;48(2):227–234. doi: 10.1016/0165-5728(93)90196-6. [DOI] [PubMed] [Google Scholar]
- MacNaul K. L., Hutchinson N. I., Parsons J. N., Bayne E. K., Tocci M. J. Analysis of IL-1 and TNF-alpha gene expression in human rheumatoid synoviocytes and normal monocytes by in situ hybridization. J Immunol. 1990 Dec 15;145(12):4154–4166. [PubMed] [Google Scholar]
- McCartney-Francis N., Allen J. B., Mizel D. E., Albina J. E., Xie Q. W., Nathan C. F., Wahl S. M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993 Aug 1;178(2):749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nussler A. K., Billiar T. R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993 Aug;54(2):171–178. [PubMed] [Google Scholar]
- Parrillo J. E. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993 May 20;328(20):1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cannella B., Brosnan C. F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991 Mar;87(3):949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira L., Houri Y., Barak V., Halabi A., Soskolne W. A., Stabholz A. Human monocyte response to cementum extracts from periodontally diseased teeth: effect of conditioning with tetracycline. J Periodontol. 1996 Jul;67(7):682–687. doi: 10.1902/jop.1996.67.7.682. [DOI] [PubMed] [Google Scholar]
- Shapira L., Soskolne W. A., Houri Y., Barak V., Halabi A., Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun. 1996 Mar;64(3):825–828. doi: 10.1128/iai.64.3.825-828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira L., Takashiba S., Amar S., Van Dyke T. E. Porphyromonas gingivalis lipopolysaccharide stimulation of human monocytes: dependence on serum and CD14 receptor. Oral Microbiol Immunol. 1994 Apr;9(2):112–117. doi: 10.1111/j.1399-302x.1994.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Shapira L., Takashiba S., Champagne C., Amar S., Van Dyke T. E. Involvement of protein kinase C and protein tyrosine kinase in lipopolysaccharide-induced TNF-alpha and IL-1 beta production by human monocytes. J Immunol. 1994 Aug 15;153(4):1818–1824. [PubMed] [Google Scholar]
- Simpson S. Q., Modi H. N., Balk R. A., Bone R. C., Casey L. C. Reduced alveolar macrophage production of tumor necrosis factor during sepsis in mice and men. Crit Care Med. 1991 Aug;19(8):1060–1066. doi: 10.1097/00003246-199108000-00015. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Haffajee A. D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992 Apr;63(4 Suppl):322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Jandinski J. J., Fujiyoshi P., Rynar J., Socransky S. S. Tissue levels of bone resorptive cytokines in periodontal disease. J Periodontol. 1991 Aug;62(8):504–509. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- Wilson M., Moore J., Kieser J. B. Identity of limulus amoebocyte lysate-active root surface materials from periodontally involved teeth. J Clin Periodontol. 1986 Sep;13(8):743–747. doi: 10.1111/j.1600-051x.1986.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Zhang X., Morrison D. C. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993 Feb 1;177(2):511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]