Abstract
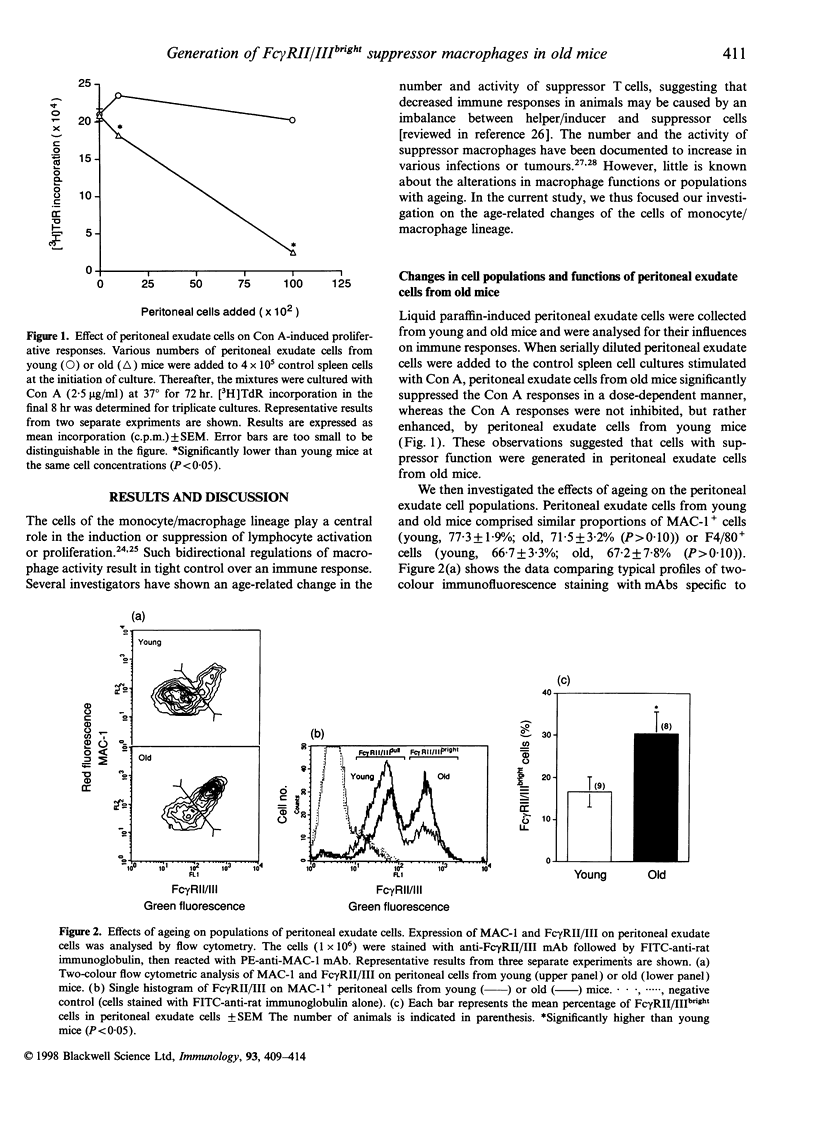
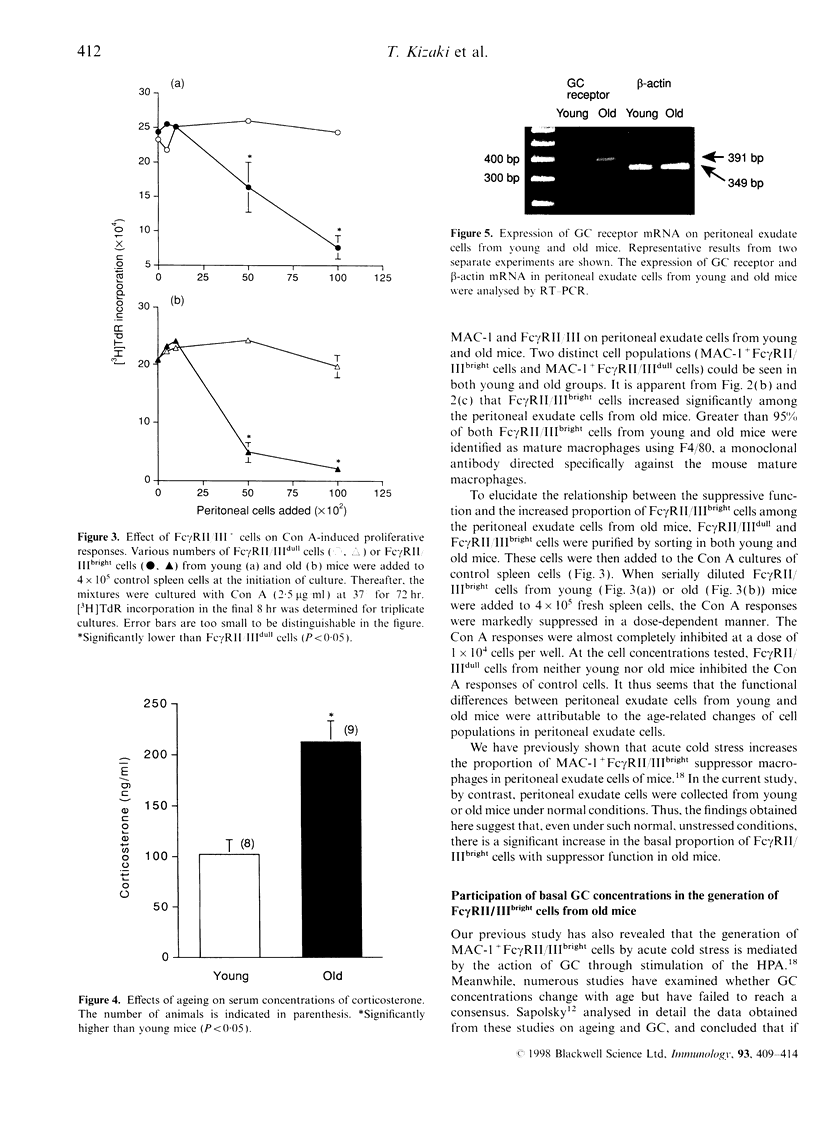
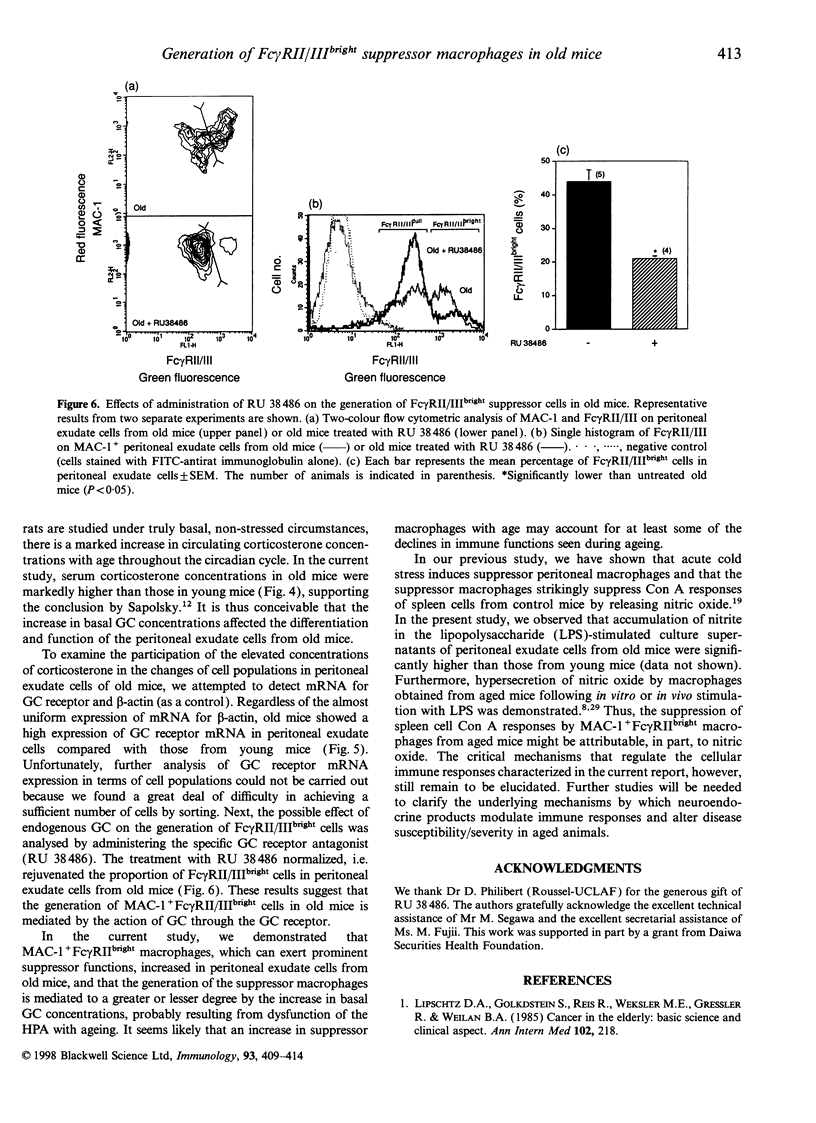
Ageing is usually accompanied by a decline in immune and neuroendocrine functions. To elucidate the mechanisms underlying age-related immunosuppression, the functions and surface phenotypes of peritoneal cells in the monocyte/macrophage lineage from old mice were investigated. The role of glucocorticoids (GC) in the immunomodulation was also examined. Proliferative responses of spleen cells from control mice stimulated with concanavalin A (Con A) were significantly suppressed by adding peritoneal exudate cells from old mice. Flow cytometry analysis revealed that the proportion of MAC-1+ cells with a high density of type II or type III receptor for the Fc portion of IgG (Fc gamma RII/IIIbright cells) was increased markedly in the periotoneal exudate cells from old mice. The prominent suppressor activity for Con A responses of control spleen cells was found in the Fc gamma RII/IIIbright cells, whereas MAC-1+ cells with a low density of Fc gamma RII/III (Fc gamma RII/IIIdull cells) did not suppress the Con A responses. On the other hand, both the basal corticosterone concentrations in serum and the mRNA expression for GC receptor in peritoneal exudate cells increased significantly in old mice. Furthermore, the proportion of Fc gamma RII/IIIbright cells in peritoneal exudate cells from old mice was normalized on administration of the GC antagonist RU 38,486 (mifepristone). These results suggest that the increase in basal corticosterone concentrations in old mice induces the generation of Fc gamma RII/IIIbright suppressor cells, possibly leading to the immune-suppressive state.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Blalock J. E. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev. 1989 Jan;69(1):1–32. doi: 10.1152/physrev.1989.69.1.1. [DOI] [PubMed] [Google Scholar]
- Chorinchath B. B., Kong L. Y., Mao L., McCallum R. E. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol. 1996 Feb 15;156(4):1525–1530. [PubMed] [Google Scholar]
- De Souza E. B. Corticotropin-releasing factor and interleukin-1 receptors in the brain-endocrine-immune axis. Role in stress response and infection. Ann N Y Acad Sci. 1993 Oct 29;697:9–27. doi: 10.1111/j.1749-6632.1993.tb49919.x. [DOI] [PubMed] [Google Scholar]
- Everitt A., Meites J. Aging and anti-aging effects of hormones. J Gerontol. 1989 Nov;44(6):B139–B147. doi: 10.1093/geronj/44.6.b139. [DOI] [PubMed] [Google Scholar]
- Higashimoto Y., Fukuchi Y., Shimada Y., Ishida K., Ohata M., Furuse T., Shu C., Teramoto S., Matsuse T., Sudo E. The effects of aging on the function of alveolar macrophages in mice. Mech Ageing Dev. 1993 Jul;69(3):207–217. doi: 10.1016/0047-6374(93)90024-l. [DOI] [PubMed] [Google Scholar]
- Jefferies W. M. Cortisol and immunity. Med Hypotheses. 1991 Mar;34(3):198–208. doi: 10.1016/0306-9877(91)90212-h. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Kizaki T., Ishige M., Bingyan W., Kumagai M., Day N. K., Good R. A., Onoé K. Interleukin-1-dependent mitogenic responses induced by protoscoleces of Echinococcus multilocularis in murine lymphocytes. J Leukoc Biol. 1993 Mar;53(3):233–239. doi: 10.1002/jlb.53.3.233. [DOI] [PubMed] [Google Scholar]
- Kizaki T., Kobayashi S., Ogasawara K., Day N. K., Good R. A., Onoé K. Immune suppression induced by protoscoleces of Echinococcus multilocularis in mice. Evidence for the presence of CD8dull suppressor cells in spleens of mice intraperitoneally infected with E. multilocularis. J Immunol. 1991 Sep 1;147(5):1659–1666. [PubMed] [Google Scholar]
- Kizaki T., Oh-ishi S., Ohno H. Acute cold stress induces suppressor macrophages in mice. J Appl Physiol (1985) 1996 Jul;81(1):393–399. doi: 10.1152/jappl.1996.81.1.393. [DOI] [PubMed] [Google Scholar]
- Kizaki T., Oh-ishi S., Ookawara T., Yamamoto M., Izawa T., Ohno H. Glucocorticoid-mediated generation of suppressor macrophages with high density Fc gamma RII during acute cold stress. Endocrinology. 1996 Oct;137(10):4260–4267. doi: 10.1210/endo.137.10.8828485. [DOI] [PubMed] [Google Scholar]
- Kizaki T., Yamashita H., Oh-Ishi S., Day N. K., Good R. A., Ohno H. Immunomodulation by cells of mononuclear phagocyte lineage in acute cold-stressed or cold-acclimatized mice. Immunology. 1995 Nov;86(3):456–462. [PMC free article] [PubMed] [Google Scholar]
- Ligthart G. J., Schuit H. R., Hijmans W. Subpopulations of mononuclear cells in ageing: expansion of the null cell compartment and decrease in the number of T and B cells in human blood. Immunology. 1985 May;55(1):15–21. [PMC free article] [PubMed] [Google Scholar]
- Lipschitz D. A., Goldstein S., Reis R., Weksler M. E., Bressler R., Neilan B. A. Cancer in the elderly: basic science and clinical aspects. Ann Intern Med. 1985 Feb;102(2):218–228. doi: 10.7326/0003-4819-102-2-218. [DOI] [PubMed] [Google Scholar]
- Lázár G., Lázár G., Jr, Husztik E., Duda E., Agarwal M. K. The influence of antiglucocorticoids on stress and shock. Ann N Y Acad Sci. 1995 Jun 12;761:276–295. doi: 10.1111/j.1749-6632.1995.tb31384.x. [DOI] [PubMed] [Google Scholar]
- Makinodan T., James S. J., Inamizu T., Chang M. P. Immunologic basis for susceptibility to infection in the aged. Gerontology. 1984;30(5):279–289. doi: 10.1159/000212647. [DOI] [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Miller R. A. Aging and immune function. Int Rev Cytol. 1991;124:187–215. doi: 10.1016/s0074-7696(08)61527-2. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nagel J. E., Chrest F. J., Adler W. H. Enumeration of T lymphocyte subsets by monoclonal antibodies in young and aged humans. J Immunol. 1981 Nov;127(5):2086–2088. [PubMed] [Google Scholar]
- Role of macrophages in the immune response. Immunol Rev. 1978;40:1–255. [PubMed] [Google Scholar]
- Sapolsky R. M. Do glucocorticoid concentrations rise with age in the rat? Neurobiol Aging. 1992 Jan-Feb;13(1):171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens B. A., Sapolsky R. M. Stress, glucocorticoids, and aging. Aging (Milano) 1992 Sep;4(3):197–210. doi: 10.1007/BF03324092. [DOI] [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. The cellular and subcellular bases of immunosenescence. Adv Immunol. 1989;46:221–261. doi: 10.1016/s0065-2776(08)60655-0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]



