Abstract
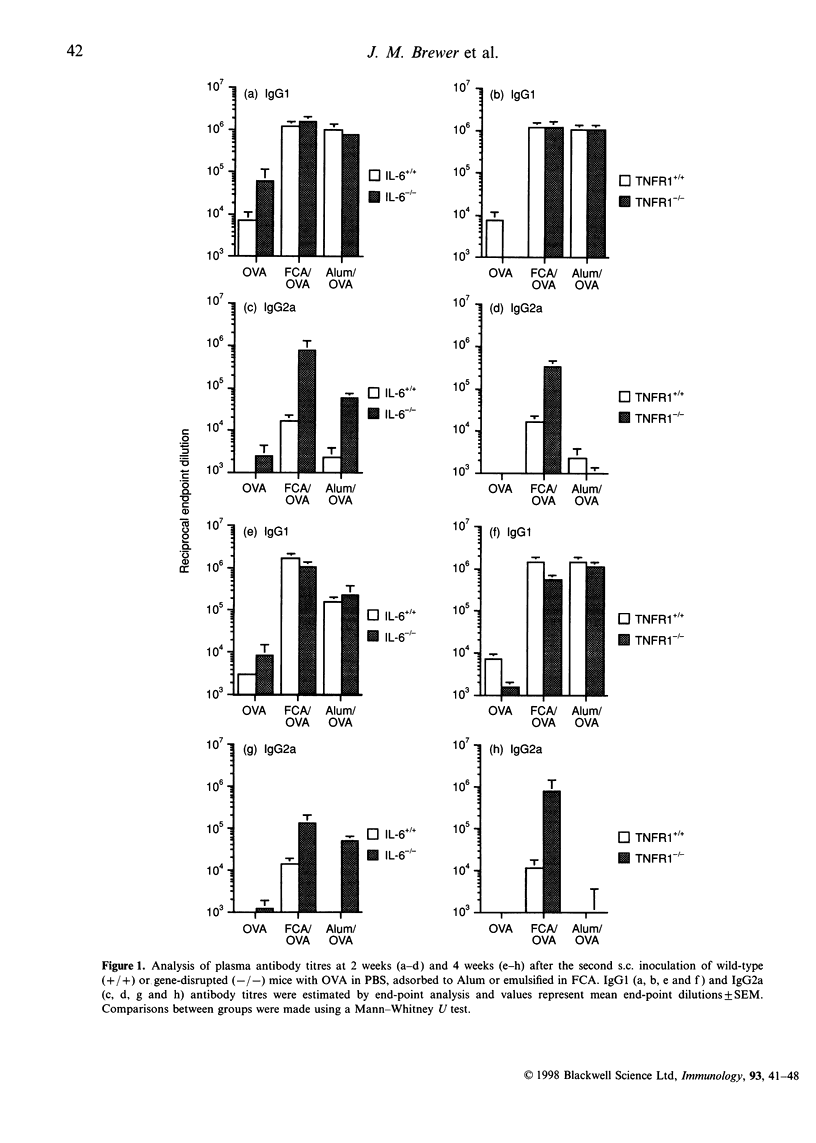
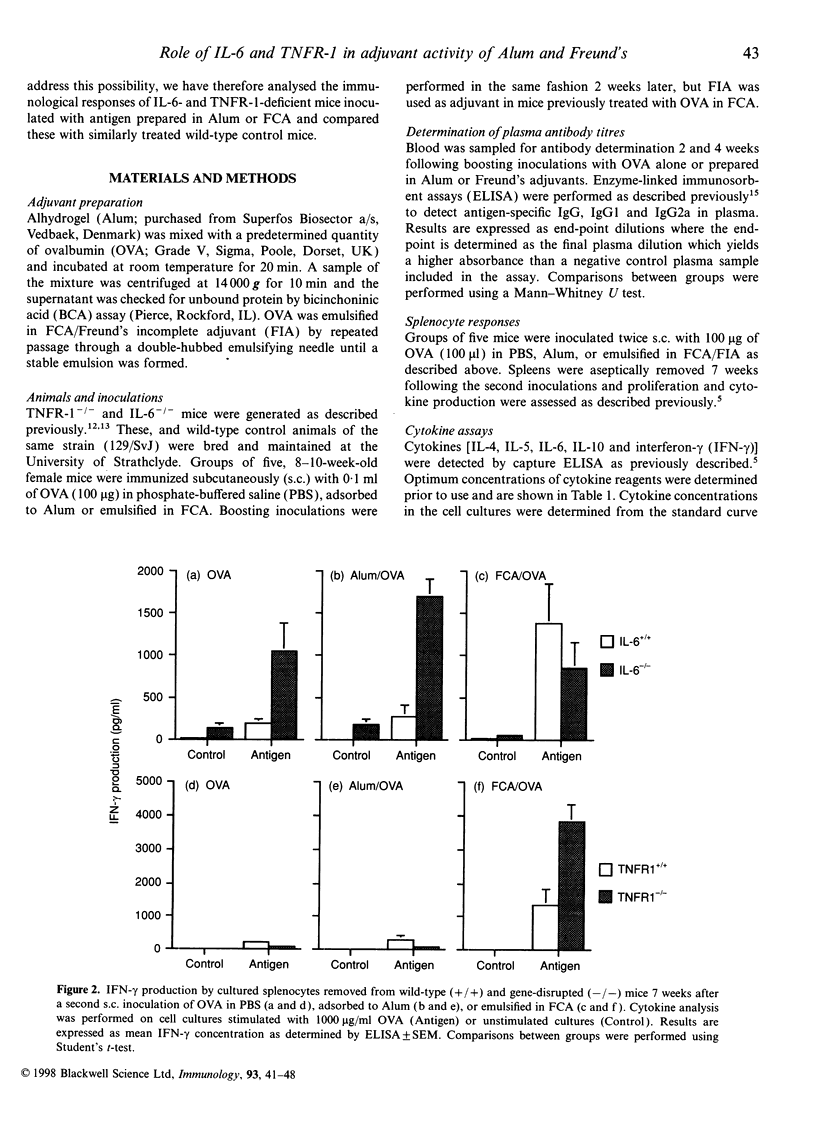
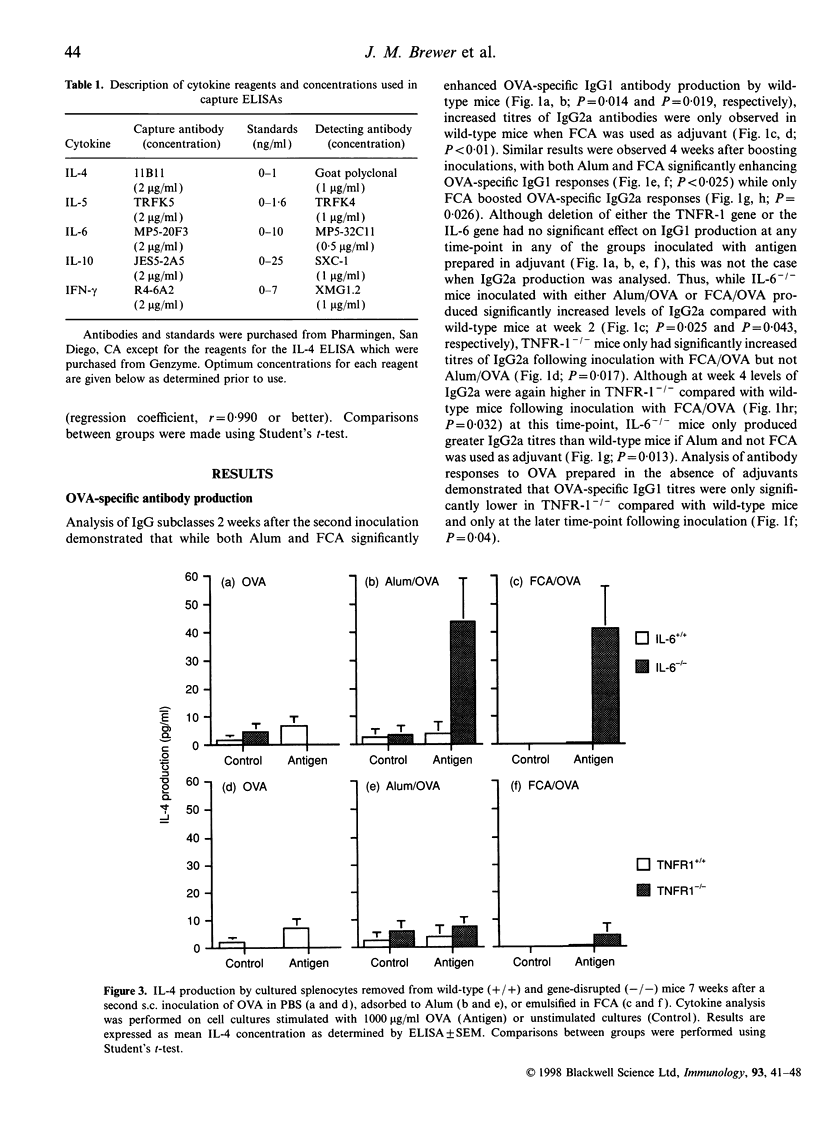
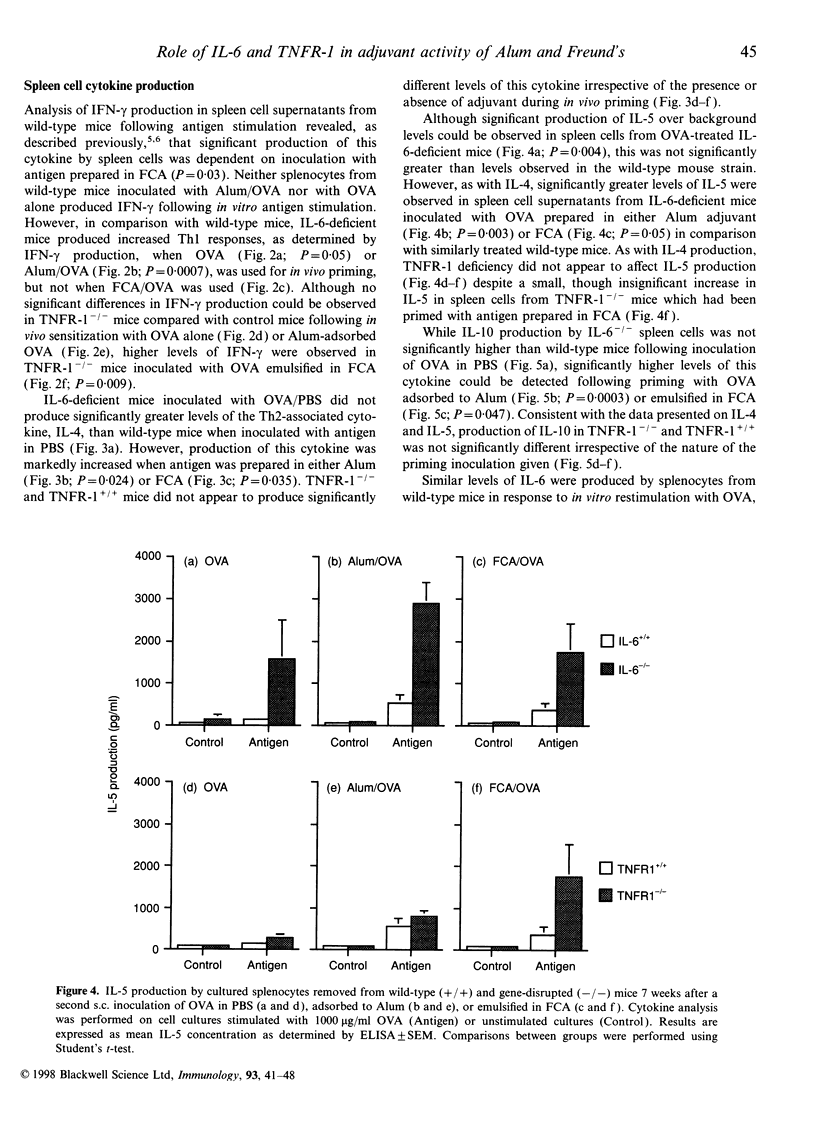
The potential contribution made by the inflammatory cytokines, interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-alpha) to the adjuvant activity of aluminium hydroxide gels (Alum) or Freund's complete adjuvant (FCA) was studied by comparing the immunological responses of IL-6- or TNF receptor 1- (p55; TNFR-1) deficient mice with immunocompetent control mice. While both TNFR-1- and IL-6-deficient mice primed with ovalbumin (OVA) prepared in either Alum or FCA produced similar IgG.1 responses in comparison to control mice, the pattern of T-helper type 1- (Th1) dependent IgG2a production was significantly altered. In TNFR-1-deficient mice, IgG2a responses were greater than in control mice when FCA, but not when Alum, was used as an adjuvant. Correspondingly, spleen cells from FCA-inoculated TNFR-1-deficient mice restimulated with antigen in vitro produced higher Th1 cytokine (interferon-gamma; IFN-gamma) levels with no alteration in Th2 cytokine (IL-4; IL-5, IL-6 and IL-10) production in comparison with wild-type mice. Higher levels of IgG2a were also detected in IL-6-deficient mice compared with wild-type mice following inoculation with OVA prepared in either FCA or in Alum. Furthermore, analysis of cytokine production by spleen cells revealed that both Th1 and Th2 cytokine production was higher in IL-6-deficient mice compared with control mice. As the majority of the effects of TNF-alpha are mediated via TNFR-1, we conclude that this cytokine inhibits the adjuvant activities of FCA. Furthermore, the results also imply that immunopotentiating effects of FCA or Alum adjuvant are both inhibited by IL-6.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Schreiber R. D., Unanue E. R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991 Dec;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bomford R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. II. Effect on delayed-type hypersensitivity in the mouse and guinea pig, and cell-mediated immunity to tumour antigens in the mouse of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bordetella pertussis, muramyl dipeptide and saponin. Clin Exp Immunol. 1980 Feb;39(2):435–441. [PMC free article] [PubMed] [Google Scholar]
- Brewer J. M., Alexander J. The adjuvant activity of non-ionic surfactant vesicles (niosomes) on the BALB/c humoral response to bovine serum albumin. Immunology. 1992 Apr;75(4):570–575. [PMC free article] [PubMed] [Google Scholar]
- Brewer J. M., Conacher M., Satoskar A., Bluethmann H., Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996 Sep;26(9):2062–2066. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- Cox F. E., Liew F. Y. T-cell subsets and cytokines in parasitic infections. Immunol Today. 1992 Nov;13(11):445–448. doi: 10.1016/0167-5699(92)90072-F. [DOI] [PubMed] [Google Scholar]
- Dalrymple S. A., Lucian L. A., Slattery R., McNeil T., Aud D. M., Fuchino S., Lee F., Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995 Jun;63(6):2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKruyff R. H., Mosmann R. R., Umetsu D. T. Induction of antibody synthesis by CD4+ T cells: IL 5 is essential for induction of antigen-specific antibody responses by TH2 but not TH1 clones. Eur J Immunol. 1990 Oct;20(10):2219–2227. doi: 10.1002/eji.1830201010. [DOI] [PubMed] [Google Scholar]
- Erickson S. L., de Sauvage F. J., Kikly K., Carver-Moore K., Pitts-Meek S., Gillett N., Sheehan K. C., Schreiber R. D., Goeddel D. V., Moore M. W. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994 Dec 8;372(6506):560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- Fattori E., Cappelletti M., Costa P., Sellitto C., Cantoni L., Carelli M., Faggioni R., Fantuzzi G., Ghezzi P., Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994 Oct 1;180(4):1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Maurer P. H. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989 Jun;121(1):134–145. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Bancroft G. J. Administration of interleukin-10 abolishes innate resistance to Listeria monocytogenes. Eur J Immunol. 1996 Feb;26(2):356–364. doi: 10.1002/eji.1830260214. [DOI] [PubMed] [Google Scholar]
- Kersten G. F., Crommelin D. J. Liposomes and ISCOMS as vaccine formulations. Biochim Biophys Acta. 1995 Jul 17;1241(2):117–138. doi: 10.1016/0304-4157(95)00002-9. [DOI] [PubMed] [Google Scholar]
- Kopf M., Baumann H., Freer G., Freudenberg M., Lamers M., Kishimoto T., Zinkernagel R., Bluethmann H., Köhler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994 Mar 24;368(6469):339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Le Hir M., Bluethmann H., Kosco-Vilbois M. H., Müller M., di Padova F., Moore M., Ryffel B., Eugster H. P. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med. 1996 May 1;183(5):2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkerson J. M., Isakson P. C. Interleukin 5 (IL-5) provides a signal that is required in addition to IL-4 for isotype switching to immunoglobulin (Ig) G1 and IgE. J Exp Med. 1992 Apr 1;175(4):973–982. doi: 10.1084/jem.175.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Spaccapelo R., Toniatti C., Puccetti P., Bistoni F., Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med. 1996 Apr 1;183(4):1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Puccetti P., Bistoni F. Biological role of Th cell subsets in candidiasis. Chem Immunol. 1996;63:115–137. [PubMed] [Google Scholar]
- Rothe J., Lesslauer W., Lötscher H., Lang Y., Koebel P., Köntgen F., Althage A., Zinkernagel R., Steinmetz M., Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993 Aug 26;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Conley F. K., Remington J. S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989 Sep 15;143(6):2045–2050. [PubMed] [Google Scholar]
- Tew J. G., Phipps R. P., Mandel T. E. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Thoma B., Grell M., Pfizenmaier K., Scheurich P. Identification of a 60-kD tumor necrosis factor (TNF) receptor as the major signal transducing component in TNF responses. J Exp Med. 1990 Oct 1;172(4):1019–1023. doi: 10.1084/jem.172.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnetti L., Spaccapelo R., Cenci E., Mencacci A., Puccetti P., Coffman R. L., Bistoni F., Romani L. Interleukin-4 and -10 exacerbate candidiasis in mice. Eur J Immunol. 1995 Jun;25(6):1559–1565. doi: 10.1002/eji.1830250614. [DOI] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]


