Abstract
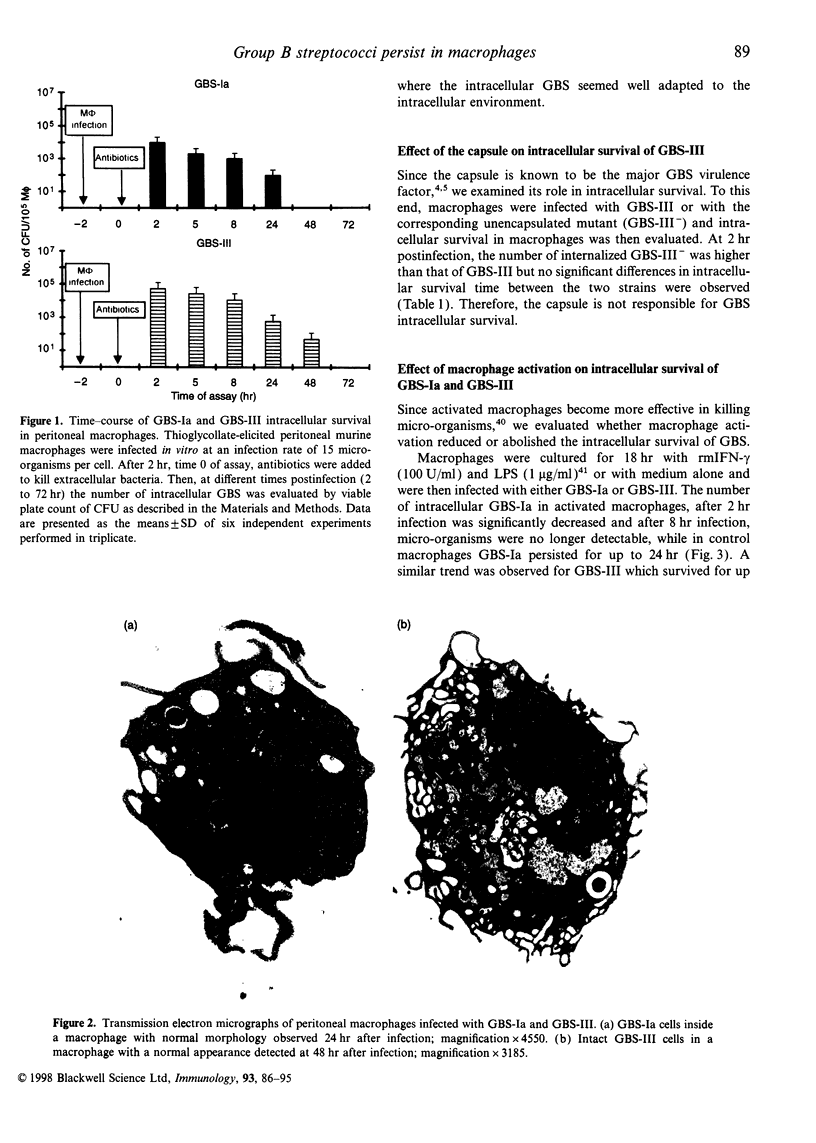
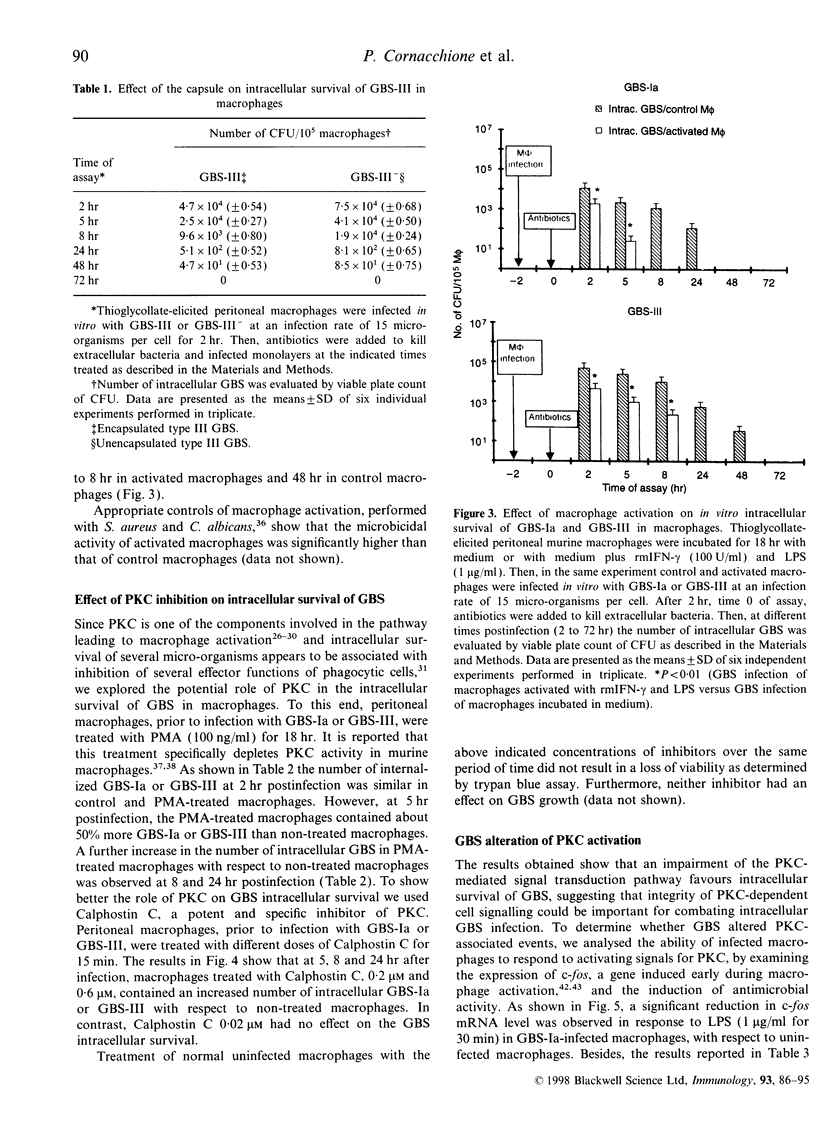
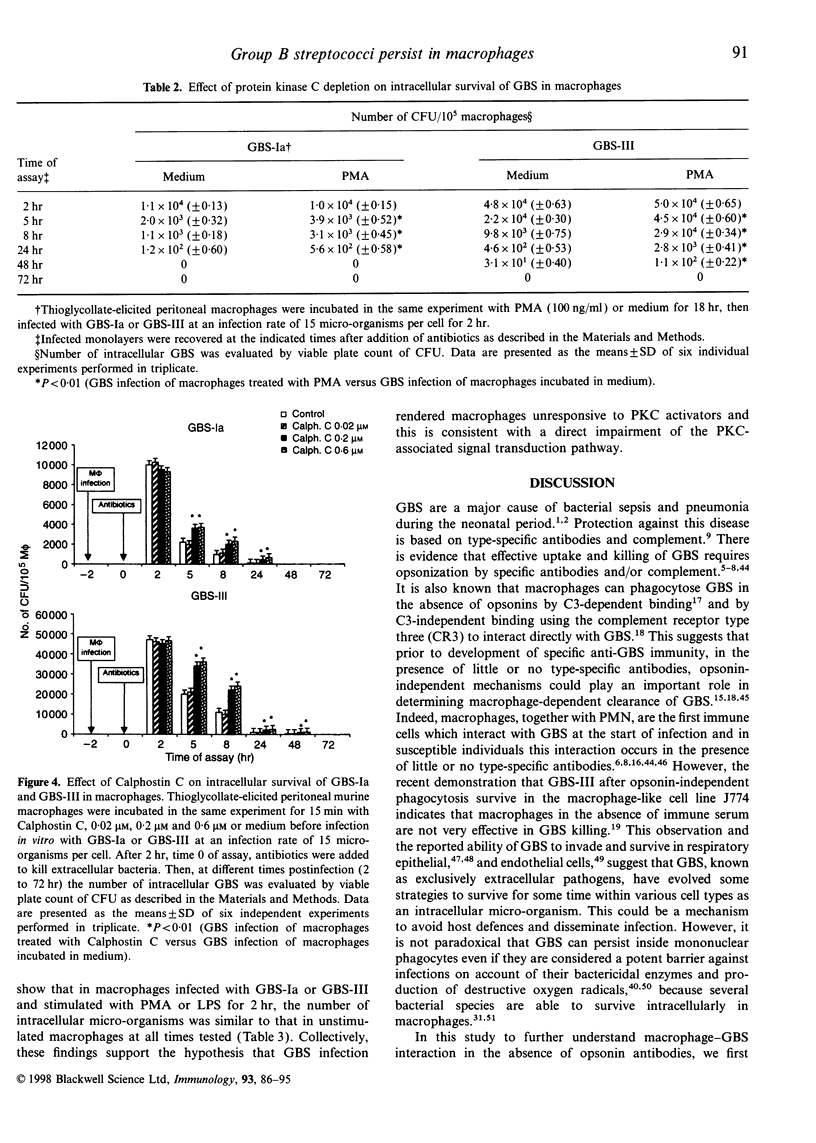
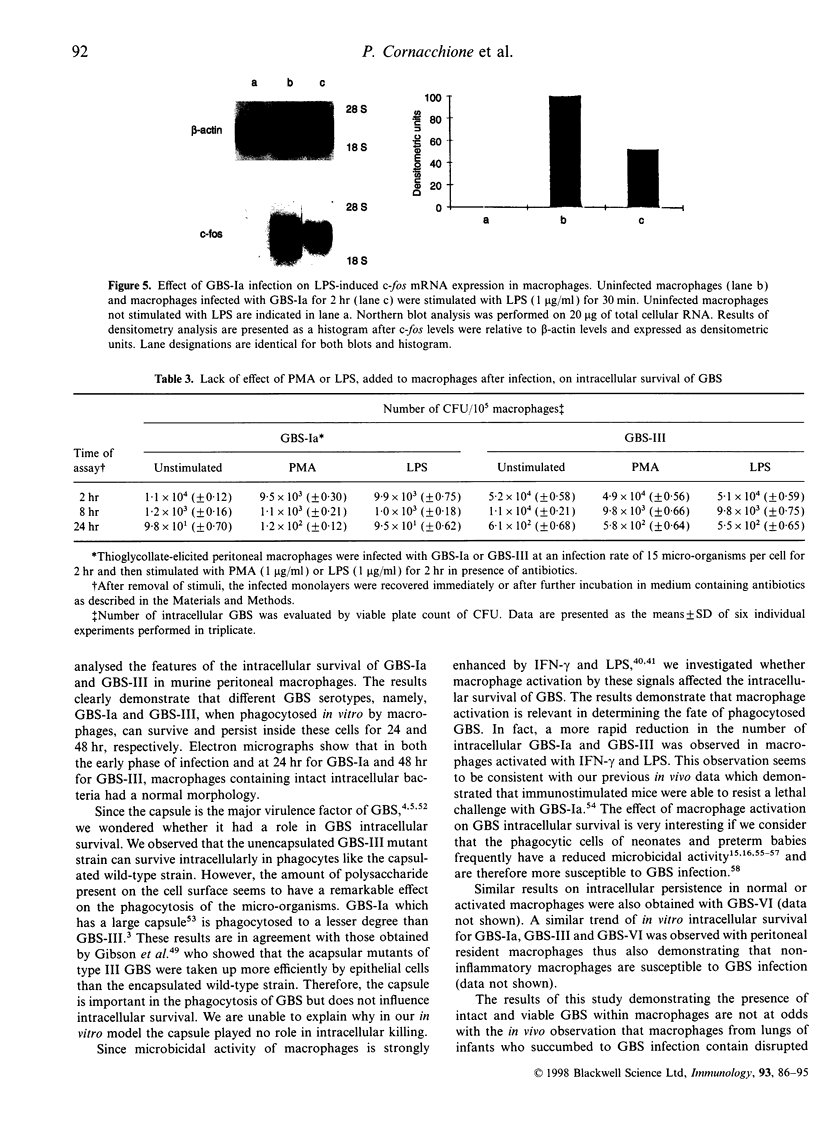
Group B streptococci (GBS) are an important cause of neonatal sepsis, pneumonia and meningitis. In the early phase of infection, macrophages and polymorphonuclear cells (PMN) are the first immune cells that interact with GBS. In this in vitro study, to gain insight into GBS-macrophage interaction in the absence of type-specific antibodies, we examined the features of GBS survival in thioglycollate-elicited murine peritoneal macrophages and the effect of GBS on the protein kinase C (PKC)-dependent transduction pathway. Our results demonstrate that type Ia GBS, strain 090 (GBS-Ia) and type III GBS strain COH 31r/s (GBS-III), after in vitro phagocytosis survive and persist intracellularly in macrophages for up to 24 and 48 hr, respectively. However, macrophage activation by interferon-gamma (IFN-gamma) and lipopolysaccharide from Escherichia coli (LPS) caused a significant reduction in the time of intracellular persistence. Macrophage activation by IFN-gamma and LPS seems to be a multifactorial event involving multiple intracellular signal pathways also including PKC. Since PKC is one of the components in the signal network leading to macrophage activation and an important target for several intracellular micro-organisms, we wondered whether PKC could have a role in intracellular GBS survival. Both PKC depletion by treatment with phorbol 12-myristate 13-acetate (PMA) for 18 hr and PKC inhibition by Calphostin C rendered macrophages more permissive for the intracellular GBS survival. Furthermore, GBS-infected macrophages were unable to respond to PMA and LPS, activators of PKC, by inducing antimicrobial activity. The ability of GBS to impair PKC-dependent cell signalling was also demonstrated by the reduced c-fos gene expression in GBS-infected macrophages with respect to control macrophages, after LPS stimulation. In conclusion, our results indicate that GBS survive in macrophages and impairment of PKC signal transduction contributes to their intracellular survival.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antal J. M., Cunningham J. V., Goodrum K. J. Opsonin-independent phagocytosis of group B streptococci: role of complement receptor type three. Infect Immun. 1992 Mar;60(3):1114–1121. doi: 10.1128/iai.60.3.1114-1121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub E. M., Swingle H. Pathogenic mechanisms in neonatal GBS infection. Antibiot Chemother (1971) 1985;35:128–141. doi: 10.1159/000410368. [DOI] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Edwards M. S. Group B streptococcal infections. Perinatal impact and prevention methods. Ann N Y Acad Sci. 1988;549:193–202. doi: 10.1111/j.1749-6632.1988.tb23972.x. [DOI] [PubMed] [Google Scholar]
- Baker C. J. Group B streptococcal infection in newborns: prevention at last? N Engl J Med. 1986 Jun 26;314(26):1702–1704. doi: 10.1056/NEJM198606263142609. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976 Apr 1;294(14):753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Immunological investigation of infants with septicemia or meningitis due to group B Streptococcus. J Infect Dis. 1977 Aug;136 (Suppl):S98–104. doi: 10.1093/infdis/136.supplement.s98. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Rench M. A., Edwards M. S., Carpenter R. J., Hays B. M., Kasper D. L. Immunization of pregnant women with a polysaccharide vaccine of group B streptococcus. N Engl J Med. 1988 Nov 3;319(18):1180–1185. doi: 10.1056/NEJM198811033191802. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Rench M. A., Kasper D. L. Response to type III polysaccharide in women whose infants have had invasive group B streptococcal infection. N Engl J Med. 1990 Jun 28;322(26):1857–1860. doi: 10.1056/NEJM199006283222606. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Kasper D. L., Vecchitto J. Mouse protection test for group B Streptococcus type III. J Infect Dis. 1979 Jul;140(1):81–88. doi: 10.1093/infdis/140.1.81. [DOI] [PubMed] [Google Scholar]
- Becker I. D., Robinson O. M., Bazán T. S., López-Osuna M., Kretschmer R. R. Bactericidal capacity of newborn phagocytes against group B beta-hemolytic streptococci. Infect Immun. 1981 Nov;34(2):535–539. doi: 10.1128/iai.34.2.535-539.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Galán J. E., Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993 Jun 4;73(5):903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- Bowdy B. D., Aziz S. M., Marple S. L., Yoneda K., Pauly T. H., Coonrod J. D., Gillespie M. N. Organ-specific disposition of group B streptococci in piglets: evidence for a direct interaction with target cells in the pulmonary circulation. Pediatr Res. 1990 Apr;27(4 Pt 1):344–348. doi: 10.1203/00006450-199004000-00005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Descoteaux A., Matlashewski G. c-fos and tumor necrosis factor gene expression in Leishmania donovani-infected macrophages. Mol Cell Biol. 1989 Nov;9(11):5223–5227. doi: 10.1128/mcb.9.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eason S., Martin W. Involvement of tyrosine kinase and protein kinase C in the induction of nitric oxide synthase by lipopolysaccharide and interferon-gamma in J774 macrophages. Arch Int Pharmacodyn Ther. 1995 Sep-Oct;330(2):225–240. [PubMed] [Google Scholar]
- Edwards M. S., Baker C. J., Kasper D. L. Opsonic specificity of human antibody to the type III polysaccharide of group B Streptococcus. J Infect Dis. 1979 Dec;140(6):1004–1008. doi: 10.1093/infdis/140.6.1004. [DOI] [PubMed] [Google Scholar]
- Eilers A., Georgellis D., Klose B., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Decker T. Differentiation-regulated serine phosphorylation of STAT1 promotes GAF activation in macrophages. Mol Cell Biol. 1995 Jul;15(7):3579–3586. doi: 10.1128/mcb.15.7.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. D., Goldberg M., Bloom B. R. Interferon-gamma-induced transcriptional activation is mediated by protein kinase C. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5122–5125. doi: 10.1073/pnas.85.14.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. L., Lee M. K., Soderland C., Chi E. Y., Rubens C. E. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect Immun. 1993 Feb;61(2):478–485. doi: 10.1128/iai.61.2.478-485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givner L. B., Edwards M. S., Baker C. J. A polyclonal human IgG preparation hyperimmune for type III, group B Streptococcus: in vitro opsonophagocytic activity and efficacy in experimental models. J Infect Dis. 1988 Oct;158(4):724–730. doi: 10.1093/infdis/158.4.724. [DOI] [PubMed] [Google Scholar]
- Gotoff S. P., Boyer K. M. Cellular and humoral aspects of host defense mechanisms against GBS. Antibiot Chemother (1971) 1985;35:142–156. doi: 10.1159/000410369. [DOI] [PubMed] [Google Scholar]
- Gotoff S. P., Odell C., Papierniak C. K., Klegerman M. E., Boyer K. M. Human IgG antibody to group b Streptococcus type III: comparison of protective levels in a murine model with levels in infected human neonates. J Infect Dis. 1986 Mar;153(3):511–519. doi: 10.1093/infdis/153.3.511. [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Setoguchi M., Yoshida S., Akizuki S., Yamamoto S. Enhancement of c-fos expression is associated with activated macrophages. Oncogene. 1988 May;2(5):515–521. [PubMed] [Google Scholar]
- Hulse M. L., Smith S., Chi E. Y., Pham A., Rubens C. E. Effect of type III group B streptococcal capsular polysaccharide on invasion of respiratory epithelial cells. Infect Immun. 1993 Nov;61(11):4835–4841. doi: 10.1128/iai.61.11.4835-4841.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M., Hamilton T. A., Kaufman R. E., Adams D. O., Bast R. C., Jr Treatment of murine peritoneal macrophages with bacterial lipopolysaccharide alters expression of c-fos and c-myc oncogenes. J Immunol. 1986 Oct 15;137(8):2711–2715. [PubMed] [Google Scholar]
- Kiyotaki C., Bloom B. R. Activation of murine macrophage cell lines. Possible involvement of protein kinases in stimulation of superoxide production. J Immunol. 1984 Aug;133(2):923–931. [PubMed] [Google Scholar]
- Levy N. J., Kasper D. L. Antibody-independent and -dependent opsonization of group B Streptococcus requires the first component of complement C1. Infect Immun. 1985 Jul;49(1):19–24. doi: 10.1128/iai.49.1.19-24.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi P., Scaringi L., Tissi L., Boccanera M., Bistoni F., Bonmassar E., Cassone A. Induction of natural killer cell activity by inactivated Candida albicans in mice. Infect Immun. 1985 Oct;50(1):297–303. doi: 10.1128/iai.50.1.297-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. Subversion of the immune system by pathogens. Cell. 1994 Jan 28;76(2):323–332. doi: 10.1016/0092-8674(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Ruzinski J. T., Rubens C. E., Chi E. Y., Wilson C. B. The effect of type-specific polysaccharide capsule on the clearance of group B streptococci from the lungs of infant and adult rats. J Infect Dis. 1992 Feb;165(2):306–314. doi: 10.1093/infdis/165.2.306. [DOI] [PubMed] [Google Scholar]
- McNeely T. B., Turco S. J. Inhibition of protein kinase C activity by the Leishmania donovani lipophosphoglycan. Biochem Biophys Res Commun. 1987 Oct 29;148(2):653–657. doi: 10.1016/0006-291x(87)90926-0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier M., Brownsey R. W., Reiner N. E. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7481–7485. doi: 10.1073/pnas.89.16.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orefici G., Recchia S., Galante L. Possible virulence marker for Streptococcus agalactiae (Lancefield Group B). Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):302–305. doi: 10.1007/BF01963108. [DOI] [PubMed] [Google Scholar]
- Paul A., Pendreigh R. H., Plevin R. Protein kinase C and tyrosine kinase pathways regulate lipopolysaccharide-induced nitric oxide synthase activity in RAW 264.7 murine macrophages. Br J Pharmacol. 1995 Jan;114(2):482–488. doi: 10.1111/j.1476-5381.1995.tb13252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzioch D., Bottazzi B., Varesio L. Augmentation of c-fos mRNA expression by activators of protein kinase C in fresh, terminally differentiated resting macrophages. Mol Cell Biol. 1987 Feb;7(2):595–599. doi: 10.1128/mcb.7.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner N. E. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol Today. 1994 Aug;15(8):374–381. doi: 10.1016/0167-5699(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Ng W., Wilson C. B., McMaster W. R., Burchett S. K. Modulation of in vitro monocyte cytokine responses to Leishmania donovani. Interferon-gamma prevents parasite-induced inhibition of interleukin 1 production and primes monocytes to respond to Leishmania by producing both tumor necrosis factor-alpha and interleukin 1. J Clin Invest. 1990 Jun;85(6):1914–1924. doi: 10.1172/JCI114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rubens C. E., Smith S., Hulse M., Chi E. Y., van Belle G. Respiratory epithelial cell invasion by group B streptococci. Infect Immun. 1992 Dec;60(12):5157–5163. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaringi L., Tissi L., Cornacchione P., Rosati E., Campanelli C., von Hunolstein C., Orefici G., Rossi R., Marconi P. Antibody-independent protection in mice against type Ia group B streptococcus lethal infection. FEMS Immunol Med Microbiol. 1994 Aug;9(2):151–162. doi: 10.1111/j.1574-695X.1994.tb00486.x. [DOI] [PubMed] [Google Scholar]
- Schwarzer E., Turrini F., Giribaldi G., Cappadoro M., Arese P. Phagocytosis of P. falciparum malarial pigment hemozoin by human monocytes inactivates monocyte protein kinase C. Biochim Biophys Acta. 1993 Mar 24;1181(1):51–54. doi: 10.1016/0925-4439(93)90089-j. [DOI] [PubMed] [Google Scholar]
- Severn A., Wakelam M. J., Liew F. Y. The role of protein kinase C in the induction of nitric oxide synthesis by murine macrophages. Biochem Biophys Res Commun. 1992 Nov 16;188(3):997–1002. doi: 10.1016/0006-291x(92)91330-s. [DOI] [PubMed] [Google Scholar]
- Sherman M. P., Johnson J. T., Rothlein R., Hughes B. J., Smith C. W., Anderson D. C. Role of pulmonary phagocytes in host defense against group B streptococci in preterm versus term rabbit lung. J Infect Dis. 1992 Oct;166(4):818–826. doi: 10.1093/infdis/166.4.818. [DOI] [PubMed] [Google Scholar]
- Shigeoka A. O., Charette R. P., Wyman M. L., Hill H. R. Defective oxidative metabolic responses of neutrophils from stressed neonates. J Pediatr. 1981 Mar;98(3):392–398. doi: 10.1016/s0022-3476(81)80701-9. [DOI] [PubMed] [Google Scholar]
- Shigeoka A. O., Hall R. T., Hemming V. G., Allred C. D., Hill H. R. Role of antibody and complement in opsonization of group B streptococci. Infect Immun. 1978 Jul;21(1):34–40. doi: 10.1128/iai.21.1.34-40.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka A. O., Santos J. I., Hill H. R. Functional analysis of neutrophil granulocytes from healthy, infected, and stressed neonates. J Pediatr. 1979 Sep;95(3):454–460. doi: 10.1016/s0022-3476(79)80535-1. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae. Infect Immun. 1988 Aug;56(8):1912–1919. doi: 10.1128/iai.56.8.1912-1919.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect Immun. 1987 Feb;55(2):446–450. doi: 10.1128/iai.55.2.446-450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Weigand P., Benkel P., Rohde M., Chhatwal G. S. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun. 1996 Jul;64(7):2467–2473. doi: 10.1128/iai.64.7.2467-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A., Mazzolla R., Farinelli S., Cassone A., Bistoni F. Immunomodulation by Candida albicans: crucial role of organ colonization and chronic infection with an attenuated agerminative strain of C. albicans for establishment of anti-infectious protection. J Gen Microbiol. 1988 Sep;134(9):2583–2592. doi: 10.1099/00221287-134-9-2583. [DOI] [PubMed] [Google Scholar]
- Weinstein S. L., Sanghera J. S., Lemke K., DeFranco A. L., Pelech S. L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992 Jul 25;267(21):14955–14962. [PubMed] [Google Scholar]
- Wolf J. E., Massof S. E., Sherwin J. R., Considine R. V. Inhibition of murine macrophage protein kinase C activity by nonviable Histoplasma capsulatum. Infect Immun. 1992 Jul;60(7):2683–2687. doi: 10.1128/iai.60.7.2683-2687.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S., Parker P. J., Ullrich A., Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987 Jun 15;244(3):775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]