Abstract
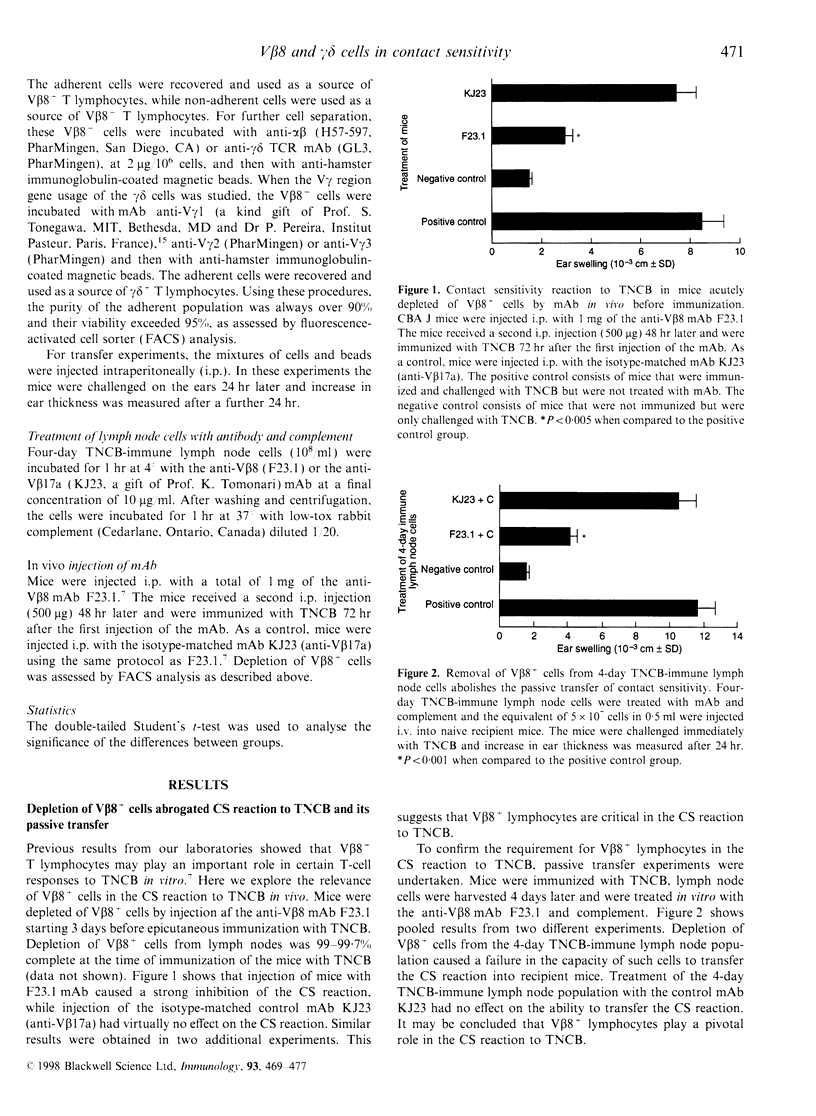
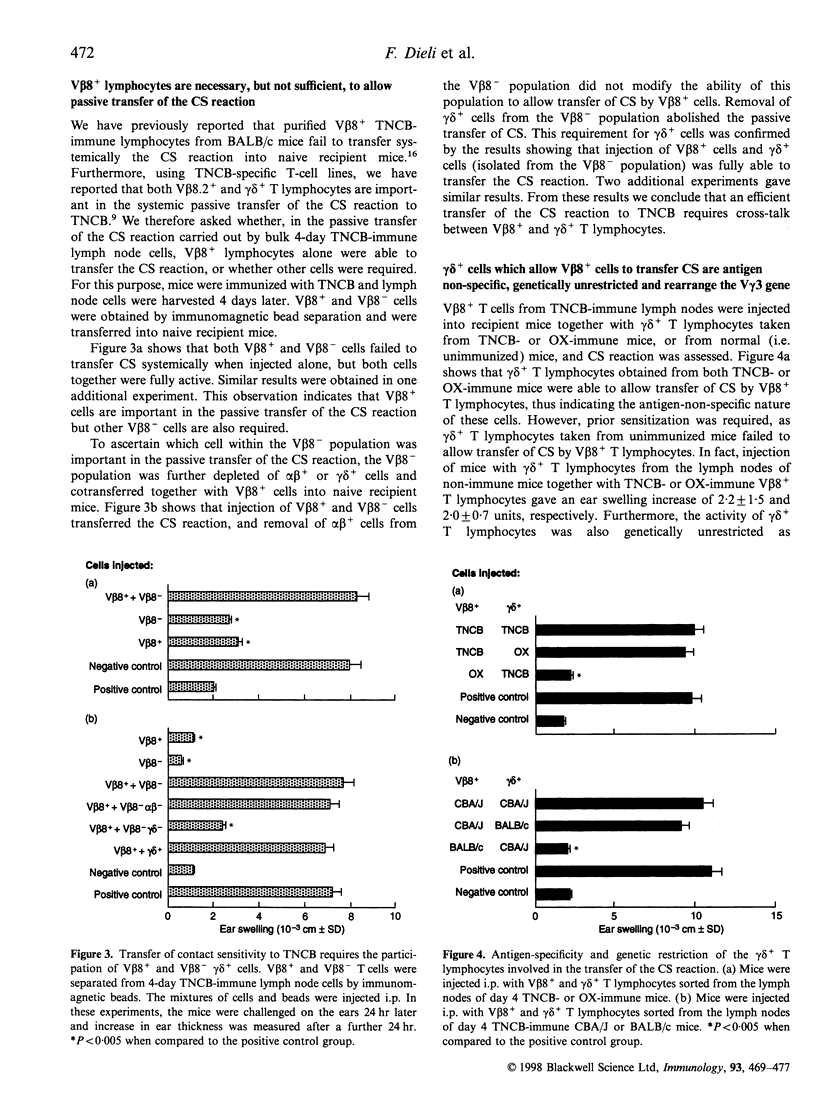
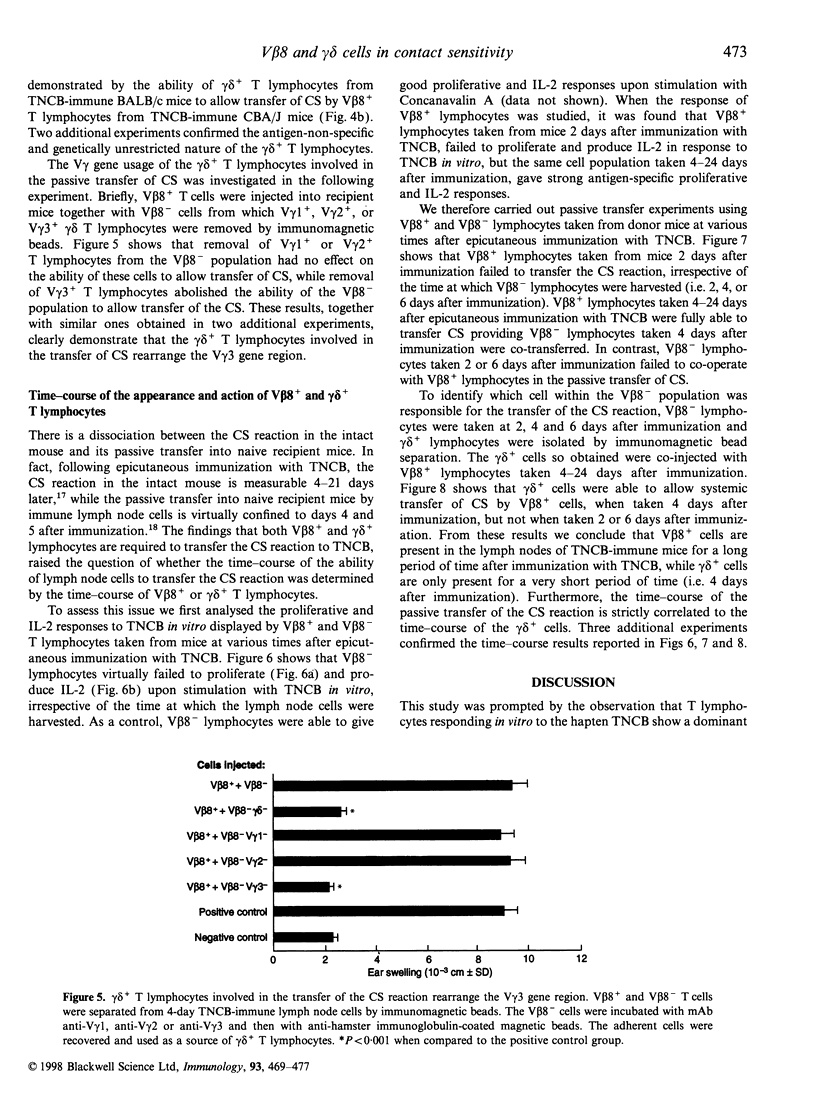
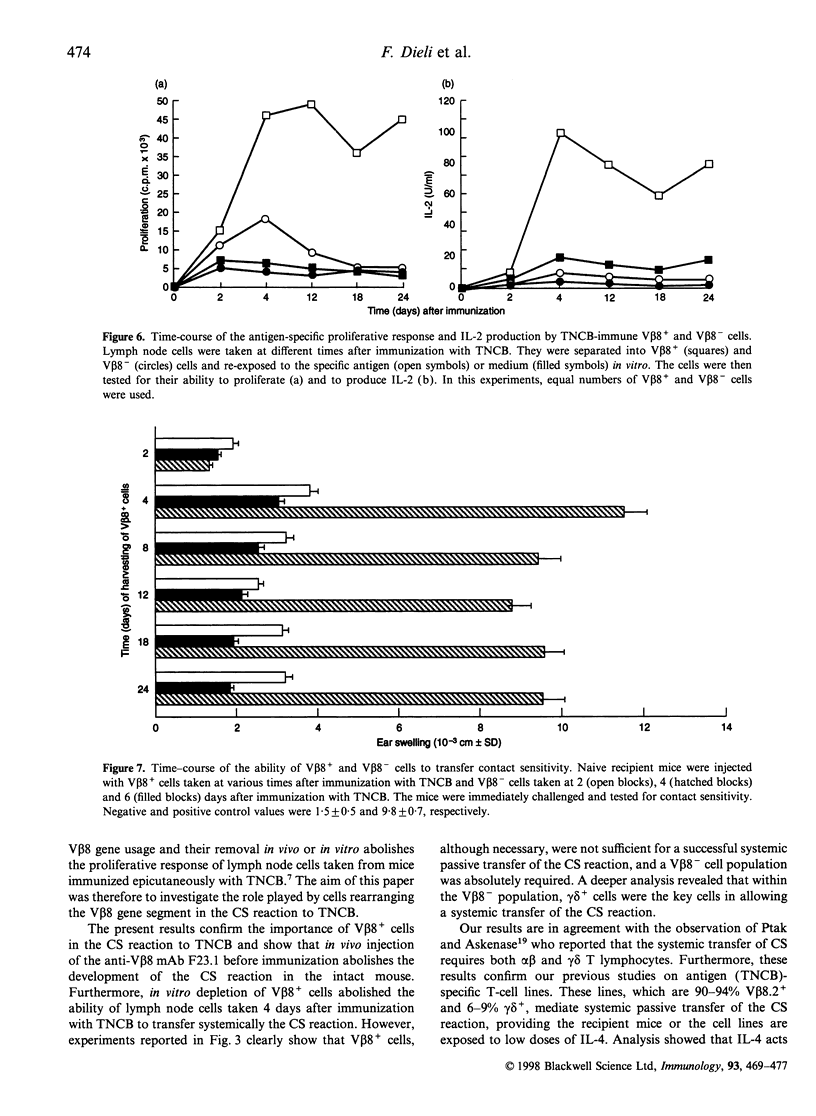
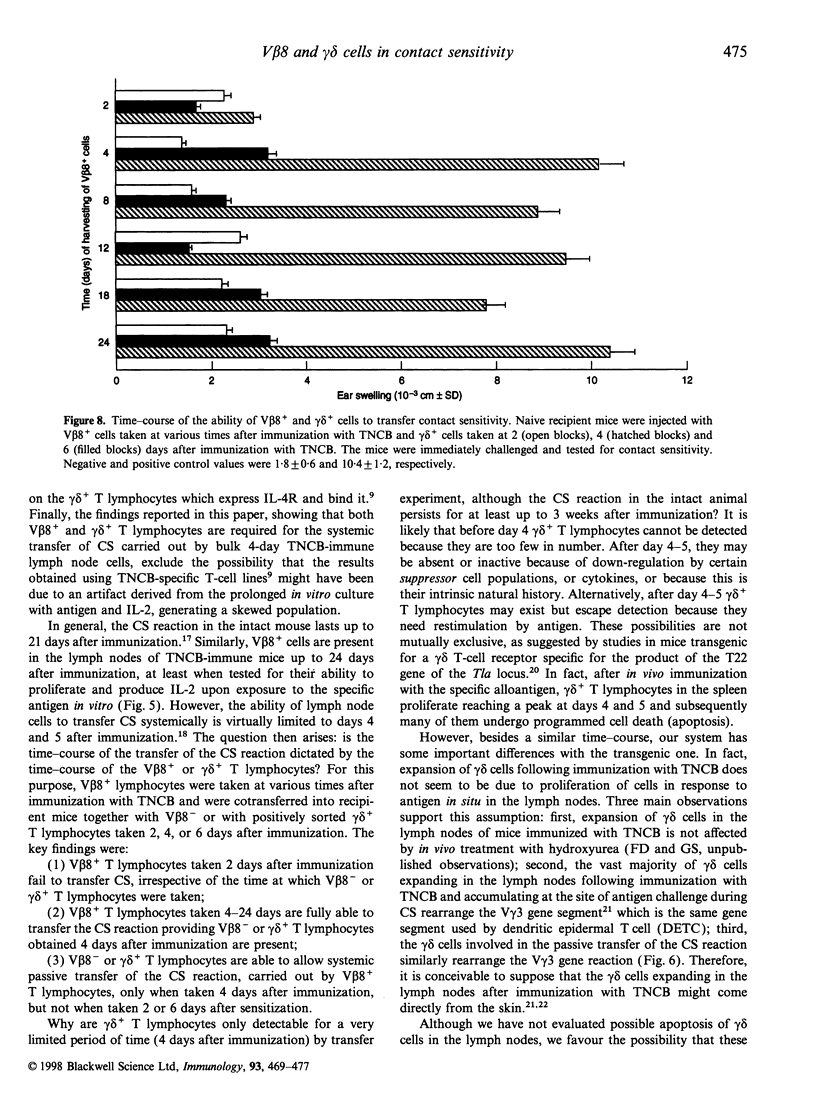
We have previously reported that T lymphocytes proliferating in vitro to the hapten trinitrochlorobenzene (TNCB) exhibit a very restricted V beta gene usage and response to TNCB is limited to T-cell receptors (TCR) composed of V beta 8.2 in combination with V alpha 3.2, V alpha 8 and V alpha 10. This paper investigates the role played by T lymphocytes expressing the V beta 8.2 gene segment in the contact sensitivity (CS) reaction to TNCB in the intact mouse and in its passive transfer into naive recipient mice. Mice injected with monoclonal antibodies to V beta 8 are unable to develop CS upon immunization with TNCB and 4-day TNCB-immune lymph node cells from mice that had been depleted in vivo or in vitro of V beta 8+ T lymphocytes fail to transfer CS. However, when separated V beta 8+ and V beta 8- cells were used for passive transfer, it was found that V beta 8+ T lymphocytes failed to transfer CS when given alone to recipient mice and a V beta 8- population was absolutely required. Further analysis revealed that within the V beta 8- population, T lymphocytes expressing the gamma delta TCR were fundamental to allow transfer of the CS reaction. These gamma delta cells were found to be antigen non-specific, genetically unrestricted and to rearrange the V gamma 3 gene segment. This indicates that transfer of the CS reaction requires cross-talk between V beta 8+ and gamma delta+ T lymphocytes, thus confirming our previous results obtained using TNCB-specific T-cell lines. Time-course experiments showed that V beta 8+ lymphocytes taken 4-24 days after immunization with TNCB were able to proliferate and produce interleukin-2 (IL-2) in response to the specific antigen in vitro. Similar time-course experiments were then undertaken using the passive transfer of the CS reaction system. The results obtained confirm that TNCB-specific V beta 8+ T lymphocytes are present in the lymph nodes of immunized mice from day 4 to day 24, and reveal that gamma delta+ T lymphocytes are active for a very short period of time, i.e. days 4 and 5 after immunization. In fact, TNCB-specific V beta 8+ cells are able to transfer CS when taken 4-24 days after immunization, providing the accompanying V beta 8- or gamma delta+ T lymphocyte are obtained 4 days after immunization. In contrast, injection of V beta 8+ T lymphocytes together with V beta 8- or gamma delta+ T lymphocytes that had been taken 2 or 6 days after immunization, failed to transfer significant CS into recipient mice. Taken together, our results confirm that cross-talk between V beta 8+ and gamma delta+ T lymphocytes is necessary for full development of the CS reaction and may explain why the CS reaction in the intact mouse lasts up to 21 days after immunization while the ability of immune lymph node cells to transfer CS is limited to days 4 and 5 after immunization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asarnow D. M., Kuziel W. A., Bonyhadi M., Tigelaar R. E., Tucker P. W., Allison J. P. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988 Dec 2;55(5):837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Dieli F. Immune deviation in the mouse: transfer of selective depression of the contact sensitivity and interleukin-2 response with retention of interferon-gamma production requires CD8+ T cells. Immunology. 1992 Jul;76(3):427–432. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Ptak W. Contact and delayed hypersensitivity in the mouse. I. Active sensitization and passive transfer. Immunology. 1968 Sep;15(3):405–416. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M. Anatomical location of cells which mediate contact sensitivity in the lympho nodes and bone marrow. Nat New Biol. 1973 Aug 8;244(136):176–177. doi: 10.1038/newbio244176a0. [DOI] [PubMed] [Google Scholar]
- Bellavia A., Mattei M., Dieli F., Salerno A., Caroleo M. C., Asherson G. L., Colizzi V. Augmented passive transfer of contact sensitivity in severe combined immunodeficiency mice and its dependence of V beta 8+ cells in the picryl system. Int Arch Allergy Immunol. 1993;101(4):402–407. doi: 10.1159/000236483. [DOI] [PubMed] [Google Scholar]
- Bürk M. R., Mori L., De Libero G. Human V gamma 9-V delta 2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol. 1995 Jul;25(7):2052–2058. doi: 10.1002/eji.1830250737. [DOI] [PubMed] [Google Scholar]
- Cavani A., Hackett C. J., Wilson K. J., Rothbard J. B., Katz S. I. Characterization of epitopes recognized by hapten-specific CD4+ T cells. J Immunol. 1995 Feb 1;154(3):1232–1238. [PubMed] [Google Scholar]
- Constant P., Davodeau F., Peyrat M. A., Poquet Y., Puzo G., Bonneville M., Fournié J. J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994 Apr 8;264(5156):267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Dieli F., Asherson G. L., Bonanno C. T., Sireci G., Salerno A. Major histocompatibility complex control of the class of the immune response to the hapten trinitrophenyl. Immunology. 1995 Mar;84(3):355–359. [PMC free article] [PubMed] [Google Scholar]
- Dieli F., Asherson G. L., Romano G. C., Sireci G., Gervasi F., Salerno A. IL-4 is essential for the systemic transfer of delayed hypersensitivity by T cell lines. Role of gamma/delta cells. J Immunol. 1994 Mar 15;152(6):2698–2704. [PubMed] [Google Scholar]
- Dieli F., Asherson G. L., Sireci G., Dominici R., Gervasi F., Vendetti S., Colizzi V., Salerno A. gamma delta cells involved in contact sensitivity preferentially rearrange the Vgamma3 region and require interleukin-7. Eur J Immunol. 1997 Jan;27(1):206–214. doi: 10.1002/eji.1830270131. [DOI] [PubMed] [Google Scholar]
- Dieli F., Asherson G. L., Sireci G., Dominici R., Sciré E., Salerno A. Development of IFN-gamma-producing CD8+ gamma delta+ T lymphocytes and IL-2-producing CD4+ alpha beta+ T lymphocytes during contact sensitivity. J Immunol. 1997 Mar 15;158(6):2567–2575. [PubMed] [Google Scholar]
- Dieli F., Salerno A. Role of the fourth complement component (C4) in the regulation of contact sensitivity. I. Analysis in mice with high and low C4 levels. Cell Immunol. 1987 Apr 1;105(2):386–396. doi: 10.1016/0008-8749(87)90086-4. [DOI] [PubMed] [Google Scholar]
- Friedmann P. S. Contact hypersensitivity. Curr Opin Immunol. 1989 Apr;1(4):690–693. doi: 10.1016/0952-7915(89)90043-5. [DOI] [PubMed] [Google Scholar]
- Fujita M., Miyachi Y., Nakata K., Imamura S. gamma delta T-cell receptor-positive cells of human skin. II. Appearance in delayed-type hypersensitivity reaction. Arch Dermatol Res. 1993;285(7):436–440. doi: 10.1007/BF00372140. [DOI] [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Havran W. L., Boismenu R. Activation and function of gamma delta T cells. Curr Opin Immunol. 1994 Jun;6(3):442–446. doi: 10.1016/0952-7915(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Heufler C., Topar G., Grasseger A., Stanzl U., Koch F., Romani N., Namen A. E., Schuler G. Interleukin 7 is produced by murine and human keratinocytes. J Exp Med. 1993 Sep 1;178(3):1109–1114. doi: 10.1084/jem.178.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H., Descossy P., van Brandwijk R., Knop J. Activation of murine epidermal TCR-gamma delta+ T cells by keratinocytes treated with contact sensitizers. J Immunol. 1995 Sep 15;155(6):2888–2894. [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Martin S., Weltzien H. U. T cell recognition of haptens, a molecular view. Int Arch Allergy Immunol. 1994 May;104(1):10–16. doi: 10.1159/000236703. [DOI] [PubMed] [Google Scholar]
- Matsue H., Bergstresser P. R., Takashima A. Keratinocyte-derived IL-7 serves as a growth factor for dendritic epidermal T cells in mice. J Immunol. 1993 Dec 1;151(11):6012–6019. [PubMed] [Google Scholar]
- Nordlind K., Lidén S. Gamma/delta T cells and human skin reactivity to heavy metals. Arch Dermatol Res. 1995;287(2):137–141. doi: 10.1007/BF01262321. [DOI] [PubMed] [Google Scholar]
- Pereira P., Gerber D., Huang S. Y., Tonegawa S. Ontogenic development and tissue distribution of V gamma 1-expressing gamma/delta T lymphocytes in normal mice. J Exp Med. 1995 Dec 1;182(6):1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K., Schoel B., Gulle H., Kaufmann S. H., Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of gamma/delta T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990 May;20(5):1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- Ptak W., Askenase P. W. Gamma delta T cells assist alpha beta T cells in adoptive transfer of contact sensitivity. J Immunol. 1992 Dec 1;149(11):3503–3508. [PubMed] [Google Scholar]
- Ptak W., Szczepanik M., Ramabhadran R., Askenase P. W. Immune or normal gamma delta T cells that assist alpha beta T cells in elicitation of contact sensitivity preferentially use V gamma 5 and V delta 4 variable region gene segments. J Immunol. 1996 Feb 1;156(3):976–986. [PubMed] [Google Scholar]
- Spaner D., Migita K., Ochi A., Shannon J., Miller R. G., Pereira P., Tonegawa S., Phillips R. A. Gamma delta T cells differentiate into a functional but nonproliferative state during a normal immune response. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8415–8419. doi: 10.1073/pnas.90.18.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J. Fate of H2-activated T lymphocytes in syngeneic hosts. I. Fate in lymphoid tissues and intestines traced with 3H-thymidine, 125I-deoxyuridine and 51chromium. Cell Immunol. 1976 Feb;21(2):278–302. doi: 10.1016/0008-8749(76)90057-5. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sano S., Nieves E., De Libero G., Rosa D., Modlin R. L., Brenner M. B., Bloom B. R., Morita C. T. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. The role of T cells in the passive transfer of contact sensitivity and their occurrence in the bone marrow. Eur J Immunol. 1973 Nov;3(11):667–680. doi: 10.1002/eji.1830031105. [DOI] [PubMed] [Google Scholar]


