Abstract
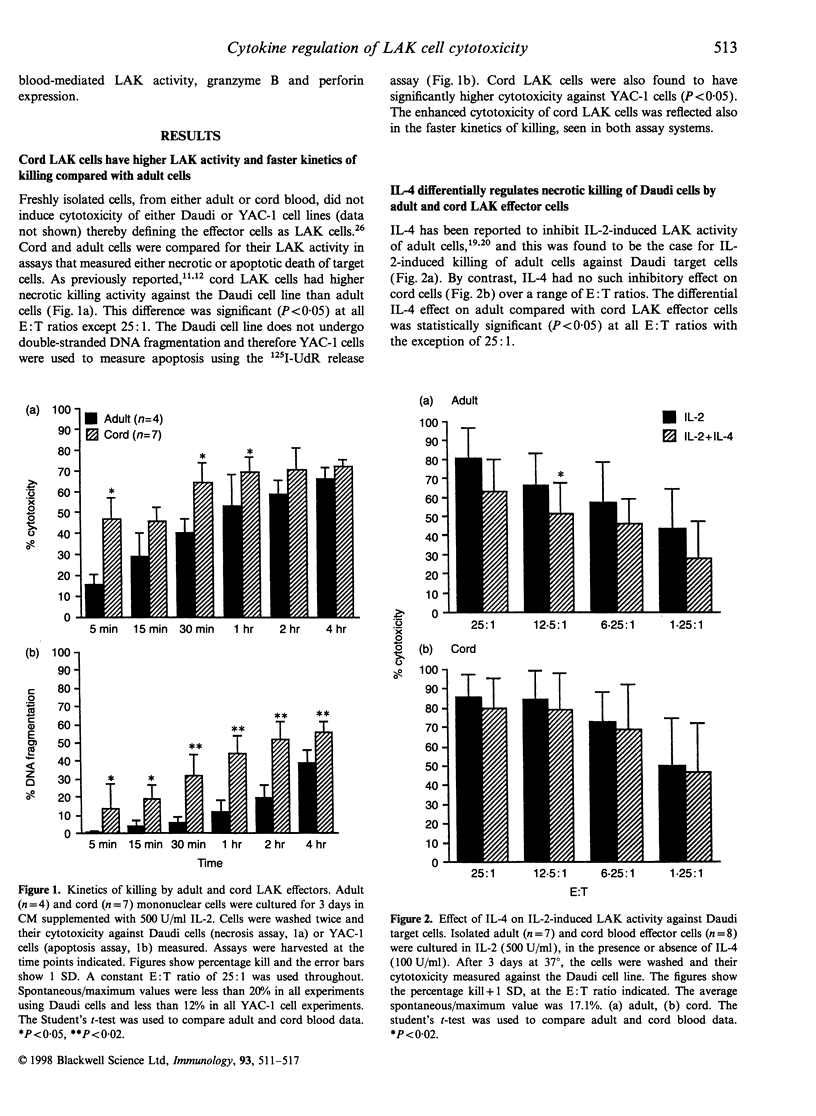
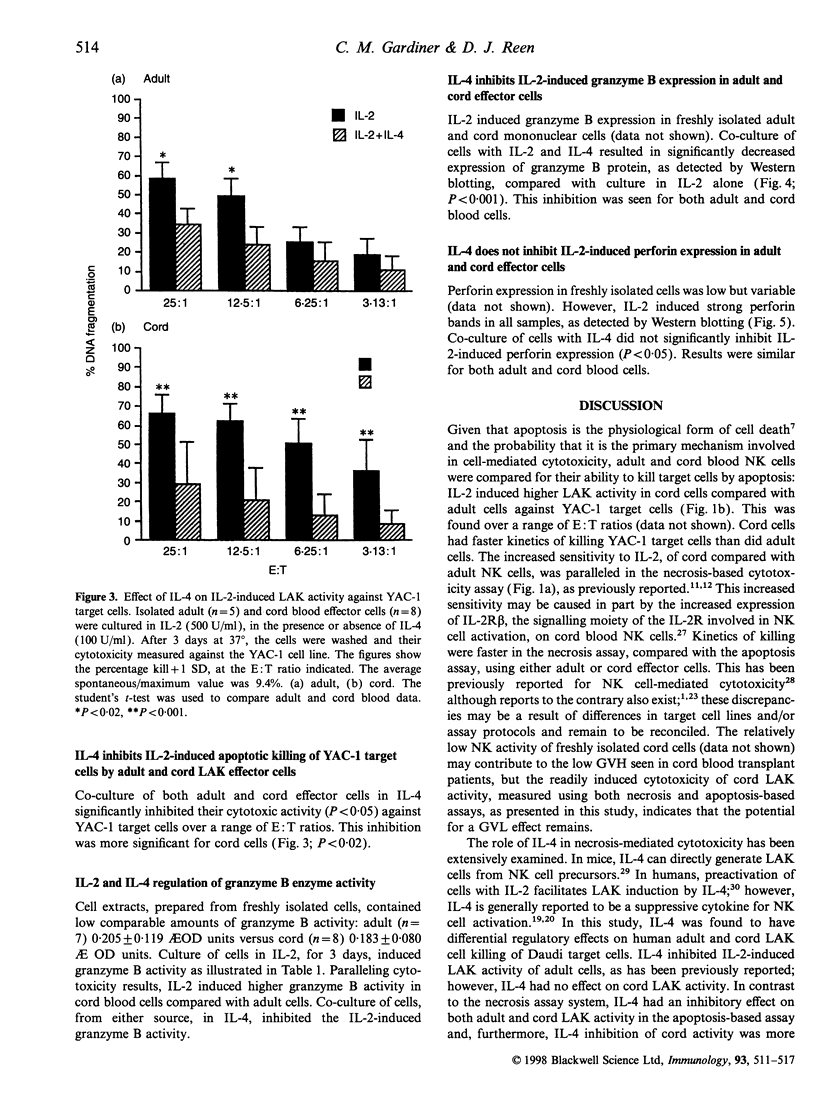
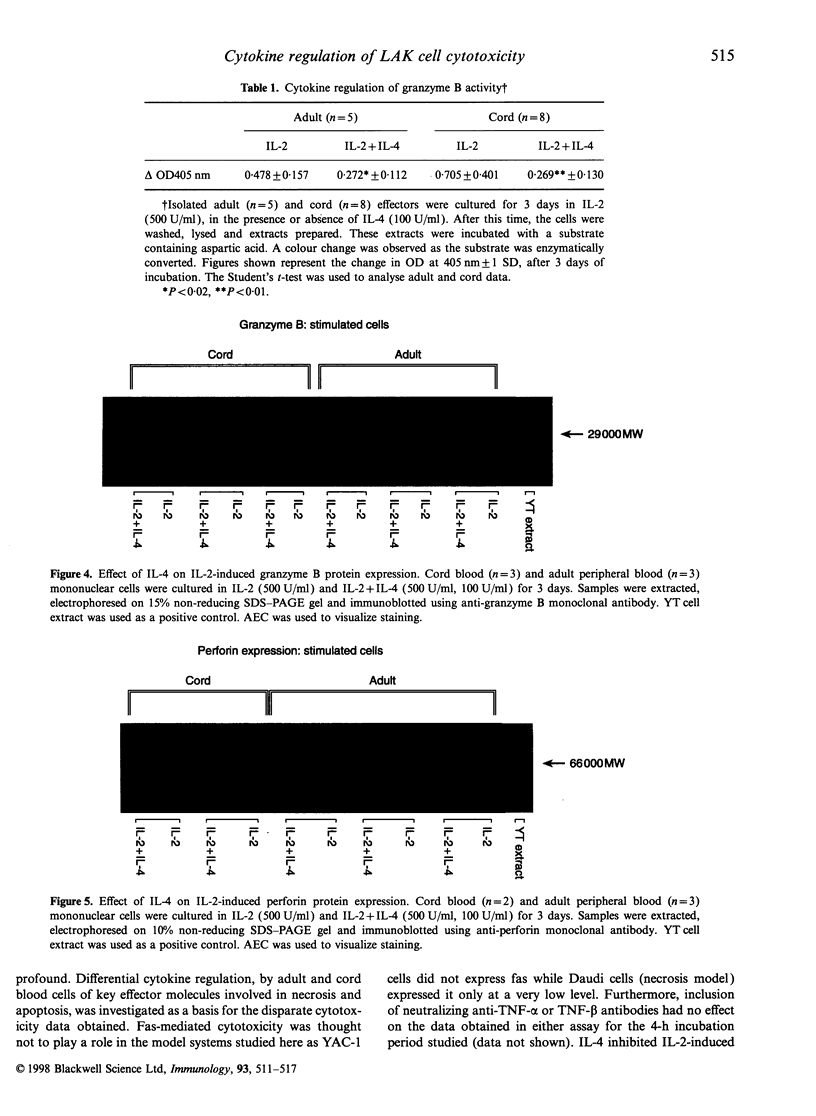
Natural killer (NK) cells can kill target cells by either necrotic or apoptotic mechanisms. Using the 51Cr-release assay to measure necrotic death of target cells, neonatal NK cells had low NK activity (K562 targets) and high lymphokine-activated killer (LAK) activity (Daudi targets) compared with adult cells, as has been previously reported. Using a 125I-deoxyuridine (125I-UdR) release assay, cord cells were shown to also have higher apoptotic LAK activity against YAC-1 target cells. Interleukin-4 (IL-4) inhibited interleukin-2 (IL-2)-induced necrotic killing of target cells by adult effectors but had no such inhibitory effect on cord cells. In contrast, IL-4 inhibited both adult and cord LAK cytotoxicity of YAC-1 target cells by apoptotic mechanisms with higher suppression observed in cord cell preparations. Using a colorimetric substrate conversion assay, IL-2 induced higher, and IL-4 had a more significant suppressive effect on, cord cell granzyme B enzyme activity compared with adult cells, paralleling apoptosis cytotoxicity data. Co-culture of either adult or cord LAK cells with IL-4 had a similar inhibitory effect on granzyme B protein expression, as detected by Western blotting. In contrast, IL-4 did not inhibit perforin expression, thereby defining IL-4 as a cytokine that can differentially regulate the NK cell-mediated cytotoxicity processes of apoptosis and necrosis. The differential sensitivity of cord cells to cytokine regulation of cytotoxicity may also have implications for cord blood transplantations, as NK cells are known to function as an effector cell in both graft-versus-host disease and in the graft-versus-leukaemia phenomena.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Skoglund A. C., Rönnholm M., Lindsten T., Lamon E. W., Collisson E. W., Walia A. S. Functional aspects of IgM and IgG Fc receptors on murine T lymphocytes. Immunol Rev. 1981;56:5–50. doi: 10.1111/j.1600-065x.1981.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Barrios C., Brawand P., Berney M., Brandt C., Lambert P. H., Siegrist C. A. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996 Jul;26(7):1489–1496. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- Brahmi Z., Tomita A., Hommel-Berrey G., Woodard G., Morse P. Mouse targets undergo double-strand DNA fragmentation when exposed to syngeneic or xenogeneic LAK cells whereas human targets undergo single-strand breaks. Nat Immun Cell Growth Regul. 1991;10(4):196–206. [PubMed] [Google Scholar]
- Brooks B., Chapman K., Lawry J., Meager A., Rees R. C. Suppression of lymphokine-activated killer (LAK) cell induction mediated by interleukin-4 and transforming growth factor-beta 1: effect of addition of exogenous tumour necrosis factor-alpha and interferon-gamma, and measurement of their endogenous production. Clin Exp Immunol. 1990 Dec;82(3):583–589. doi: 10.1111/j.1365-2249.1990.tb05494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7–7. [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J., Chervenak R. Differences in target cell DNA fragmentation induced by mouse cytotoxic T lymphocytes and natural killer cells. J Immunol. 1986 Sep 1;137(5):1442–1447. [PubMed] [Google Scholar]
- Early E. M., Reen D. J. Antigen-independent responsiveness to interleukin-4 demonstrates differential regulation of newborn human T cells. Eur J Immunol. 1996 Dec;26(12):2885–2889. doi: 10.1002/eji.1830261212. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Robb R. J., Roth J. A., Neckers L. M., Lachman L. B., Wilson D. J., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. III. Evidence that IL-2 is sufficient for direct activation of peripheral blood lymphocytes into lymphokine-activated killer cells. J Exp Med. 1983 Oct 1;158(4):1356–1361. doi: 10.1084/jem.158.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercend T., Takvorian T., Nowill A., Tantravahi R., Moingeon P., Anderson K. C., Murray C., Bohuon C., Ythier A., Ritz J. Characterization of natural killer cells with antileukemia activity following allogeneic bone marrow transplantation. Blood. 1986 Mar;67(3):722–728. [PubMed] [Google Scholar]
- Heusel J. W., Wesselschmidt R. L., Shresta S., Russell J. H., Ley T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994 Mar 25;76(6):977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Higuchi C. M., Thompson J. A., Lindgren C. G., Gillis S., Widmer M. B., Kern D. E., Fefer A. Induction of lymphokine-activated killer activity by interleukin 4 in human lymphocytes preactivated by interleukin 2 in vivo or in vitro. Cancer Res. 1989 Dec 1;49(23):6487–6492. [PubMed] [Google Scholar]
- Lee R. K., Spielman J., Zhao D. Y., Olsen K. J., Podack E. R. Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. J Immunol. 1996 Sep 1;157(5):1919–1925. [PubMed] [Google Scholar]
- Lewis D. B., Yu C. C., Meyer J., English B. K., Kahn S. J., Wilson C. B. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Invest. 1991 Jan;87(1):194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994 Aug 25;370(6491):650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Malygin A. M., Timonen T. Non-major histocompatibility complex-restricted killer cells in human cord blood: generation and cytotoxic activity in recombinant interleukin-2-supplemented cultures. Immunology. 1993 Jul;79(3):506–508. [PMC free article] [PubMed] [Google Scholar]
- Masson D., Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9069–9072. [PubMed] [Google Scholar]
- Montagna D., Maccario R., Ugazio A. G., Mingrat G., Burgio G. R. Natural cytotoxicity in the neonate: high levels of lymphokine activated killer (LAK) activity. Clin Exp Immunol. 1988 Jan;71(1):177–181. [PMC free article] [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Phillips J. H. The effects of IL-4 on human natural killer cells. A potent regulator of IL-2 activation and proliferation. J Immunol. 1988 Oct 1;141(7):2349–2351. [PubMed] [Google Scholar]
- Nishikawa K., Saito S., Morii T., Kato Y., Narita N., Ichijo M., Ohashi Y., Takeshita T., Sugamura K. Differential expression of the interleukin 2 receptor beta (p75) chain on human peripheral blood natural killer subsets. Int Immunol. 1990;2(6):481–486. doi: 10.1093/intimm/2.6.481. [DOI] [PubMed] [Google Scholar]
- Robinet E., Kamoun M., Farace F., Chouaib S. Interleukin-4 differentially regulates interleukin-2-mediated and CD2-mediated induction of human lymphokine-activated killer effectors. Eur J Immunol. 1992 Nov;22(11):2861–2865. doi: 10.1002/eji.1830221116. [DOI] [PubMed] [Google Scholar]
- Saito S., Morii T., Umekage H., Makita K., Nishikawa K., Narita N., Ichijo M., Morikawa H., Ishii N., Nakamura M. Expression of the interleukin-2 receptor gamma chain on cord blood mononuclear cells. Blood. 1996 Apr 15;87(8):3344–3350. [PubMed] [Google Scholar]
- Shi L., Kraut R. P., Aebersold R., Greenberg A. H. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992 Feb 1;175(2):553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S., Heusel J. W., Macivor D. M., Wesselschmidt R. L., Russell J. H., Ley T. J. Granzyme B plays a critical role in cytotoxic lymphocyte-induced apoptosis. Immunol Rev. 1995 Aug;146:211–221. doi: 10.1111/j.1600-065x.1995.tb00690.x. [DOI] [PubMed] [Google Scholar]
- Smyth M. J., Trapani J. A. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995 Apr;16(4):202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- Swisher S. G., Economou J. S., Holmes E. C., Golub S. H. TNF-alpha and IFN-gamma reverse IL-4 inhibition of lymphokine-activated killer cell function. Cell Immunol. 1990 Jul;128(2):450–461. doi: 10.1016/0008-8749(90)90040-x. [DOI] [PubMed] [Google Scholar]
- Trapani J. A., Browne K. A., Dawson M., Smyth M. J. Immunopurification of functional Asp-ase (natural killer cell granzyme B) using a monoclonal antibody. Biochem Biophys Res Commun. 1993 Sep 15;195(2):910–920. doi: 10.1006/bbrc.1993.2131. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. E., Kernan N. A., Steinbuch M., Broxmeyer H. E., Gluckman E. Allogeneic sibling umbilical-cord-blood transplantation in children with malignant and non-malignant disease. Lancet. 1995 Jul 22;346(8969):214–219. doi: 10.1016/s0140-6736(95)91268-1. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Bryant J., Day R., Herberman R. B. Natural killer cytotoxicity in the diagnosis of immune dysfunction: criteria for a reproducible assay. J Clin Lab Anal. 1990;4(2):102–114. doi: 10.1002/jcla.1860040207. [DOI] [PubMed] [Google Scholar]
- Xun C. Q., Thompson J. S., Brown S. A., Jennings C. D., Henslee-Downey P. J. Acute graft-versus-host-like disease induced by human CD56+, CD16+, CD8-dim+ cells in SCID mice. Transplant Proc. 1993 Feb;25(1 Pt 2):1227–1229. [PubMed] [Google Scholar]
- Yabuhara A., Kawai H., Komiyama A. Development of natural killer cytotoxicity during childhood: marked increases in number of natural killer cells with adequate cytotoxic abilities during infancy to early childhood. Pediatr Res. 1990 Oct;28(4):316–322. doi: 10.1203/00006450-199010000-00002. [DOI] [PubMed] [Google Scholar]
- Zola H., Fusco M., Macardle P. J., Flego L., Roberton D. Expression of cytokine receptors by human cord blood lymphocytes: comparison with adult blood lymphocytes. Pediatr Res. 1995 Sep;38(3):397–403. doi: 10.1203/00006450-199509000-00021. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Zheng L. M., Liu C. C., Young J. D. Cytolytic lymphocytes induce both apoptosis and necrosis in target cells. J Immunol. 1991 Jan 1;146(1):393–400. [PubMed] [Google Scholar]




