Abstract
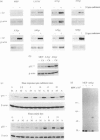
In humans, expression of the cellular proto-oncogene c-fgr is normally restricted to mature cells of the myeloid lineage, mantle zone B cells and various myeloid and B-cell lines. Previous studies of the monoblastoid cell line, U937, showed that c-fgr expression increased following differentiation, but its role in monocytes and related cells has not been defined in functional terms. We therefore investigated the role of c-fgr in U937 cells transfected with the c-fgr gene such that its expression could be manipulated independent of differentiation. Induction of the transfected c-fgr gene by cadmium ions did not affect cell proliferation, responses to phorbol 12-myristate 13-acetate (PMA), dihydroxycholecalciferol (DHCC), tumour necrosis factor-alpha (TNF-alpha) or retinoic acid, or phagocytosis of antibody-coated sheep red blood cells. However, there was increased surface expression of CD54 (intracellular adhesion molecule-1; ICAM-1) and CD102 (ICAM-2) and decreased surface expression of CD50 (ICAM-3) compared with cells that had been transfected with plasmid only and treated in the same way. These findings suggest that the product of the c-fgr gene may be important in control of relative adhesive properties of mature monocytic cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berton G., Fumagalli L., Laudanna C., Sorio C. Beta 2 integrin-dependent protein tyrosine phosphorylation and activation of the FGR protein tyrosine kinase in human neutrophils. J Cell Biol. 1994 Aug;126(4):1111–1121. doi: 10.1083/jcb.126.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnerts M. E., van Kooyk Y., Simmons D. L., Figdor C. G. Distinct binding of T lymphocytes to ICAM-1, -2 or -3 upon activation of LFA-1. Eur J Immunol. 1994 Sep;24(9):2155–2160. doi: 10.1002/eji.1830240933. [DOI] [PubMed] [Google Scholar]
- Brickell P. M., Patel M. Structure and expression of c-fgr protooncogene mRNA in Epstein-Barr virus converted cell lines. Br J Cancer. 1988 Dec;58(6):704–709. doi: 10.1038/bjc.1988.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickell P. M. The p60c-src family of protein-tyrosine kinases: structure, regulation, and function. Crit Rev Oncog. 1992;3(4):401–446. [PubMed] [Google Scholar]
- Campanero M. R., del Pozo M. A., Arroyo A. G., Sánchez-Mateos P., Hernández-Caselles T., Craig A., Pulido R., Sánchez-Madrid F. ICAM-3 interacts with LFA-1 and regulates the LFA-1/ICAM-1 cell adhesion pathway. J Cell Biol. 1993 Nov;123(4):1007–1016. doi: 10.1083/jcb.123.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah M. S., Ley T. J., Tronick S. R., Robbins K. C. fgr proto-oncogene mRNA induced in B lymphocytes by Epstein-Barr virus infection. Nature. 1986 Jan 16;319(6050):238–240. doi: 10.1038/319238a0. [DOI] [PubMed] [Google Scholar]
- Cushley W., Harnett M. M. Cellular signalling mechanisms in B lymphocytes. Biochem J. 1993 Jun 1;292(Pt 2):313–332. doi: 10.1042/bj2920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits A. J., Dimjati W., van de Winkel J. G., Capel P. J. Synergism of interleukin 6 and 1 alpha,25-dihydroxyvitamin D3 in induction of myeloid differentiation of human leukemic cell lines. J Leukoc Biol. 1992 Mar;51(3):237–243. doi: 10.1002/jlb.51.3.237. [DOI] [PubMed] [Google Scholar]
- Faulkner L., Patel M., Brickell P. M., Katz D. R. Regulation of c-fgr messenger RNA levels in U937 cells treated with different modulating agents. Immunology. 1992 May;76(1):65–71. [PMC free article] [PubMed] [Google Scholar]
- Gutkind J. S., Robbins K. C. Translocation of the FGR protein-tyrosine kinase as a consequence of neutrophil activation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8783–8787. doi: 10.1073/pnas.86.22.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada F., Aoki M., Akiyama T., Toyoshima K. Association of immunoglobulin G Fc receptor II with Src-like protein-tyrosine kinase Fgr in neutrophils. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6305–6309. doi: 10.1073/pnas.90.13.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M., Barker S., Brennan A., Nathan J., Katz D. R., O'Riordan J. L. Autocrine regulation of 1,25-dihydroxycholecalciferol metabolism in myelomonocytic cells. Immunology. 1989 Oct;68(2):247–252. [PMC free article] [PubMed] [Google Scholar]
- Hewison M., Dabrowski M., Faulkner L., Hughson E., Vadher S., Rut A., Brickell P. M., O'Riordan J. L., Katz D. R. Transfection of vitamin D receptor cDNA into the monoblastoid cell line U937. The role of vitamin D3 in homotypic macrophage adhesion. J Immunol. 1994 Dec 15;153(12):5709–5719. [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995 Jan 27;80(2):225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Inoue K., Yamamoto T., Toyoshima K. Specific expression of human c-fgr in natural immunity effector cells. Mol Cell Biol. 1990 Apr;10(4):1789–1792. doi: 10.1128/mcb.10.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahroudi N., Foster R., Price-Haughey J., Beitel G., Gedamu L. Cell-type specific and differential regulation of the human metallothionein genes. Correlation with DNA methylation and chromatin structure. J Biol Chem. 1990 Apr 15;265(11):6506–6511. [PubMed] [Google Scholar]
- Link D. C., Zutter M. The proto-oncogene c-fgr is expressed in normal mantle zone B lymphocytes and is developmentally regulated during myelomonocytic differentiation in vivo. Blood. 1995 Jan 15;85(2):472–479. [PubMed] [Google Scholar]
- Lowell C. A., Soriano P., Varmus H. E. Functional overlap in the src gene family: inactivation of hck and fgr impairs natural immunity. Genes Dev. 1994 Feb 15;8(4):387–398. doi: 10.1101/gad.8.4.387. [DOI] [PubMed] [Google Scholar]
- Notario V., Gutkind J. S., Imaizumi M., Katamine S., Robbins K. C. Expression of the fgr protooncogene product as a function of myelomonocytic cell maturation. J Cell Biol. 1989 Dec;109(6 Pt 1):3129–3136. doi: 10.1083/jcb.109.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohh M., Smith C. A., Carpenito C., Takei F. Regulation of intercellular adhesion molecule-1 gene expression involves multiple mRNA stabilization mechanisms: effects of interferon-gamma and phorbol myristate acetate. Blood. 1994 Oct 15;84(8):2632–2639. [PubMed] [Google Scholar]
- Rudd C. E., Janssen O., Cai Y. C., da Silva A. J., Raab M., Prasad K. V. Two-step TCR zeta/CD3-CD4 and CD28 signaling in T cells: SH2/SH3 domains, protein-tyrosine and lipid kinases. Immunol Today. 1994 May;15(5):225–234. doi: 10.1016/0167-5699(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Sharp N. A., Luscombe M. J., Clemens M. J. Regulation of c-fgr proto-oncogene expression in Burkitt's lymphoma cells: effect of interferon treatment and relationship to EBV status and c-myc mRNA levels. Oncogene. 1989 Aug;4(8):1043–1046. [PubMed] [Google Scholar]
- Szekanecz Z., Haines G. K., Lin T. R., Harlow L. A., Goerdt S., Rayan G., Koch A. E. Differential distribution of intercellular adhesion molecules (ICAM-1, ICAM-2, and ICAM-3) and the MS-1 antigen in normal and diseased human synovia. Their possible pathogenetic and clinical significance in rheumatoid arthritis. Arthritis Rheum. 1994 Feb;37(2):221–231. doi: 10.1002/art.1780370211. [DOI] [PubMed] [Google Scholar]
- Tiisala S., Majuri M. L., Carpén O., Renkonen R. Genistein enhances the ICAM-mediated adhesion by inducing the expression of ICAM-1 and its counter-receptors. Biochem Biophys Res Commun. 1994 Aug 30;203(1):443–449. doi: 10.1006/bbrc.1994.2202. [DOI] [PubMed] [Google Scholar]
- Wu H., Parsons J. T. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993 Mar;120(6):1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambruno G., Cossarizza A., Zacchi V., Ottani D., Luppi A. M., Giannetti A., Girolomoni G. Functional intercellular adhesion molecule-3 is expressed by freshly isolated epidermal Langerhans cells and is not regulated during culture. J Invest Dermatol. 1995 Aug;105(2):215–219. doi: 10.1111/1523-1747.ep12317494. [DOI] [PubMed] [Google Scholar]
- Ziegler S. F., Wilson C. B., Perlmutter R. M. Augmented expression of a myeloid-specific protein tyrosine kinase gene (hck) after macrophage activation. J Exp Med. 1988 Nov 1;168(5):1801–1810. doi: 10.1084/jem.168.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles A. R., Klickstein L. B., Springer T. A. Cloning and expression of intercellular adhesion molecule 3 reveals strong homology to other immunoglobulin family counter-receptors for lymphocyte function-associated antigen 1. J Exp Med. 1993 Apr 1;177(4):1187–1192. doi: 10.1084/jem.177.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo M. A., Pulido R., Muñoz C., Alvarez V., Humbría A., Campanero M. R., Sánchez-Madrid F. Regulation of ICAM-3 (CD50) membrane expression on human neutrophils through a proteolytic shedding mechanism. Eur J Immunol. 1994 Nov;24(11):2586–2594. doi: 10.1002/eji.1830241104. [DOI] [PubMed] [Google Scholar]