Abstract
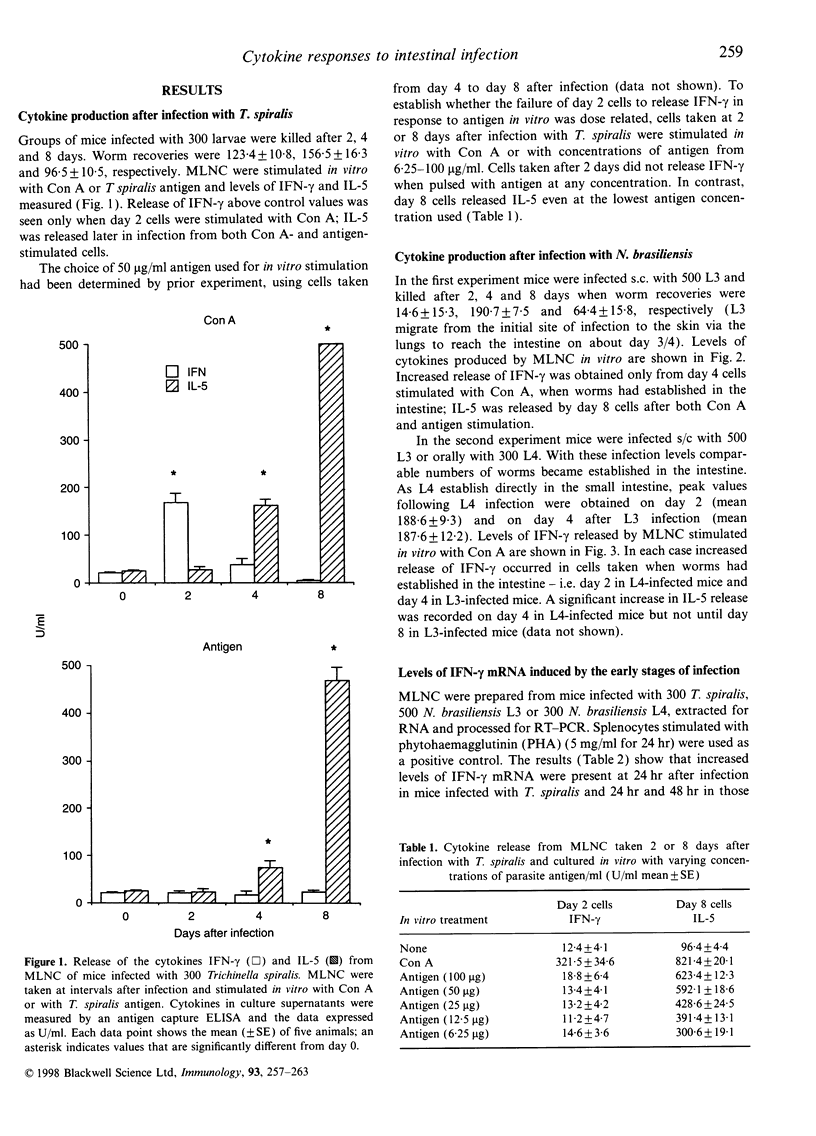
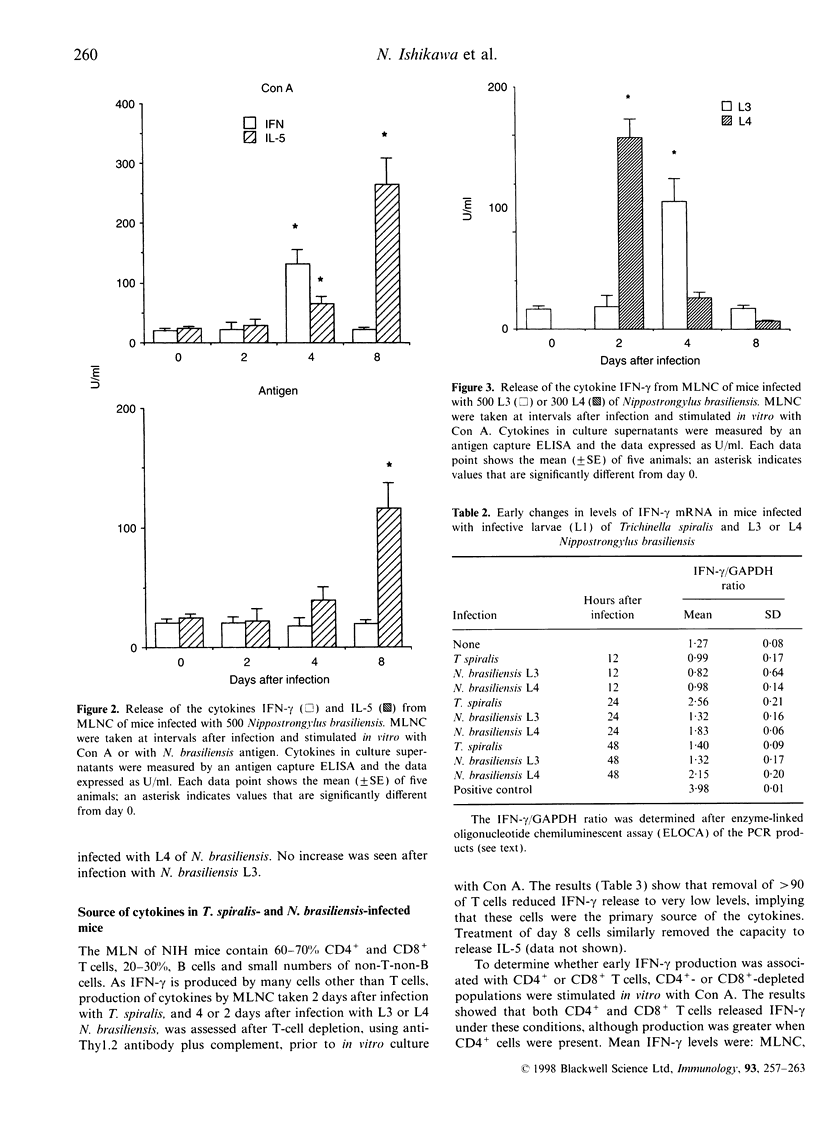
Infections with gastro-intestinal nematodes elicit immune and inflammatory responses mediated by cytokines released from T-helper type-2 (Th2) cells. In vitro assays of cells from the mesenteric lymph nodes (MLN) of experimentally infected rodents confirm that, after about 1 week, the dominant cytokine responses to mitogens and antigens are those associated with this Th-cell subset. Polarization of the Th response in this way implies an initial local cytokine environment that favours Th2 development. However, experimental infections with Trichinella spiralis and Nippostrongylus brasiliensis show that, within 2 days of worms reaching the intestine, MLN cells (MLNC) respond with a Th1 rather than a Th2 response [i.e. there is an increase in mRNA for the type 1 cytokine interferon-gamma (IFN-gamma), and mitogen-stimulated MLNC release IFN-gamma rather than interleukin-5 (IL-5)]. Antigen stimulation at this time does not elicit IFN-gamma release and the MLNC cannot adoptively transfer immunity. Within a few days the MLNC phenotype changes. There is a Th2 response (IL-5 release) to both mitogen and antigen stimulation and MLNC can adoptively transfer immunity. Early release of IFN-gamma is T-cell dependent, with CD4+ T cells playing the major role. The data are discussed in relation to factors regulating the mucosal response to invasion by parasites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Else K. J., Hültner L., Grencis R. K. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology. 1992 Feb;75(2):232–237. [PMC free article] [PubMed] [Google Scholar]
- Ferrick D. A., Schrenzel M. D., Mulvania T., Hsieh B., Ferlin W. G., Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995 Jan 19;373(6511):255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Madden K. B., Cheever A. W., Katona I. M., Morris S. C., Gately M. K., Hubbard B. R., Gause W. C., Urban J. F., Jr Effects of interleukin 12 on immune responses and host protection in mice infected with intestinal nematode parasites. J Exp Med. 1994 May 1;179(5):1563–1572. doi: 10.1084/jem.179.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Shea-Donohue T., Goldhill J., Sullivan C. A., Morris S. C., Madden K. B., Gause W. C., Urban J. F., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- Goyal P. K., Hermánek J., Wakelin D. Lymphocyte proliferation and cytokine production in mice infected with different geographical isolates of Trichinella spiralis. Parasite Immunol. 1994 Feb;16(2):105–110. doi: 10.1111/j.1365-3024.1994.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Grencis R. K., Hültner L., Else K. J. Host protective immunity to Trichinella spiralis in mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology. 1991 Oct;74(2):329–332. [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., O'Garra A., Murphy K. M. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995 Feb 1;181(2):713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa N., Horii Y., Nawa Y. Immune-mediated alteration of the terminal sugars of goblet cell mucins in the small intestine of Nippostrongylus brasiliensis-infected rats. Immunology. 1993 Feb;78(2):303–307. [PMC free article] [PubMed] [Google Scholar]
- Itohara S., Nakanishi N., Kanagawa O., Kubo R., Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E. A., Cruz E. S., Hauda K. M., Wassom D. L. IFN-gamma- and IL-5-producing cells compartmentalize to different lymphoid organs in Trichinella spiralis-infected mice. J Immunol. 1991 Jul 1;147(1):306–311. [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Bachmann M., Lamers M. C., Bluethmann H., Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993 Mar 18;362(6417):245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlan J. M., Seth R., Vautier G., Robins R. A., Scott B. B., Hawkey C. J., Jenkins D. Interleukin-8 and inducible nitric oxide synthase mRNA levels in inflammatory bowel disease at first presentation. J Pathol. 1997 Jan;181(1):87–92. doi: 10.1002/(SICI)1096-9896(199701)181:1<87::AID-PATH736>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Pond L., Wassom D. L., Hayes C. E. Evidence for differential induction of helper T cell subsets during Trichinella spiralis infection. J Immunol. 1989 Dec 15;143(12):4232–4237. [PubMed] [Google Scholar]
- Robinson K., Bellaby T., Wakelin D. Immune response profiles in vaccinated and non-vaccinated high- and low-responder mice during infection with the intestinal nematode Trichinella spiralis. Parasitology. 1995 Jan;110(Pt 1):71–78. doi: 10.1017/s0031182000081063. [DOI] [PubMed] [Google Scholar]
- Scott P. Selective differentiation of CD4+ T helper cell subsets. Curr Opin Immunol. 1993 Jun;5(3):391–397. doi: 10.1016/0952-7915(93)90058-z. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R. L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- Svetić A., Madden K. B., Zhou X. D., Lu P., Katona I. M., Finkelman F. D., Urban J. F., Jr, Gause W. C. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. J Immunol. 1993 Apr 15;150(8 Pt 1):3434–3441. [PubMed] [Google Scholar]
- Taguchi T., McGhee J. R., Coffman R. L., Beagley K. W., Eldridge J. H., Takatsu K., Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+ T cells that secrete IFN-gamma and IL-5. J Immunol. 1990 Jul 1;145(1):68–77. [PubMed] [Google Scholar]
- Tonkonogy S. L., Swain S. L. Distinct lymphokine production by CD4+ T cells isolated from mucosal and systemic lymphoid organs. Immunology. 1993 Dec;80(4):574–580. [PMC free article] [PubMed] [Google Scholar]
- Urban J. F., Jr, Katona I. M., Paul W. E., Finkelman F. D. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. F., Jr, Madden K. B., Cheever A. W., Trotta P. P., Katona I. M., Finkelman F. D. IFN inhibits inflammatory responses and protective immunity in mice infected with the nematode parasite, Nippostrongylus brasiliensis. J Immunol. 1993 Dec 15;151(12):7086–7094. [PubMed] [Google Scholar]
- Wahid F. N., Behnke J. M., Grencis R. K., Else K. J., Ben-Smith A. W. Immunological relationships during primary infection with Heligmosomoides polygyrus: Th2 cytokines and primary response phenotype. Parasitology. 1994 May;108(Pt 4):461–471. doi: 10.1017/s0031182000076022. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Goyal P. K., Dehlawi M. S., Hermanek J. Immune responses to Trichinella spiralis and T. pseudospiralis in mice. Immunology. 1994 Mar;81(3):475–479. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Lloyd M. Immunity to primary and challenge infections of Trichinella spiralis in mice: a re-examination of conventional parameters. Parasitology. 1976 Apr;72(2):173–182. doi: 10.1017/s0031182000048472. [DOI] [PubMed] [Google Scholar]


