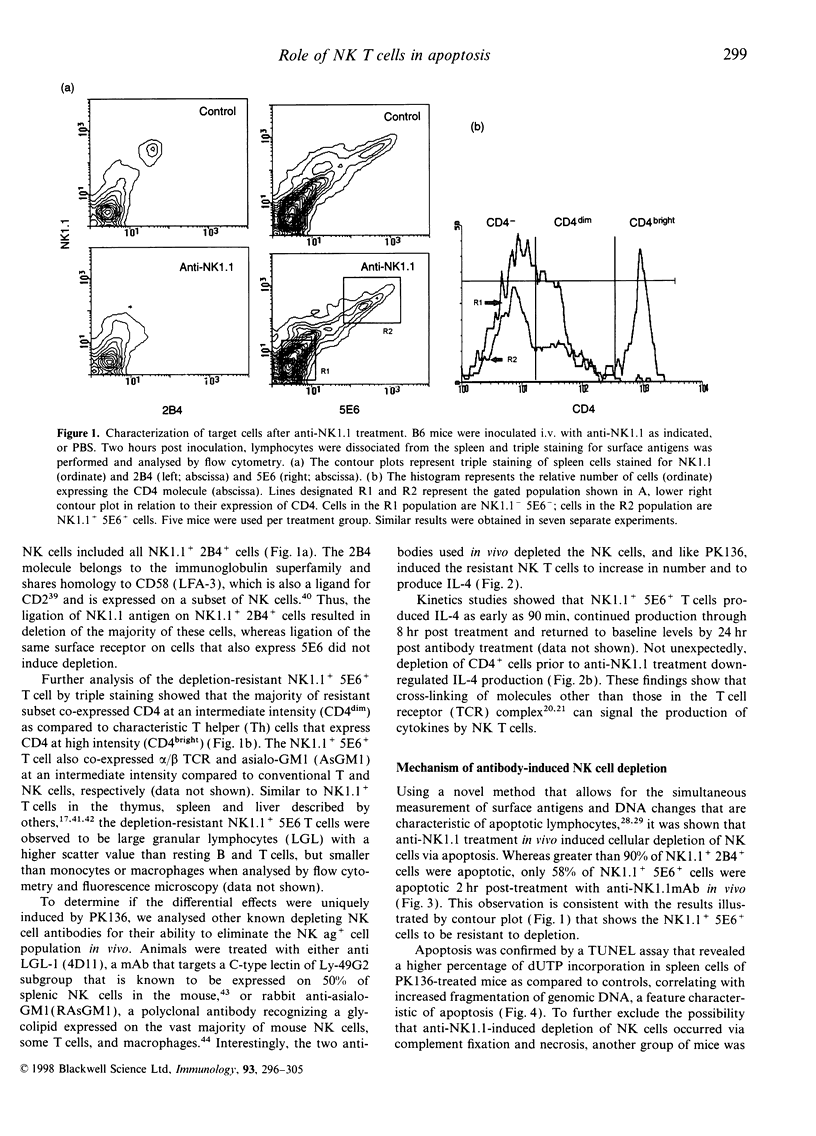
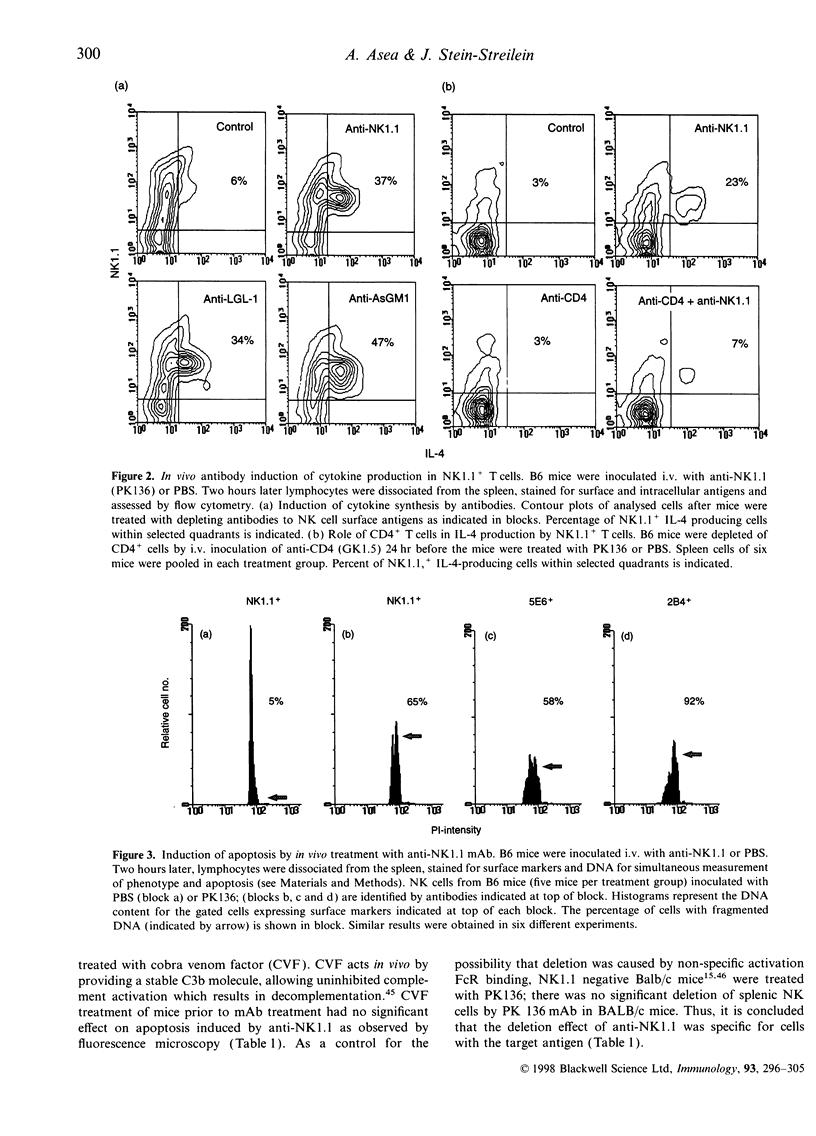
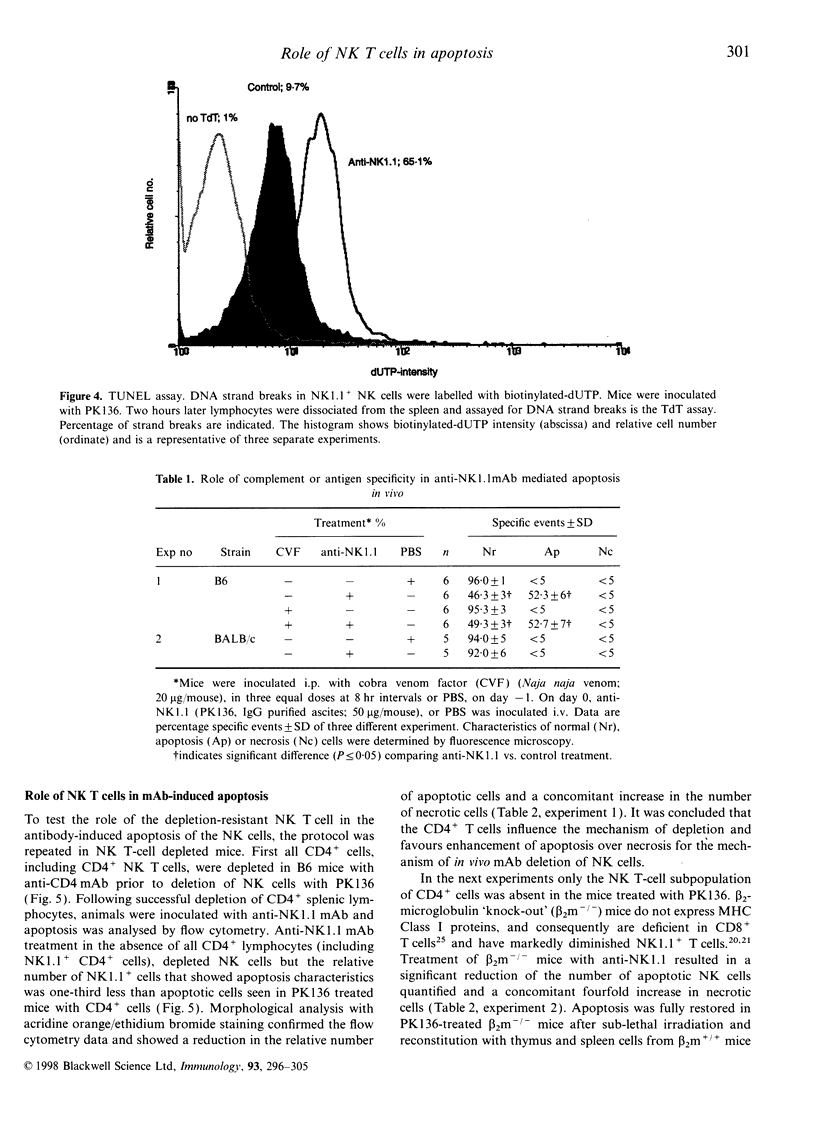
Abstract
In vivo inoculation of specific antibody is an accepted protocol for elimination of specific cell populations. Except for anti-CD3 and anti-CD4, it is not known if the depleted cells are eliminated by signalling through the target molecule or through a more non-specific mechanism. C57BL/6 mice were inoculated with anti-natural killer (NK1.1) monoclonal antibody (mAb). Thereafter spleen cells were harvested, stained for both surface and intracellular markers, and analysed by flow cytometry. As early as 2 hr post inoculation, NK cells were signalled to become apoptotic while signalling through the NK1.1 molecule activated NK1.1+ T-cell receptor (TCR)+ (NK T) cells to increase in number, and produce interleukin-4 (IL-4). Anti NK1.1 mAb was less efficient at signalling apoptosis in NK cells when NK T-cell deficient [beta 2-microglobulin beta 2m-deficient] mice were used compared with wild type mice. Efficient apoptotic signalling was restored when beta 2m-deficient mice were reconstituted with NK T cells. NK-specific antibody best signals the apoptotic process in susceptible NK cells when resistant NK T cells are present, activated, and secrete IL-4.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Watanabe H., Sato K., Iiai T., Moroda T., Takeda K., Seki S. Extrathymic T cells stand at an intermediate phylogenetic position between natural killer cells and thymus-derived T cells. Nat Immun. 1995 Apr;14(4):173–187. [PubMed] [Google Scholar]
- Adkins B., Ghanei A., Hamilton K. Developmental regulation of IL-4, IL-2, and IFN-gamma production by murine peripheral T lymphocytes. J Immunol. 1993 Dec 15;151(12):6617–6626. [PubMed] [Google Scholar]
- Araneo B. A., Dowell T., Moon H. B., Daynes R. A. Regulation of murine lymphokine production in vivo. Ultraviolet radiation exposure depresses IL-2 and enhances IL-4 production by T cells through an IL-1-dependent mechanism. J Immunol. 1989 Sep 15;143(6):1737–1744. [PubMed] [Google Scholar]
- Arase H., Arase-Fukushi N., Good R. A., Onoé K. Lymphokine-activated killer cell activity of CD4-CD8- TCR alpha beta + thymocytes. J Immunol. 1993 Jul 15;151(2):546–555. [PubMed] [Google Scholar]
- Arase H., Arase N., Nakagawa K., Good R. A., Onoé K. NK1.1+ CD4+ CD8- thymocytes with specific lymphokine secretion. Eur J Immunol. 1993 Jan;23(1):307–310. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- Ayroldi E., Cannarile L., D'Adamio F., Riccardi C. Superantigen-induced peripheral T-cell deletion: the effects of chemical modification of antigen-presenting cells, interleukin-4 and glucocorticoid hormones. Immunology. 1995 Apr;84(4):528–535. [PMC free article] [PubMed] [Google Scholar]
- Bendelac A., Killeen N., Littman D. R., Schwartz R. H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994 Mar 25;263(5154):1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Lantz O., Quimby M. E., Yewdell J. W., Bennink J. R., Brutkiewicz R. R. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995 May 12;268(5212):863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Berguer R., Ferrick D. A. Differential production of intracellular gamma interferon in alpha beta and gamma delta T-cell subpopulations in response to peritonitis. Infect Immun. 1995 Dec;63(12):4957–4958. doi: 10.1128/iai.63.12.4957-4958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J., Mager D., Jefferies W., Takei F. Expression of different members of the Ly-49 gene family defines distinct natural killer cell subsets and cell adhesion properties. J Exp Med. 1994 Dec 1;180(6):2287–2295. doi: 10.1084/jem.180.6.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C. G., Burton R. C., Pollack S. B., Henney C. S. The presence of NK alloantigens on cloned cytotoxic T lymphocytes. J Immunol. 1983 Sep;131(3):1391–1395. [PubMed] [Google Scholar]
- Carson W. E., Haldar S., Baiocchi R. A., Croce C. M., Caligiuri M. A. The c-kit ligand suppresses apoptosis of human natural killer cells through the upregulation of bcl-2. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7553–7557. doi: 10.1073/pnas.91.16.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Garni-Wagner B. A., Purohit A., Mathew P. A., Bennett M., Kumar V. A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J Immunol. 1993 Jul 1;151(1):60–70. [PubMed] [Google Scholar]
- Gorczyca W., Gong J., Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993 Apr 15;53(8):1945–1951. [PubMed] [Google Scholar]
- Hansson M., Asea A., Hermodsson S., Hellstrand K. Histaminergic regulation of NK-cells: protection against monocyte-induced apoptosis. Scand J Immunol. 1996 Aug;44(2):193–196. doi: 10.1046/j.1365-3083.1996.d01-291.x. [DOI] [PubMed] [Google Scholar]
- Hercend T., Schmidt R. E. Characteristics and uses of natural killer cells. Immunol Today. 1988 Oct;9(10):291–293. doi: 10.1016/0167-5699(88)91317-5. [DOI] [PubMed] [Google Scholar]
- Howie S. E., Harrison D. J., Wyllie A. H. Lymphocyte apoptosis--mechanisms and implications in disease. Immunol Rev. 1994 Dec;142:141–156. doi: 10.1111/j.1600-065x.1994.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen F. W., Keller J. R., Ruscetti F. W., Veiby O. P., Jacobsen S. E. Direct synergistic effects of IL-4 and IL-11 on proliferation of primitive hematopoietic progenitor cells. Exp Hematol. 1995 Aug;23(9):990–995. [PubMed] [Google Scholar]
- Karlhofer F. M., Yokoyama W. M. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen. IL-2-activated NK cells possess additional specific stimulation pathways. J Immunol. 1991 May 15;146(10):3662–3673. [PubMed] [Google Scholar]
- Kasai M., Iwamori M., Nagai Y., Okumura K., Tada T. A glycolipid on the surface of mouse natural killer cells. Eur J Immunol. 1980 Mar;10(3):175–180. doi: 10.1002/eji.1830100304. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Koo G. C., Dumont F. J., Tutt M., Hackett J., Jr, Kumar V. The NK-1.1(-) mouse: a model to study differentiation of murine NK cells. J Immunol. 1986 Dec 15;137(12):3742–3747. [PubMed] [Google Scholar]
- Koo G. C., Peppard J. R. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma. 1984 Fall;3(3):301–303. doi: 10.1089/hyb.1984.3.301. [DOI] [PubMed] [Google Scholar]
- Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991 Nov 1;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- Mason L., Giardina S. L., Hecht T., Ortaldo J., Mathieson B. J. LGL-1: a non-polymorphic antigen expressed on a major population of mouse natural killer cells. J Immunol. 1988 Jun 15;140(12):4403–4412. [PubMed] [Google Scholar]
- Mathieson P. W., Qasim F. J., Thiru S., Oldroyd R. G., Oliveira D. B. Effects of decomplementation with cobra venom factor on experimental vasculitis. Clin Exp Immunol. 1994 Sep;97(3):474–477. doi: 10.1111/j.1365-2249.1994.tb06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moingeon P., Chang H. C., Sayre P. H., Clayton L. K., Alcover A., Gardner P., Reinherz E. L. The structural biology of CD2. Immunol Rev. 1989 Oct;111:111–144. doi: 10.1111/j.1600-065x.1989.tb00544.x. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Raziuddin A., Mason L., Kumar V., Bennett M., Longo D. L. NK cell subsets in the regulation of murine hematopoiesis. I. 5E6+ NK cells promote hematopoietic growth in H-2d strain mice. J Immunol. 1995 Sep 15;155(6):2911–2917. [PubMed] [Google Scholar]
- Openshaw P., Murphy E. E., Hosken N. A., Maino V., Davis K., Murphy K., O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995 Nov 1;182(5):1357–1367. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharton T. M., Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993 Aug 1;178(2):567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Seki S., Abo T., Ohteki T., Sugiura K., Kumagai K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol. 1991 Aug 15;147(4):1214–1221. [PubMed] [Google Scholar]
- Shi Y. F., Bissonnette R. P., Parfrey N., Szalay M., Kubo R. T., Green D. R. In vivo administration of monoclonal antibodies to the CD3 T cell receptor complex induces cell death (apoptosis) in immature thymocytes. J Immunol. 1991 May 15;146(10):3340–3346. [PubMed] [Google Scholar]
- Shi Y. F., Sahai B. M., Green D. R. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989 Jun 22;339(6226):625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Sonoda Y. Interleukin-4--a dual regulatory factor in hematopoiesis. Leuk Lymphoma. 1994 Jul;14(3-4):231–240. doi: 10.3109/10428199409049673. [DOI] [PubMed] [Google Scholar]
- Spinozzi F., Nicoletti I., Agea E., Belia S., Moraca R., Migliorati G., Riccardi C., Grignani F., Bertotto A. IL-4 is able to reverse the CD2-mediated negative apoptotic signal to CD4-CD8- alpha beta and/or gamma delta T lymphocytes. Immunology. 1995 Nov;86(3):379–384. [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J., Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986 Feb 15;136(4):1435–1441. [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. The two faces of interleukin 12: a pro-inflammatory cytokine and a key immunoregulatory molecule produced by antigen-presenting cells. Ciba Found Symp. 1995;195:203–220. doi: 10.1002/9780470514849.ch14. [DOI] [PubMed] [Google Scholar]
- Van Parijs L., Ibraghimov A., Abbas A. K. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996 Mar;4(3):321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Miyaji C., Kawachi Y., Iiai T., Ohtsuka K., Iwanage T., Takahashi-Iwanaga H., Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995 Sep 15;155(6):2972–2983. [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Bendelac A., Watson C., Hu-Li J., Paul W. E. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995 Dec 15;270(5243):1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Paul W. E. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994 Apr 1;179(4):1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. Y., Kumar V., Bennett M. Murine natural killer cells and marrow graft rejection. Annu Rev Immunol. 1992;10:189–213. doi: 10.1146/annurev.iy.10.040192.001201. [DOI] [PubMed] [Google Scholar]