Abstract
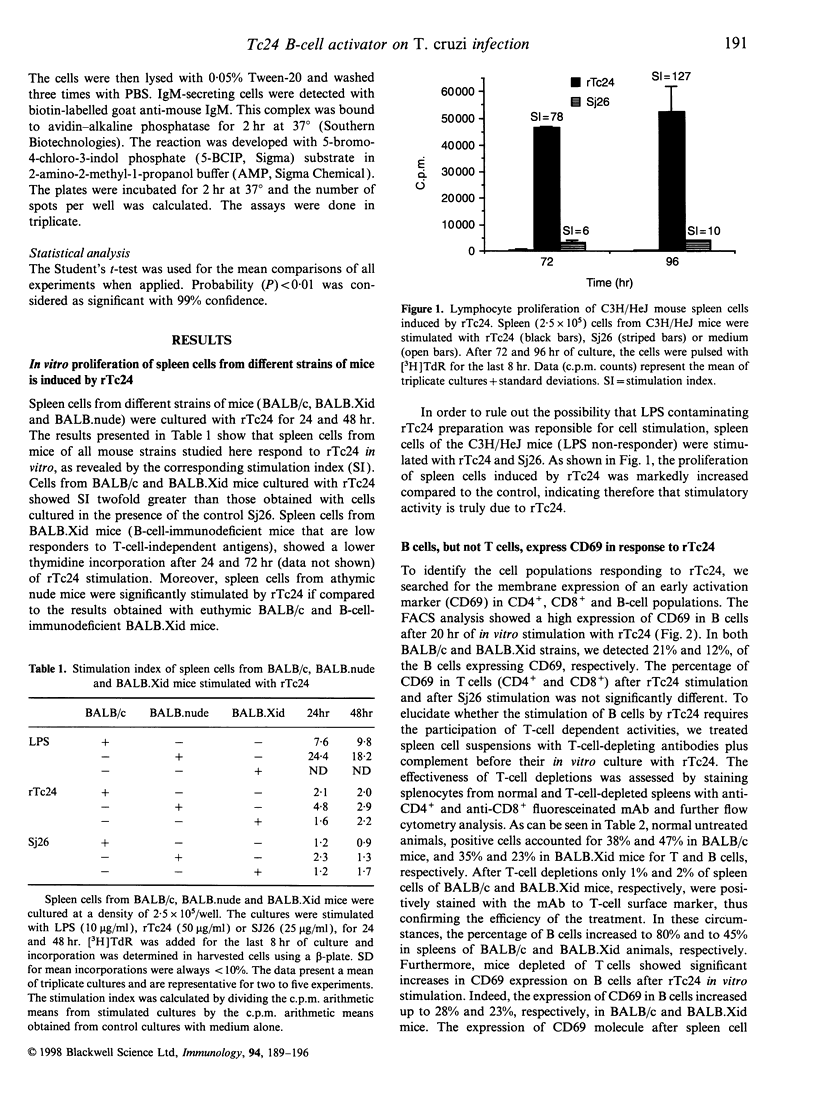
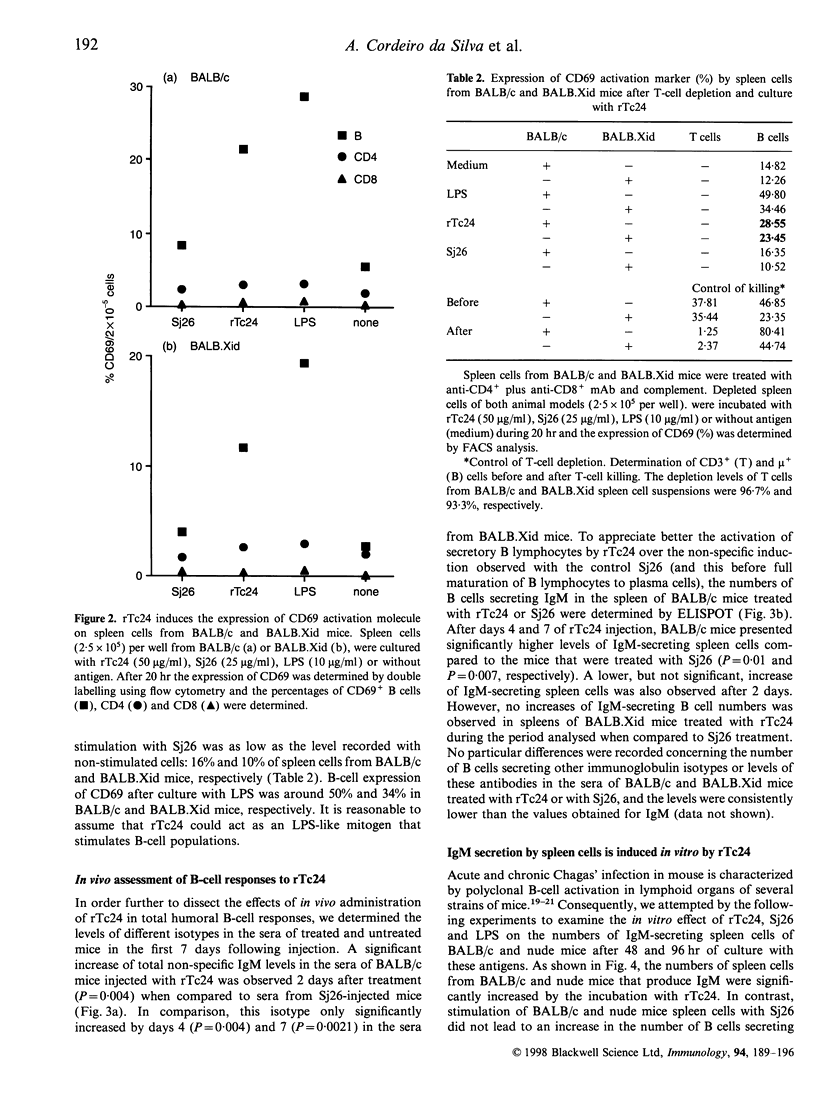
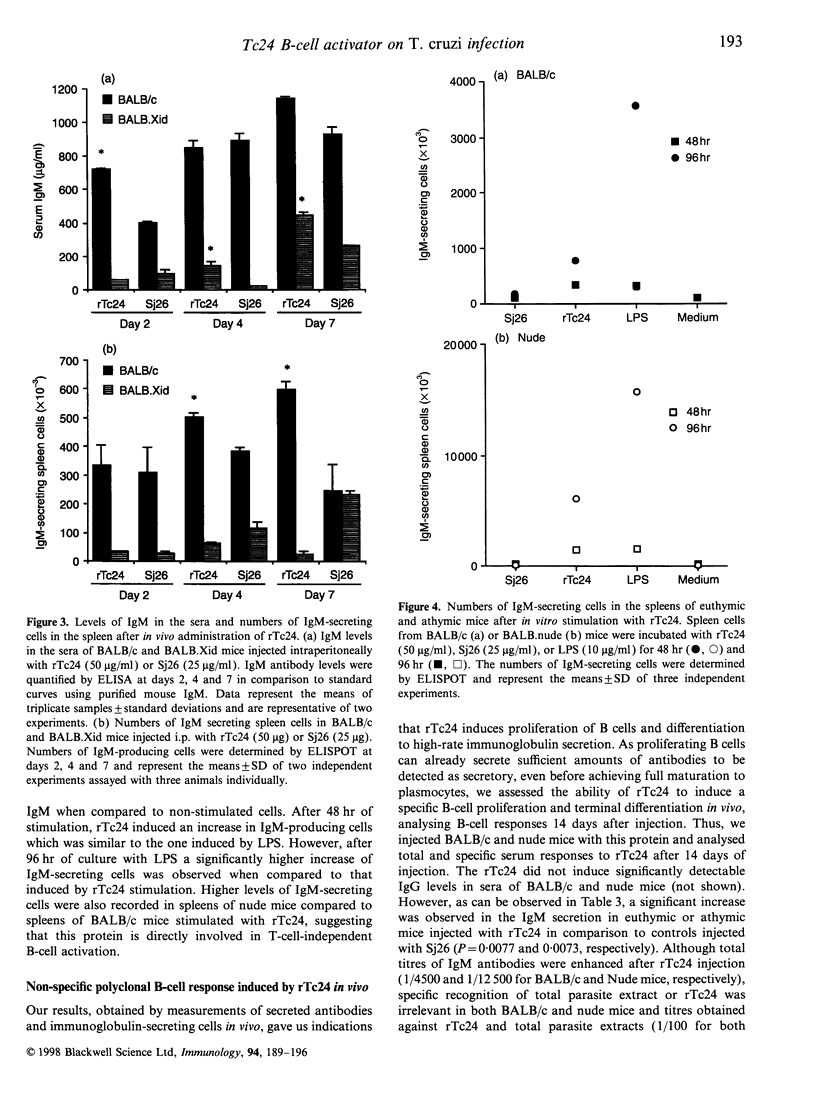
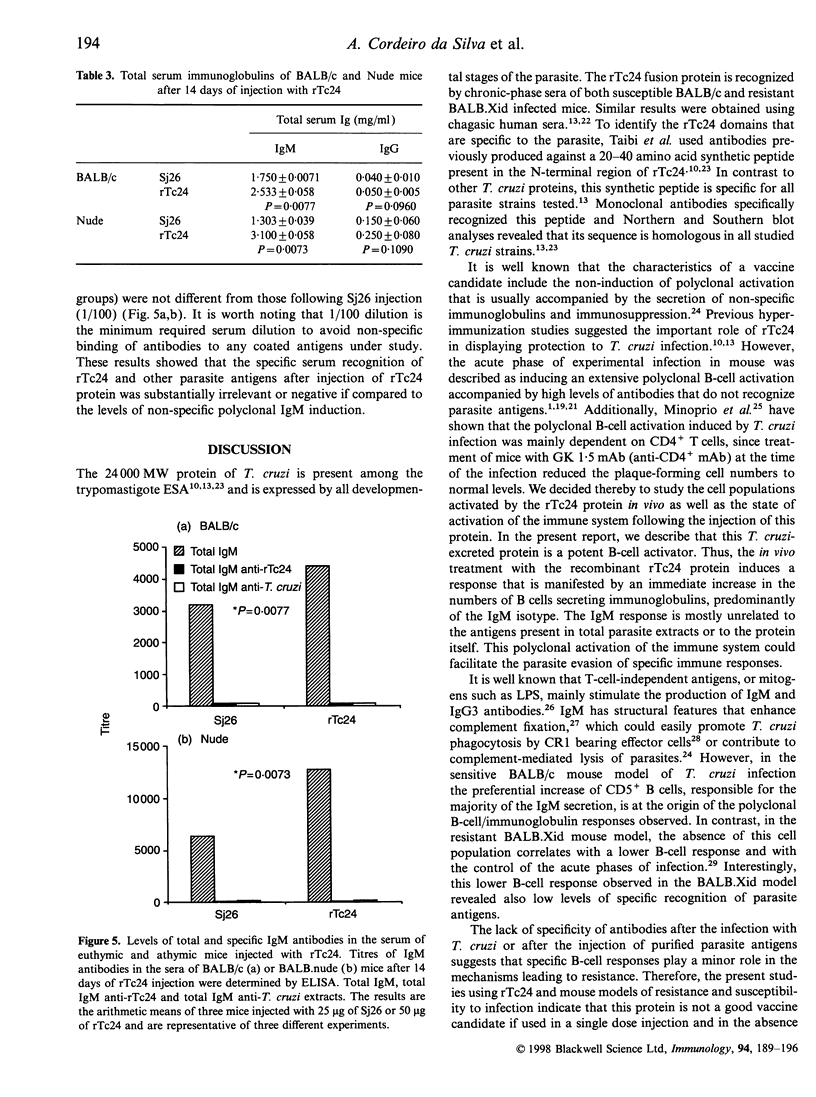
Trypanosoma cruzi, the causative agent of Chagas' disease, is a protozoan parasite that infects humans and other mammals in Central and Latin America. Several alterations of the immune response after infection have been described, such as severe immunosuppression of both cellular and humoral responses and massive polyclonal B- and T-cell activation, including the expansion of self-reactive clones. We have investigated the effects of the intraperitoneal injection of a recombinant 24,000 MW T. cruzi-specific antigen (rTc24) on the immune response of normal and deficient strains of mice. We analysed the in vivo and ex vivo levels of lymphocyte activation and the proliferative responses to rTc24 by determining the expression of CD69 activation marker and the levels of thymidine incorporation by spleen cells. The numbers of antibody-producing cells were determined by ELISPOT and the levels of immunoglobulin in the sera by isotype-specific enzyme-linked immunosorbent assay. We observed an increased [3H]thymidine ([3H]TdR) incorporation by spleen cells after rTc24 stimulation in vivo and in vitro. This proliferative activity induced by rTc24 was independent of the mouse strain used in the experiments (including C3H/HeJ mice) and ruled out the possibility that rTc24 preparations were contaminated by lipopolysaccharide. The injection of rTc24 protein induced preferentially the activation of B cells, as determined by the increased expression of CD69 molecules on IgM+ spleen cells. Considerable increases of IgM-secreting B cells were determined in both athymic and euthymic BALB/c mice. Mice that are deficient in B cells (BALB.Xid) responded to rTc24 but to a lesser extent. These increases in IgM B-cell numbers were accompanied by elevated levels of IgM immunoglobulins in the sera of injected animals. Our results suggest a role for rTc24 in B-cell activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björklund M., Coutinho A. Isotype commitment in the in vivo immune responses. II. Polyclonal plaque-forming cell responses to lipopolysaccharide in the spleen and bone marrow. Eur J Immunol. 1983 Jan;13(1):44–50. doi: 10.1002/eji.1830130111. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Benner R., Björklund M., Forni L., Holmberg D., Ivars F., Martinez-A C., Pettersson S. A "trans" perspective on the control of immunoglobulin c gene expression. Immunol Rev. 1982;67:87–114. doi: 10.1111/j.1600-065x.1982.tb01057.x. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Dec 16;65(1-2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- D'Imperio Lima M. R., Joskowicz M., Coutinho A., Kipnis T., Eisen H. Very large and isotypically atypical polyclonal plaque-forming cell responses in mice infected with Trypanosoma cruzi. Eur J Immunol. 1985 Feb;15(2):201–203. doi: 10.1002/eji.1830150219. [DOI] [PubMed] [Google Scholar]
- D'Império Lima M. R., Alvarez J. M., Furtado G. C., Kipnis T. L., Coutinho A., Minóprio P. Ig-isotype patterns of primary and secondary B cell responses to Plasmodium chabaudi chabaudi correlate with IFN-gamma and IL-4 cytokine production with CD45RB expression by CD4+ spleen cells. Scand J Immunol. 1996 Mar;43(3):263–270. doi: 10.1046/j.1365-3083.1996.d01-35.x. [DOI] [PubMed] [Google Scholar]
- Dangl J. L., Wensel T. G., Morrison S. L., Stryer L., Herzenberg L. A., Oi V. T. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988 Jul;7(7):1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Falanga P. B., D'Imperio Lima M. R., Coutinho A., Pereira da Silva L. Isotypic pattern of the polyclonal B cell response during primary infection by Plasmodium chabaudi and in immune-protected mice. Eur J Immunol. 1987 May;17(5):599–603. doi: 10.1002/eji.1830170504. [DOI] [PubMed] [Google Scholar]
- Hang L., Slack J. H., Amundson C., Izui S., Theofilopoulos A. N., Dixon F. J. Induction of murine autoimmune disease by chronic polyclonal B cell activation. J Exp Med. 1983 Mar 1;157(3):874–883. doi: 10.1084/jem.157.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L. Autoimmune phenomena in chronic chagasic cardiopathy. Parasitol Today. 1985 Jul;1(1):6–9. doi: 10.1016/0169-4758(85)90099-7. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lopes M. F., Cunha J. M., Bezerra F. L., Gonzalez M. S., Gomes J. E., Lapa e Silva J. R., Garcia E. S., Dos Reis G. A. Trypanosoma cruzi: both chemically induced and triatomine-derived metacyclic trypomastigotes cause the same immunological disturbances in the infected mammalian host. Exp Parasitol. 1995 Mar;80(2):194–204. doi: 10.1006/expr.1995.1024. [DOI] [PubMed] [Google Scholar]
- Minoprio P. M., Eisen H., Forni L., D'Imperio Lima M. R., Joskowicz M., Coutinho A. Polyclonal lymphocyte responses to murine Trypanosoma cruzi infection. I. Quantitation of both T- and B-cell responses. Scand J Immunol. 1986 Dec;24(6):661–668. doi: 10.1111/j.1365-3083.1986.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Burlen O., Pereira P., Guilbert B., Andrade L., Hontebeyrie-Joskowicz M., Coutinho A. Most B cells in acute Trypanosoma cruzi infection lack parasite specificity. Scand J Immunol. 1988 Nov;28(5):553–561. doi: 10.1111/j.1365-3083.1988.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Coutinho A., Spinella S., Hontebeyrie-Joskowicz M. Xid immunodeficiency imparts increased parasite clearance and resistance to pathology in experimental Chagas' disease. Int Immunol. 1991 May;3(5):427–433. doi: 10.1093/intimm/3.5.427. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Eisen H., Joskowicz M., Pereira P., Coutinho A. Suppression of polyclonal antibody production in Trypanosoma cruzi-infected mice by treatment with anti-L3T4 antibodies. J Immunol. 1987 Jul 15;139(2):545–550. [PubMed] [Google Scholar]
- Minoprio P., Itohara S., Heusser C., Tonegawa S., Coutinho A. Immunobiology of murine T. cruzi infection: the predominance of parasite-nonspecific responses and the activation of TCRI T cells. Immunol Rev. 1989 Dec;112:183–207. doi: 10.1111/j.1600-065x.1989.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Ouaissi A., Aguirre T., Plumas-Marty B., Piras M., Schöneck R., Gras-Masse H., Taibi A., Loyens M., Tartar A., Capron A. Cloning and sequencing of a 24-kDa Trypanosoma cruzi specific antigen released in association with membrane vesicles and defined by a monoclonal antibody. Biol Cell. 1992;75(1):11–17. doi: 10.1016/0248-4900(92)90119-l. [DOI] [PubMed] [Google Scholar]
- Ouaissi M. A., Taibi A., Cornette J., Velge P., Marty B., Loyens M., Esteva M., Rizvi F. S., Capron A. Characterization of major surface and excretory-secretory immunogens of Trypanosoma cruzi trypomastigotes and identification of potential protective antigen. Parasitology. 1990 Feb;100(Pt 1):115–124. doi: 10.1017/s0031182000060182. [DOI] [PubMed] [Google Scholar]
- Plata F. Enhancement of tumor growth correlates with suppression of the tumor-specific cytolytic T lymphocyte response in mice chronically infected by Trypanosoma cruzi. J Immunol. 1985 Feb;134(2):1312–1319. [PubMed] [Google Scholar]
- Reed S. G. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988 Jun 15;140(12):4342–4347. [PubMed] [Google Scholar]
- Rimoldi M. T., Tenner A. J., Bobak D. A., Joiner K. A. Complement component C1q enhances invasion of human mononuclear phagocytes and fibroblasts by Trypanosoma cruzi trypomastigotes. J Clin Invest. 1989 Dec;84(6):1982–1989. doi: 10.1172/JCI114388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sveen K., Hofstad T. Blastogenesis and polyclonal immunoglobulin synthesis in murine spleen cells stimulated with lipopolysaccharide, lipid A and acid-degraded polysaccharide from Fusobacterium nucleatum. FEMS Microbiol Immunol. 1990 May;2(1):29–33. doi: 10.1111/j.1574-6968.1990.tb03475.x. [DOI] [PubMed] [Google Scholar]
- Taibi A., Espinoza A. G., Ouaissi A. Trypanosoma cruzi: analysis of cellular and humoral response against a protective recombinant antigen during experimental Chagas' disease. Immunol Lett. 1995 Dec;48(3):193–200. doi: 10.1016/0165-2478(95)02465-4. [DOI] [PubMed] [Google Scholar]
- Taibi A., Guevara-Espinoza A., Schöneck R., Yahiaoui B., Ouaissi A. Improved specificity of Trypanosoma cruzi identification by polymerase chain reaction using an oligonucleotide derived from the amino-terminal sequence of a Tc24 protein. Parasitology. 1995 Dec;111(Pt 5):581–590. doi: 10.1017/s0031182000077064. [DOI] [PubMed] [Google Scholar]
- Taibi A., Plumas-Marty B., Guevara-Espinoza A., Schöneck R., Pessoa H., Loyens M., Piras R., Aguirre T., Gras-Masse H., Bossus M. Trypanosoma cruzi: immunity-induced in mice and rats by trypomastigote excretory-secretory antigens and identification of a peptide sequence containing a T cell epitope with protective activity. J Immunol. 1993 Sep 1;151(5):2676–2689. [PubMed] [Google Scholar]
- d'Imperio Lima M. R., Eisen H., Minoprio P., Joskowicz M., Coutinho A. Persistence of polyclonal B cell activation with undetectable parasitemia in late stages of experimental Chagas' disease. J Immunol. 1986 Jul 1;137(1):353–356. [PubMed] [Google Scholar]
- el-Cheikh M. C., Dutra H. S., Minóprio P., Borojevic R. Increase of B-lymphocyte number and activity during experimental murine schistosomiasis mansoni. Braz J Med Biol Res. 1994 Jul;27(7):1605–1617. [PubMed] [Google Scholar]


