Abstract
Although it is well known that cases with silicosis exhibit various immunological abnormalities, the mechanisms involved in the occurrence of immuno-dysfunction or dysregulation induced by silica compounds have not yet been determined. Fas is a well-known cell surface molecule that is involved in the apoptosis pathway that belongs to the tumour necrosis factor-receptor family. Soluble Fas (sFas) is produced as an alternatively spliced product of the Fas gene and protects cells from apoptosis due to antagonization of the binding between membrane form of the Fas gene (mFas) and the Fas ligand. To determine the role of the Fas/Fas ligand system in silica-induced immunological abnormalities, we investigated Fas and Fas-ligand message expression levels using the multiplex reverse transcription-polymerase chain reaction (RT-PCR) method with peripheral blood mononuclear cells from silicosis cases with no clinical symptoms of autoimmune diseases. Although the relative expression levels of the Fas or Fas-ligand genes were not remarkably altered in these cases, we observed the sFas message was dominantly expressed compared with mFas expression. These results suggest that self-recognizing clones in cases with silicosis survive for decades, escaping the exclusion mechanisms induced by apoptosis. Then they cause the appearance of autoantibodies and the acquisition of autoimmune diseases sequentially.
Full text
PDF


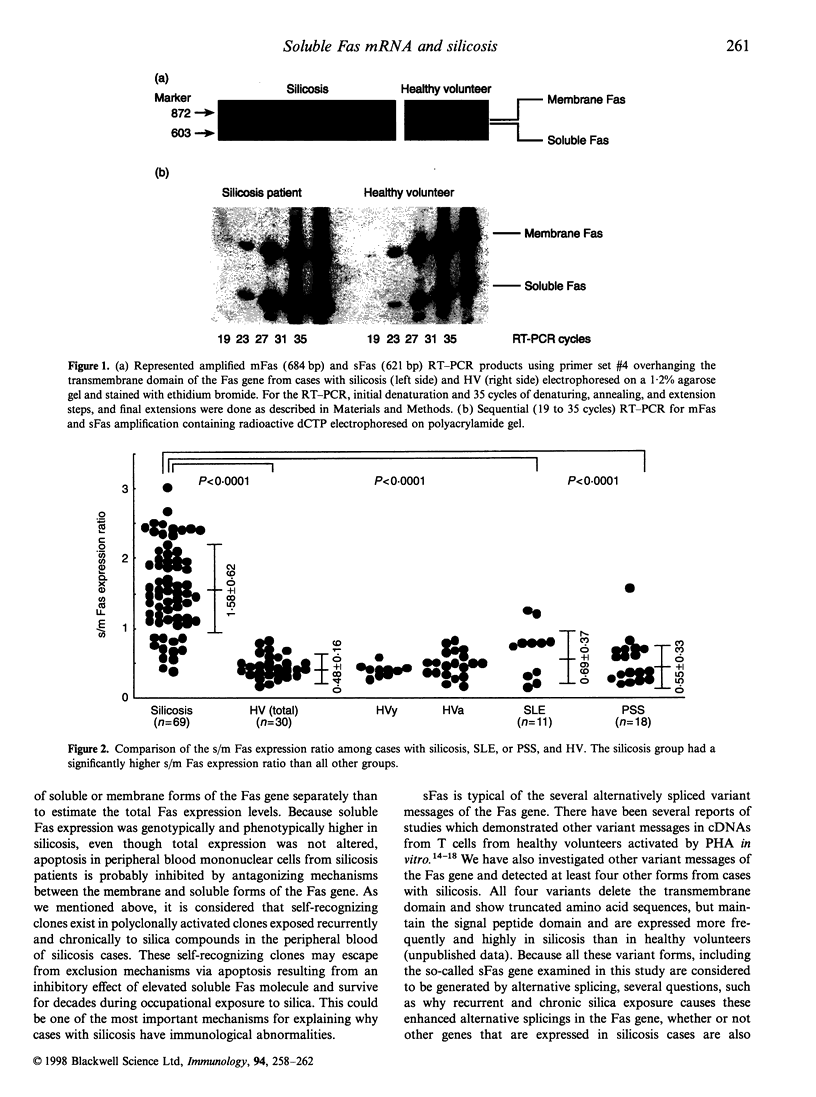

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAPLAN A. Certain unusual radiological appearances in the chest of coal-miners suffering from rheumatoid arthritis. Thorax. 1953 Mar;8(1):29–37. doi: 10.1136/thx.8.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascino I., Fiucci G., Papoff G., Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995 Mar 15;154(6):2706–2713. [PubMed] [Google Scholar]
- Cheng J., Zhou T., Liu C., Shapiro J. P., Brauer M. J., Kiefer M. C., Barr P. J., Mountz J. D. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994 Mar 25;263(5154):1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- Drappa J., Vaishnaw A. K., Sullivan K. E., Chu J. L., Elkon K. B. Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med. 1996 Nov 28;335(22):1643–1649. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- ERASMUS L. D. Scleroderma in goldminers on the Witwatersrand with particular reference to pulmonary manifestations. S Afr J Lab Clin Med. 1957 Sep;3(3):209–231. [PubMed] [Google Scholar]
- Fisher G. H., Rosenberg F. J., Straus S. E., Dale J. K., Middleton L. A., Lin A. Y., Strober W., Lenardo M. J., Puck J. M. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995 Jun 16;81(6):935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Itoh N., Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993 May 25;268(15):10932–10937. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jodo S., Kobayashi S., Kayagaki N., Ogura N., Feng Y., Amasaki Y., Fujisaku A., Azuma M., Yagita H., Okumura K. Serum levels of soluble Fas/APO-1 (CD95) and its molecular structure in patients with systemic lupus erythematosus (SLE) and other autoimmune diseases. Clin Exp Immunol. 1997 Jan;107(1):89–95. doi: 10.1046/j.1365-2249.1997.d01-901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinugawa K., Ueki A., Yamaguchi M., Watanabe Y., Kawakami Y., Hyodoh F., Tsushima H. Activation of human CD4+CD45RA+ T cells by chrysotile asbestos in vitro. Cancer Lett. 1992 Sep 30;66(2):99–106. doi: 10.1016/0304-3835(92)90221-g. [DOI] [PubMed] [Google Scholar]
- Knipping E., Debatin K. M., Stricker K., Heilig B., Eder A., Krammer P. H. Identification of soluble APO-1 in supernatants of human B- and T-cell lines and increased serum levels in B- and T-cell leukemias. Blood. 1995 Mar 15;85(6):1562–1569. [PubMed] [Google Scholar]
- Knipping E., Krammer P. H., Onel K. B., Lehman T. J., Mysler E., Elkon K. B. Levels of soluble Fas/APO-1/CD95 in systemic lupus erythematosus and juvenile rheumatoid arthritis. Arthritis Rheum. 1995 Dec;38(12):1735–1737. doi: 10.1002/art.1780381205. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L. Systemic lupus erythematosus. Cell. 1996 May 3;85(3):303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- Liu C., Cheng J., Mountz J. D. Differential expression of human Fas mRNA species upon peripheral blood mononuclear cell activation. Biochem J. 1995 Sep 15;310(Pt 3):957–963. doi: 10.1042/bj3100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountz J. D., Zhou T., Cheng J. Use of sensitive assays to detect soluble Fas in patients with systemic lupus erythematosus: comment on the article by Knipping et al and the article by Goel et al. Arthritis Rheum. 1996 Sep;39(9):1611–1612. doi: 10.1002/art.1780390925. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997 Feb 7;88(3):355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Nagata S., Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995 Jan;16(1):39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Okubo M., Ishida H., Kasukawa R. Elevated levels of soluble Fas in systemic lupus erythematosus: comment on the article by Knipping et al. Arthritis Rheum. 1996 Sep;39(9):1612–1614. doi: 10.1002/art.1780390926. [DOI] [PubMed] [Google Scholar]
- Papoff G., Cascino I., Eramo A., Starace G., Lynch D. H., Ruberti G. An N-terminal domain shared by Fas/Apo-1 (CD95) soluble variants prevents cell death in vitro. J Immunol. 1996 Jun 15;156(12):4622–4630. [PubMed] [Google Scholar]
- Rieux-Laucat F., Le Deist F., Hivroz C., Roberts I. A., Debatin K. M., Fischer A., de Villartay J. P. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995 Jun 2;268(5215):1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- Rodnan G. P., Benedek T. G., Medsger T. A., Jr, Cammarata R. J. The association of progressive systemic sclerosis (scleroderma) with coal miners' pneumoconiosis and other forms of silicosis. Ann Intern Med. 1967 Feb;66(2):323–334. doi: 10.7326/0003-4819-66-2-323. [DOI] [PubMed] [Google Scholar]
- Ruberti G., Cascino I., Papoff G., Eramo A. Fas splicing variants and their effect on apoptosis. Adv Exp Med Biol. 1996;406:125–134. doi: 10.1007/978-1-4899-0274-0_13. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tomokuni A., Aikoh T., Matsuki T., Isozaki Y., Otsuki T., Kita S., Ueki H., Kusaka M., Kishimoto T., Ueki A. Elevated soluble Fas/APO-1 (CD95) levels in silicosis patients without clinical symptoms of autoimmune diseases or malignant tumours. Clin Exp Immunol. 1997 Nov;110(2):303–309. doi: 10.1111/j.1365-2249.1997.tb08332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki A., Yamaguchi M., Ueki H., Watanabe Y., Ohsawa G., Kinugawa K., Kawakami Y., Hyodoh F. Polyclonal human T-cell activation by silicate in vitro. Immunology. 1994 Jun;82(2):332–335. [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wu J., Wilson J., He J., Xiang L., Schur P. H., Mountz J. D. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest. 1996 Sep 1;98(5):1107–1113. doi: 10.1172/JCI118892. [DOI] [PMC free article] [PubMed] [Google Scholar]



