Abstract
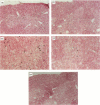
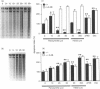
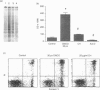
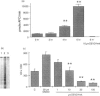
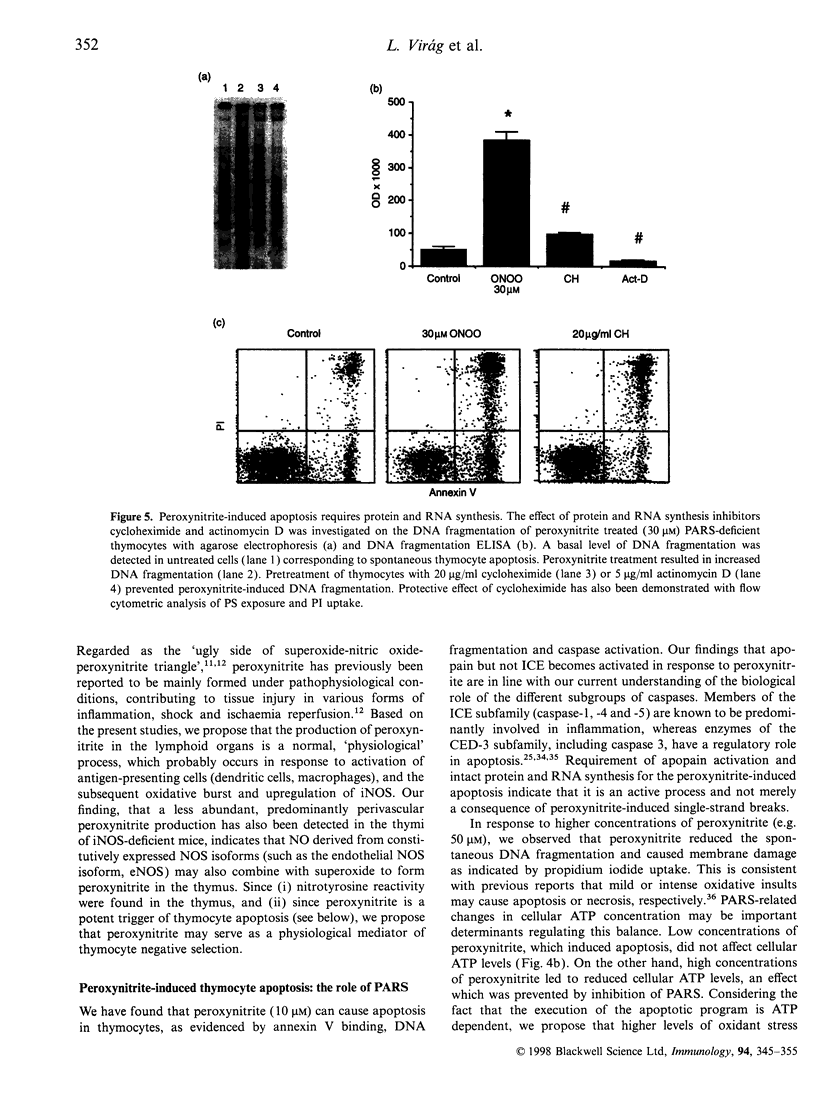
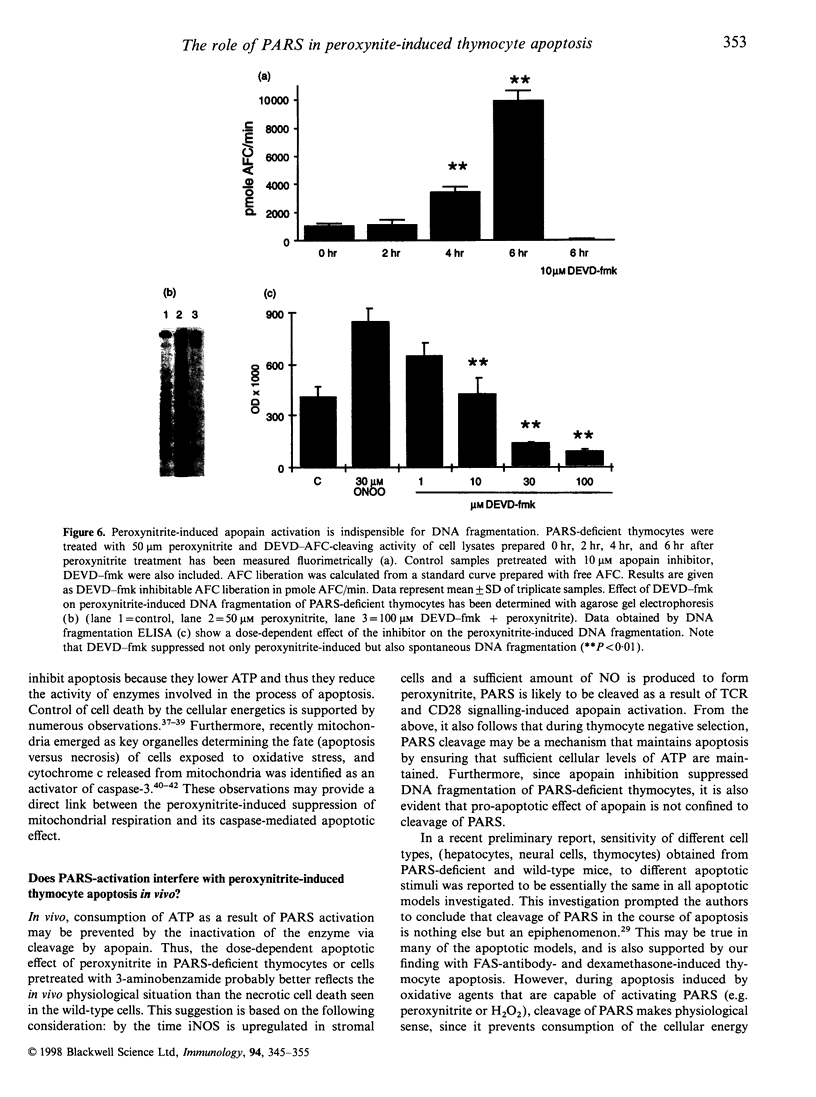
The mechanisms by which immature thymocyte apoptosis is induced during negative selection are poorly defined. Reports demonstrated that cross-linking of T-cell receptor leads to stromal cell activation, expression of inducible nitric oxide synthase (iNOS) and, subsequently, to thymocyte apoptosis. Therefore we examined, whether NO directly or indirectly, through peroxynitrite formation, causes thymocyte apoptosis. Immuno-histochemical detection of nitrotyrosine revealed in vivo peroxynitrite formation in the thymi of naive mice. Nitrotyrosine, the footprint of peroxynitrite, was predominantly found in the corticomedullary junction and the medulla of naive mice. In the thymi of mice deficient in the inducible isoform of nitric oxide synthase, considerably less nitrotyrosine was found. Exposure of thymocytes in vitro to low concentrations (10 microM) of peroxynitrite led to apoptosis, whereas higher concentrations (50 microM) resulted in intense cell death with the characteristics of necrosis. We also investigated the effect of poly (ADP-ribose) synthetase (PARS) inhibition on thymocyte apoptosis. Using the PARS inhibitor 3-aminobenzamide (3-AB), or thymocytes from PARS-deficient animals, we established that PARS determines the fate of thymocyte death. Suppression of cellular ATP levels, and the cellular necrosis in response to peroxynitrite were prevented by PARS inhibition. Therefore, in the absence of PARS, cells are diverted towards the pathway of apoptotic cell death. Similar results were obtained with H2O2 treatment, while apoptosis induced by non-oxidative stimuli such as dexamethasone or anti-FAS antibody was unaffected by PARS inhibition. In conclusion, we propose that peroxynitrite-induced apoptosis may play a role in the process of thymocyte negative selection. Furthermore, we propose that the physiological role of PARS cleavage by apopain during apoptosis may serve as an energy-conserving step, enabling the cell to complete the process of apoptosis.
Full text
PDF


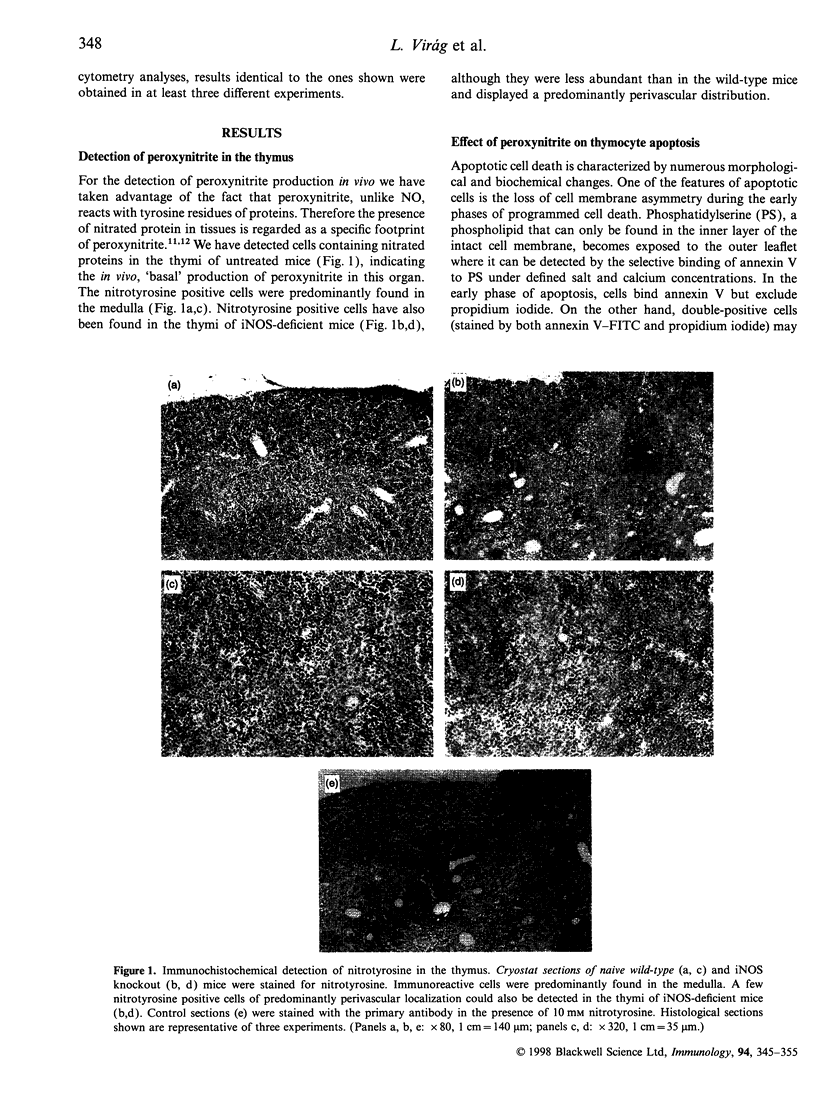
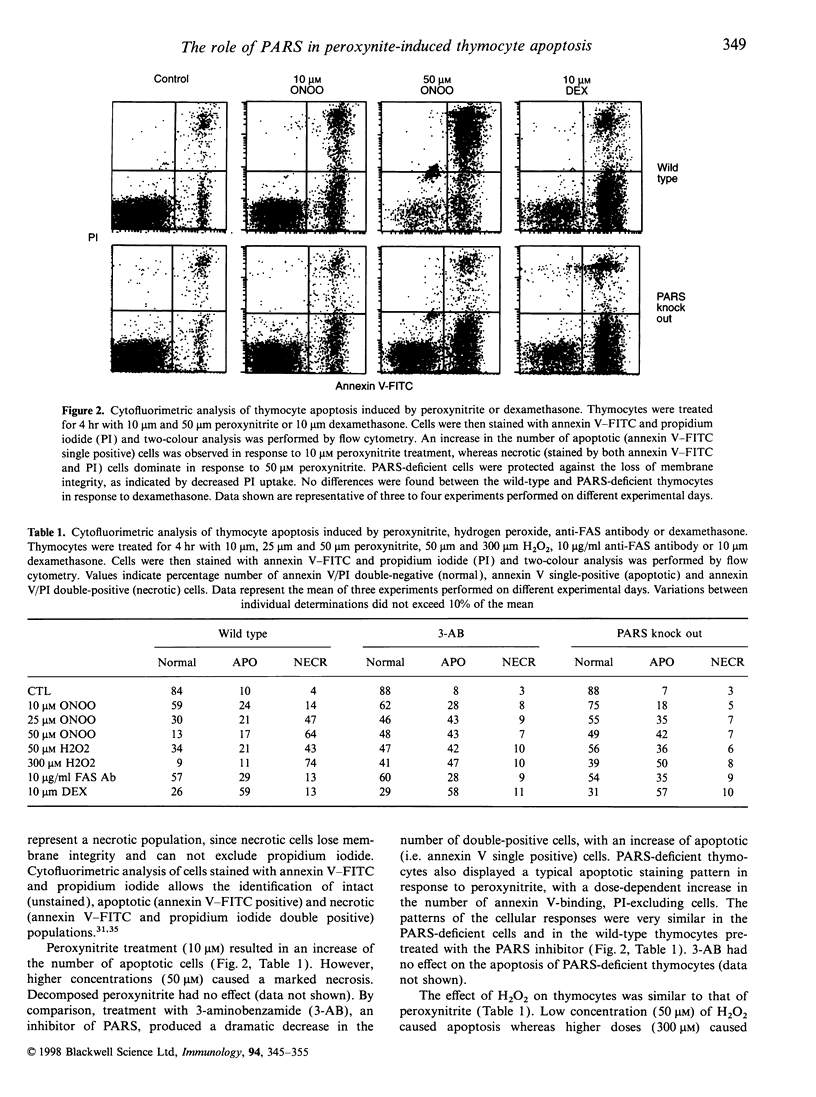
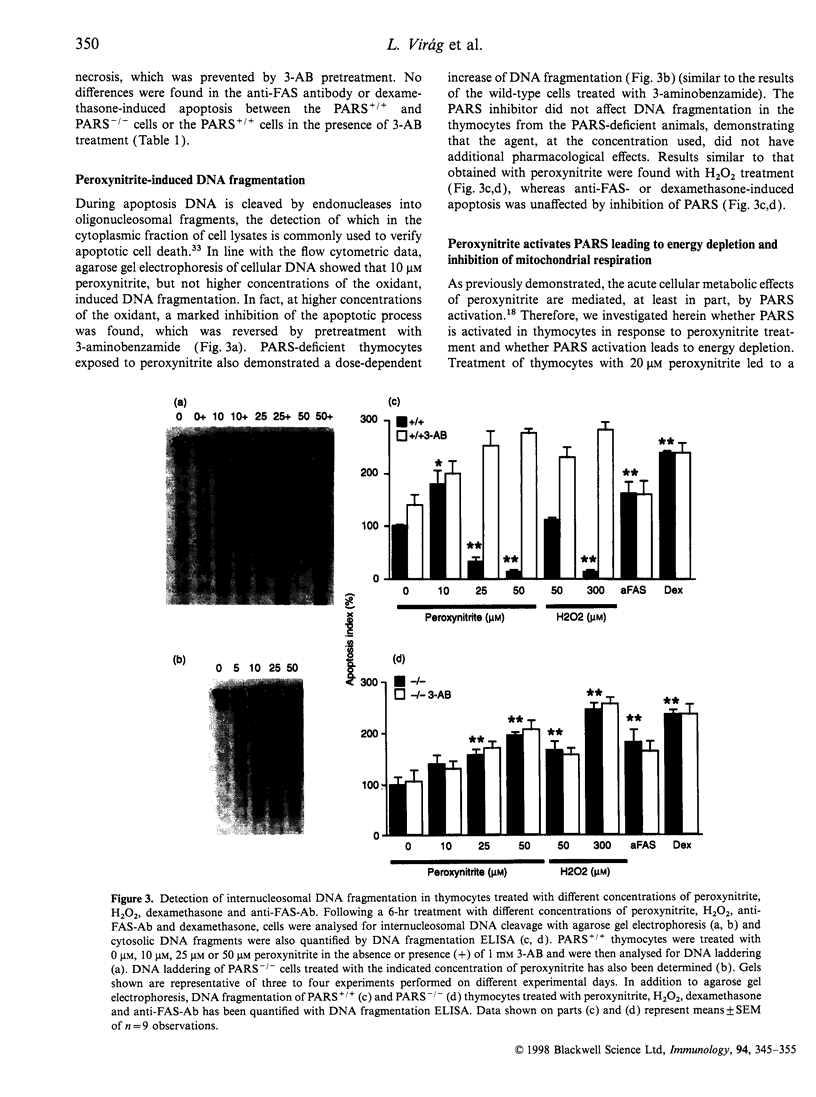
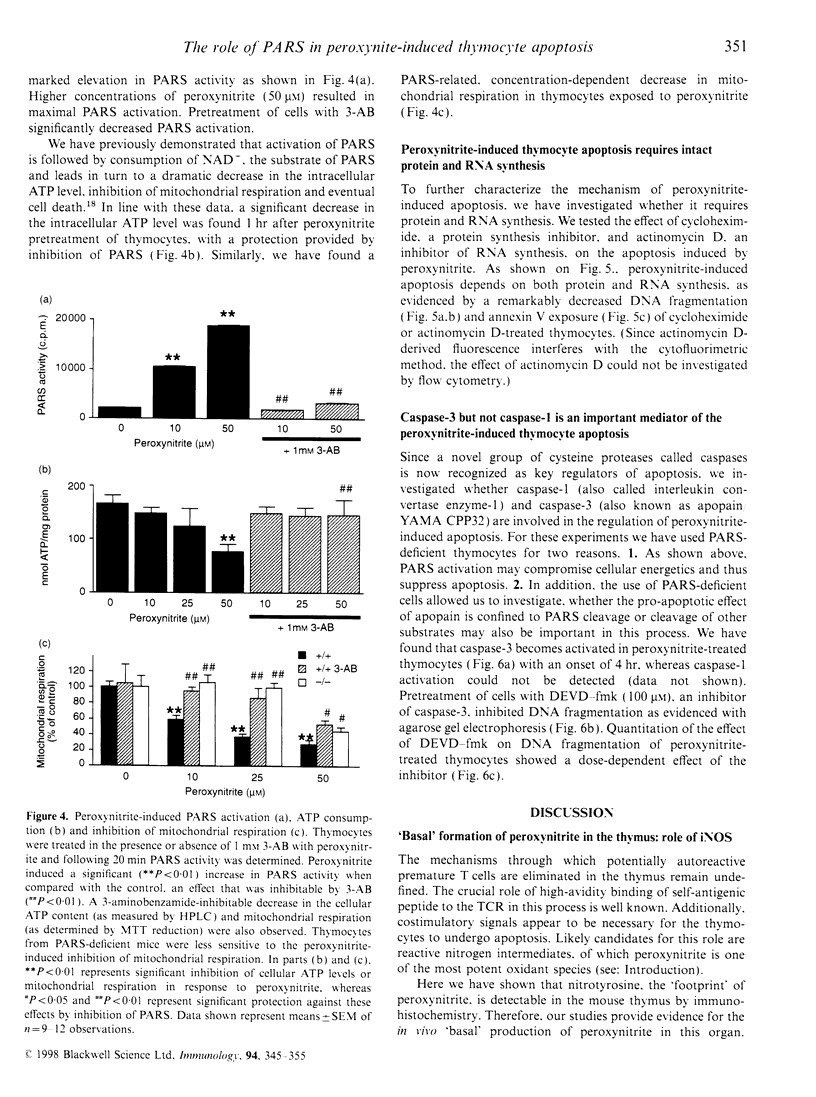


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. L., Anderson G., Michell R. H., Jenkinson E. J., Owen J. J. Intracellular signaling pathways involved in the induction of apoptosis in immature thymic T lymphocytes. J Immunol. 1996 Jun 1;156(11):4083–4091. [PubMed] [Google Scholar]
- Bonfoco E., Krainc D., Ankarcrona M., Nicotera P., Lipton S. A. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G. Mechanisms of oxidant injury of cells. Mol Aspects Med. 1991;12(2):137–147. doi: 10.1016/0098-2997(91)90009-b. [DOI] [PubMed] [Google Scholar]
- Coppola S., Nosseri C., Maresca V., Ghibelli L. Different basal NAD levels determine opposite effects of poly(ADP-ribosyl)polymerase inhibitors on H2O2-induced apoptosis. Exp Cell Res. 1995 Dec;221(2):462–469. doi: 10.1006/excr.1995.1397. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S., Zingarelli B., Costantino G., Szabó A., Salzman A. L., Caputi A. P., Szabó C. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br J Pharmacol. 1997 Jul;121(6):1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M. Role of ICE-proteases in apoptosis. Adv Exp Med Biol. 1996;406:113–117. doi: 10.1007/978-1-4899-0274-0_11. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C. Apoptosis: lessons from in vitro systems. Trends Cell Biol. 1995 Jun;5(6):217–220. doi: 10.1016/s0962-8924(00)89006-6. [DOI] [PubMed] [Google Scholar]
- Eastman A. Assays for DNA fragmentation, endonucleases, and intracellular pH and Ca2+ associated with apoptosis. Methods Cell Biol. 1995;46:41–55. doi: 10.1016/s0091-679x(08)61923-8. [DOI] [PubMed] [Google Scholar]
- Eliasson M. J., Sampei K., Mandir A. S., Hurn P. D., Traystman R. J., Bao J., Pieper A., Wang Z. Q., Dawson T. M., Snyder S. H. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997 Oct;3(10):1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Fehsel K., Kröncke K. D., Meyer K. L., Huber H., Wahn V., Kolb-Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995 Sep 15;155(6):2858–2865. [PubMed] [Google Scholar]
- Hueber A. O., Raposo G., Pierres M., He H. T. Thy-1 triggers mouse thymocyte apoptosis through a bcl-2-resistant mechanism. J Exp Med. 1994 Mar 1;179(3):785–796. doi: 10.1084/jem.179.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Desnoyers S., Ottaviano Y., Davidson N. E., Poirier G. G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993 Sep 1;53(17):3976–3985. [PubMed] [Google Scholar]
- Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989 Nov 1;49(21):5870–5878. [PubMed] [Google Scholar]
- Kaushal G. P., Ueda N., Shah S. V. Role of caspases (ICE/CED 3 proteases) in DNA damage and cell death in response to a mitochondrial inhibitor, antimycin A. Kidney Int. 1997 Aug;52(2):438–445. doi: 10.1038/ki.1997.350. [DOI] [PubMed] [Google Scholar]
- Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997 Feb 21;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Kumar S. The apoptotic cysteine protease CPP32. Int J Biochem Cell Biol. 1997 Mar;29(3):393–396. doi: 10.1016/s1357-2725(96)00146-x. [DOI] [PubMed] [Google Scholar]
- Leist M., Single B., Castoldi A. F., Kühnle S., Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997 Apr 21;185(8):1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M., Single B., Künstle G., Volbracht C., Hentze H., Nicotera P. Apoptosis in the absence of poly-(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1997 Apr 17;233(2):518–522. doi: 10.1006/bbrc.1997.6491. [DOI] [PubMed] [Google Scholar]
- Lerner A., Clayton L. K., Mizoguchi E., Ghendler Y., van Ewijk W., Koyasu S., Bhan A. K., Reinherz E. L. Cross-linking of T-cell receptors on double-positive thymocytes induces a cytokine-mediated stromal activation process linked to cell death. EMBO J. 1996 Nov 1;15(21):5876–5887. [PMC free article] [PubMed] [Google Scholar]
- Lin K. T., Xue J. Y., Nomen M., Spur B., Wong P. Y. Peroxynitrite-induced apoptosis in HL-60 cells. J Biol Chem. 1995 Jul 14;270(28):16487–16490. doi: 10.1074/jbc.270.28.16487. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Reutelingsperger C. P., McGahon A. J., Rader J. A., van Schie R. C., LaFace D. M., Green D. R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995 Nov 1;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan A. J., Ruiz-Ruiz M. C., Gorman A. M., Lopez-Rivas A., Cotter T. G. Reactive oxygen intermediate(s) (ROI): common mediator(s) of poly(ADP-ribose)polymerase (PARP) cleavage and apoptosis. FEBS Lett. 1996 Sep 2;392(3):299–303. doi: 10.1016/0014-5793(96)00838-1. [DOI] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995 Jul 6;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Thornberry N. A. Caspases: killer proteases. Trends Biochem Sci. 1997 Aug;22(8):299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Page D. M., Kane L. P., Allison J. P., Hedrick S. M. Two signals are required for negative selection of CD4+CD8+ thymocytes. J Immunol. 1993 Aug 15;151(4):1868–1880. [PubMed] [Google Scholar]
- Polverino A. J., Patterson S. D. Selective activation of caspases during apoptotic induction in HL-60 cells. Effects Of a tetrapeptide inhibitor. J Biol Chem. 1997 Mar 14;272(11):7013–7021. doi: 10.1074/jbc.272.11.7013. [DOI] [PubMed] [Google Scholar]
- Punt J. A., Osborne B. A., Takahama Y., Sharrow S. O., Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994 Feb 1;179(2):709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C., Schweizer M., Cossarizza A., Franceschi C. Control of apoptosis by the cellular ATP level. FEBS Lett. 1996 Jan 8;378(2):107–110. doi: 10.1016/0014-5793(95)01431-4. [DOI] [PubMed] [Google Scholar]
- Salgo M. G., Squadrito G. L., Pryor W. A. Peroxynitrite causes apoptosis in rat thymocytes. Biochem Biophys Res Commun. 1995 Oct 24;215(3):1111–1118. doi: 10.1006/bbrc.1995.2578. [DOI] [PubMed] [Google Scholar]
- Szabó C., Cuzzocrea S., Zingarelli B., O'Connor M., Salzman A. L. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J Clin Invest. 1997 Aug 1;100(3):723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Lim L. H., Cuzzocrea S., Getting S. J., Zingarelli B., Flower R. J., Salzman A. L., Perretti M. Inhibition of poly (ADP-ribose) synthetase attenuates neutrophil recruitment and exerts antiinflammatory effects. J Exp Med. 1997 Oct 6;186(7):1041–1049. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Zingarelli B., O'Connor M., Salzman A. L. DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai X. G., Toyo-oka K., Yamamoto N., Yashiro Y., Mu J., Hamaoka T., Fujiwara H. Expression of an inducible type of nitric oxide (NO) synthase in the thymus and involvement of NO in deletion of TCR-stimulated double-positive thymocytes. J Immunol. 1997 May 15;158(10):4696–4703. [PubMed] [Google Scholar]
- Tanigawa Y., Shimoyama M. Mg2+-dependent/poly(ADP-ribose)-sensitive endonuclease. J Biol Chem. 1983 Aug 10;258(15):9184–9191. [PubMed] [Google Scholar]
- Tentori L., Orlando L., Lacal P. M., Benincasa E., Faraoni I., Bonmassar E., D'Atri S., Graziani G. Inhibition of O6-alkylguanine DNA-alkyltransferase or poly(ADP-ribose) polymerase increases susceptibility of leukemic cells to apoptosis induced by temozolomide. Mol Pharmacol. 1997 Aug;52(2):249–258. doi: 10.1124/mol.52.2.249. [DOI] [PubMed] [Google Scholar]
- Vermes I., Haanen C., Steffens-Nakken H., Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995 Jul 17;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Wang Z. Q., Stingl L., Morrison C., Jantsch M., Los M., Schulze-Osthoff K., Wagner E. F. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997 Sep 15;11(18):2347–2358. doi: 10.1101/gad.11.18.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997 Feb 21;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zingarelli B., O'Connor M., Wong H., Salzman A. L., Szabó C. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol. 1996 Jan 1;156(1):350–358. [PubMed] [Google Scholar]
- de Murcia J. M., Niedergang C., Trucco C., Ricoul M., Dutrillaux B., Mark M., Oliver F. J., Masson M., Dierich A., LeMeur M. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]