Abstract
Bronchiolar epithelial cells are the prime targets for influenza A virus infection. It still remains to be clarified which signals are generated from these cells to initiate an immune response. Among chemokines, viral infection of primary lung epithelial cells triggered exclusively the release of CXCL8/interleukin-8 (IL-8), which contrasts with our previous observation that influenza A virus induced in monocytes the expression of mononuclear-leukocyte-attracting chemokines and even suppressed the production of neutrophil-attracting chemokines. Therefore, we speculated that it may be advantageous for respiratory epithelial cells to release primarily neutrophil-attracting CXCL8/IL-8 since neutrophils rapidly remove necrotic debris and are the first line of defense against bacterial superinfections. This concept has also been supported by our finding that influenza A virus infection led to necrosis of lung epithelial cells. This is in striking contrast to previous studies where influenza A virus infection induced apoptosis in monocytes and epithelial cells from origins other than the lung. Thus, the cell type instead of the virus determines which death pathway will be followed. In addition to the release of CXCL8/IL-8, we obtained a massive release of macrophage migration inhibitory factor (MIF) from virus-infected lung cells. However, whereas the CXCL8/IL-8 secretion was accompanied by induced gene activation, the transcription rate of MIF remained unchanged during the infection course and the virus-induced MIF release was predominantly a discharge from intracellular stores, suggesting that MIF is passively released upon cell death. Despite virus induced necrosis, the passively liberated MIF remained bioactive. Considering the well-established immunostimulatory effects of MIF on different leukocyte subsets, is its very likely that enhanced levels of MIF may contribute to the host immune response during the acute phase of influenza A virus infection in humans.
An infection by influenza A virus has been shown to affect initially the respiratory tract and in particular the ciliated bronchiolar epithelial cells (17, 18, 19). Little is known about further consequences of bronchiolar epithelial cell infection, except that some cytokine mRNAs are expressed (11, 26, 27), which suggests that these cells signal to the immune system. Among various candidate cytokines, macrophage migration inhibitory factor (MIF) has been shown to be constitutively expressed in bronchiolar cells of the lung (1). In a clinical study, the MIF levels were higher in the bronchoalveolar fluids obtained from acute respiratory distress syndrome patients than in those from control subjects (13), which indicated that this cytokine may play an important role in lung diseases.
The cytokine MIF was initially described as a T-cell-derived factor that inhibited the random migration of macrophages (6, 7, 12). Subsequent work showed that macrophages and T cells secrete MIF in response to glucocorticoids and to various proinflammatory stimuli (5, 9). Several studies involving experimental animal models indicate that MIF plays a critical role in the expression and ultimate outcome of an inflammatory or immune response. Neutralizing antibodies to MIF protected mice from septic shock, and a new report confirmed that mice lacking the MIF gene were resistant to the lethal effects of a high dose of bacterial lipopolysaccharide (6, 8). In addition, the development of the classic, tuberculin-induced delayed-type hypersensitivity reaction was significantly reduced in animals treated with blocking antibodies to MIF (4). More recently, the expression of MIF has been found to be critical for the generation of an antigen-specific immune response. Activated T cells produced MIF and neutralizing anti-MIF antibodies inhibited T-cell proliferation and interleukin-2 (IL-2) production in vitro and suppressed antigen-driven T-cell activation and antibody production in vivo (2).
In the present in vitro study, we infected normal human bronchiolar epithelial (NHBE) cells and two human cell lines, bronchiolar epithelial cell line U1752 (3) and alveolar cell line A549 (24), with influenza A virus and show the release of MIF and the selective production of the chemokine CXCL8/IL-8.
MATERIALS AND METHODS
Cell cultures.
Primary normal NHBE cells were obtained from CellSystems (St. Katharinen, Germany) and maintained in serum-free bronchiolar/tracheal epithelial cell growth medium (CellSystems) supplemented with 50 μg of bovine pituitary extract, 5 ng of human epidermal growth factor, 0.5 μg of hydrocortisone, 0.5 μg of epinephrine, 10 μg of transferrin, 5 μg of insulin, 6.5 ng of tri-iodothyronine, and 50 μg of gentamicin, each per milliliter.
Bronchiolar epithelial cell line U1752 was kindly donated by G. Jaques (Department of Hematology and Oncology, University of Marburg, Marburg, Germany). The characteristics of this cell line have been described elsewhere (3). The A549 alveolar epithelial cell line (23) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). A549 cells have been used as a model of type II alveolar epithelial cells (23). Cell lines U1752 and A549 were maintained in RPMI 1640 (Linaris, Bettingen, Germany) supplemented with 5% heat-inactivated, virus- and mycoplasma-free fetal calf serum (FCS), 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM l-glutamine (all from Biochrom, Berlin, Germany).
Virus preparation and infection of cells.
Influenza A virus strain A/PR/8/34 (H1N1) was kindly donated by H.-D. Klenk, Institute of Virology, Marburg, Germany, and was propagated and purified as previously outlined in detail (29). The infection rate was assessed by a standard plaque assay as cytopathic effect on confluent cultures of mycoplasma-free Madin-Darby canine kidney II (MDCK II) cells, with a 0.5% agarose overlay.
Lung epithelial cells (5 × 105/ml) were infected by exposure to A/PR/8/34 virus at a multiplicity of infection (MOI) of 2 (2 PFU per cell) under serum-free conditions, the medium was exchanged after a 1-h exposure, and the culture was further continued for up to 48 h in RPMI 1640 medium containing 1% FCS.
RNA preparation and Northern blot analysis.
U1752 and A549 lung epithelial cells (107) were incubated in 10-cm-diameter petri dishes and exposed to A/PR/8/34 at an MOI of 2 for 1 h in serum-free RPMI 1640 medium. After exchange of the medium, the culture was continued up to 4, 8, 24, and 48 h in medium containing 1% FCS. Finally, the medium was removed and total RNA was prepared by the TRIzol method (Gibco-Life Technologies, Eggenstein, Germany). Total RNA (5 μg) was separated on 1% agarose gels. The RNA was blotted by 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) to a positively charged nylon membrane (Boehringer GmbH, Mannheim, Germany). After UV cross-linking, hybridization was performed under continuous rotation in a hybridization oven (Biometra, Göttingen, Germany). The membranes were hybridized with digoxigenin (DIG)-labeled antisense riboprobes overnight under highly stringent conditions in 50% formamide at 68°C and finally washed in 0.1% SSC-0.1% sodium dodecyl sulfate at the same temperature. Hybridized DIG-labeled riboprobes were visualized with the DIG nucleic acid detection kit (Boehringer) and CPD-Star chemiluminescence substrate (Tropix, Bedford, Mass.; distributed by Serva, Heidelberg, Germany). Equal loading of the RNA was confirmed by hybridization of the RNA with a specific GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cRNA probe.
Analysis of mRNA for chemokines by reverse transcription-PCR.
Two micrograms of total RNA from U1752, A549, and NHBE cells was reverse transcribed into cDNA by use of the Ready To Go system (Pharmacia Biotech, Freiburg, Germany) according to the manufacturer's protocol. The PCRs were performed with 1 μl of cDNA by using an automated Touch-Down thermal cycler (Hybaid Ltd., Heidelberg, Germany) and 1 U of Taq polymerase (Perkin-Elmer, Rodgau, Germany). The amplification reactions were performed for 30 cycles with denaturation at 95°C for 1 min, annealing at 62°C for 1 min, and extension at 72°C for 1 min. The PCR products were analyzed in ethidium bromide-stained agarose gels. To prove equal loading of cDNAs, a PCR with the housekeeping gene encoding GAPDH was performed (25 cycles). The primers used are shown in Table 1.
TABLE 1.
Human primers
| Gene product | Sequence (5′-3′) | Size (bp) |
|---|---|---|
| MIF | TCCTTCTGCCATGCCGA | 370 |
| TGCGGCTCTTAGGCGAAGGT | ||
| CXCL8/IL-8 | TTCTGCAGCTCTGTGTGAAGGTG | 710 |
| AAGGATCCTGGCTAGCAGACTAG | ||
| CCL5/RANTES | GATGTACTCCCGAACCCA | 235 |
| TCCCCATATTCCTCGGAC | ||
| CXCL1/GRO-α | AACTGCGCTGCCAGTGCTTGC | 313 |
| TTGGATCCAGGTGGCCTCTGC | ||
| GAPDH | CGTCTTCACCACCATGGAGA | 300 |
| GCTATACCAGGAAATGAGCTT |
Human recombinant MIF.
Human MIF cDNA was cloned into the pET-17b vector (Novagen, Madison, Wis.) and expressed in Escherichia coli BL-21 (DE3) strain after induction with 0.4 mM isopropyl β-d-thiogalactopyranoside (IPTG) and purified from the soluble fraction of the cell lysate by two-step high-pressure liquid chromatography (HPLC): (i) size exclusion HPLC on Bio-Sil TSK 250 (Bio-Rad, Munich, Germany) and (ii) ion-exchange HPLC on Ultropac TSK CM-3SW (LKB/Pharmacia, Freiburg, Germany).
MIF inhibitor.
(S,R)-3-(4-Hydrophenyl)-4,5-dihydro-5-isoazole acetic acid methyl ester (ISO-1) has been recently shown to covalently bind to the d-dopachrome tautomerase activity site of MIF, and this interaction has been reported to inhibit several biological activities of MIF (25).
Measurement of calcium fluxes.
All calcium fluxes were measured by a newly established method using peritubular cells from rats (37). Stromal prostatic cells were cultured for 3 days in six-well plates on sterile coverslips under normal conditions. The medium was removed, and the cells were incubated for 2 h at 37°C in Hanks' HEPES-buffered salt solution (HHBSS; pH 7.4) incubation buffer containing 3 μM FURA 2/AM fluorescence dye (1 mM stock solution in dimethyl sulfoxide; Sigma, Deisenhofen, Germany) as described by Glaum et al. (16). The supernatants were removed, and the cells were incubated in normal HHBSS buffer for 30 min at 37°C. The coverslips were transferred to the measuring chamber and covered with 500 μl of HHBSS buffer. Before stimulation, the cells were monitored for 10 min to exclude spontaneous calcium fluxes. Stimulation was performed with 500 μl of HHBSS buffer containing 50 ng of recombinant MIF or supernatants from virus-infected U1752 or A549 cells. The calcium fluxes were monitored with a computer-assisted cell fluorescence photomultiplier system (DeltaScan system; PTI, Wedel, Germany), and the results were recorded as the ratios of intensities at 340 nm to those at 380 nm. Buffer without MIF served as a negative control. Calcium ionophore A 23187 (calcimycin; 10−5 M; Sigma) served as a positive control to demonstrate the integrity of the cells used. The specificity of MIF from cell supernatants for inducing calcium fluxes was ascertained by blocking experiments with a specific murine monoclonal antibody, followed by immunoprecipitation with protein G (Sigma).
Determination of MIF and chemokines.
MIF and chemokine levels in culture supernatants from U1752, A549, and NHBE cells were determined by specific sandwich enzyme-linked immunosorbent assays (ELISAs) that were developed in our laboratory. Briefly, 96-well microtiter plates (Maxisorp; Nunc, Wiesbaden, Germany) were coated in phosphate-buffered saline (PBS) with monoclonal antibodies specific for MIF, CXCL8/IL-8, CXCL1/GRO-α, or CCL5/RANTES (R&D Systems, Wiesbaden, Germany) or CCL2/MCP-1 (PharMingen, Hamburg, Germany). For detection of bound chemokines or MIF, a second specific, biotinylated monoclonal antibody was used. Detection was performed with streptavidin peroxidase and subsequent conversion of the o-phenylenediamine substrate according to standard procedures.
Western blotting.
For determination of intracellular MIF, U1752, A549, and NHBE cells (5 × 105/ml) were incubated for 0, 8, 24, and 48 h and NHBE cells were incubated additionally for 72 and 96 h, with or without A/PR/8/34. The cells were harvested, lysed three times by freezing and thawing, and centrifuged, and the cell-free supernatant was used at 2 μg of protein/lane. Concentration of cell supernatants was necessary for detection of MIF by Western blotting. A 1-ml sample volume was concentrated with a Centricon-10 (Amicon, Beverly, Mass.) at 4,600 × g for 70 min. Western blotting was performed by the NuPAGE electrophoresis system (Novex, San Diego, Calif.) using 4 to 12% N,N-methylenebisacrylamide-Tris gels. Proteins were transferred onto Optitran BA-S83 membranes (Schleicher & Schüll, Dassel, Germany). The antibodies used were polyclonal anti-MIF rabbit immunoglobulin G (IgG) diluted 1/5,000 in PBS-0.5% Tween 20 and peroxidase-labeled goat anti-rabbit IgG diluted 1/250,000 in PBS-0.5% Tween 20 (Pierce; distributed by Perbio, Bonn, Germany). The bands were visualized by an enhanced chemiluminescence detection system, as recommended by the manufacturer (SuperSignal ULTRA; Pierce; distributed by Perbio).
DNA analysis for apoptosis.
DNA from 2 × 106 cells was examined for apoptosis by the apoptotic DNA ladder kit (Boehringer GmbH).
Detection of necrotic cells.
To distinguish apoptotic from necrotic cells, the annexin V/phosphatidylserine method was used (14, 35, 36). Cells were grown on chamber slides (Nunc, Roskilde, Denmark) and infected for 24 h as described previously (15). Unfixed cells were incubated with annexin V-Fluos (Roche Diagnostics GmbH, Mannheim, Germany) by using 20 μl of reagent and 20 μl of propidium iodide (50 mg/ml) in 1,000 μl of RPMI 1640 without FCS for 10 min. After being washed with PBS, the apoptotic or necrotic cells were identified by fluorescence microscopy. Necrotic lysis of virus-infected lung epithelial cells (A549 and U1752) was also measured by the CytoTox96 assay (Promega, Mannheim, Germany) by following the instructions of the manufacturer's protocol. This assay quantitatively measures lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lysis. Released LDH in culture supernatant is measured with a coupled enzymatic assay. Briefly, 50 μl of culture supernatants was transferred into a 96-well plate and incubated for 30 min in the dark with 50 μl of substrate mixture, 50 μl of the stop solution was added, and absorbance was measured at 490 nm.
Immunohistochemistry.
Paraffin sections from human lung were dewaxed in xylene and graded alcohols; thereafter the endogenous peroxidase activity was blocked with 3% hydrogen peroxide solution in methanol (30 min at room temperature). For MIF detection, the sections were subsequently incubated with 0.1 M sodium citrate buffer under microwave exposure (three times for 5 min each at 600 W). After treatment with 0.001% trypsin at 37°C for 15 min, sections were stained with the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif.) according to the manufacturer's protocol. The sections were incubated with the murine polyclonal MIF antibody at a dilution of 1:1,000 for 1 h at 37°C. Sections were then developed with 3,3-diaminobenzidine, giving a brown product.
RESULTS
MIF protein expression in human lung.
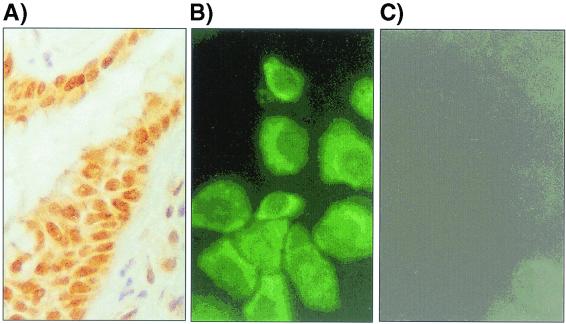
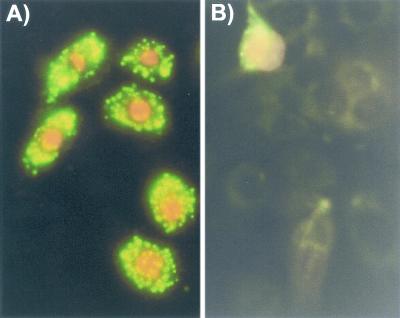
Immunohistochemical studies of lung sections showed that the bronchiolar epithelial cells contained large amounts of preformed MIF (Fig. 1A) and confirmed the initial study about the cellular MIF localization within human lung tissue (21). Positive immunostaining was also found in glandular bronchioles, smooth muscle cells, and nerve fibers. By immunofluorescence staining, MIF was observed in the cytoplasm of cultured primary NHBE cells (Fig. 1B). Human bronchiolar cell line U1752 and alveolar cell line A549 were also investigated and were found to display a similar pattern of MIF expression (data not shown).
FIG. 1.
Constitutive expression of MIF. (A) Immunohistochemical staining of the normal bronchiolar epithelium of a lung section. (B) Constitutive expression of MIF in the primary NHBE cells shows uniform MIF-positive fluorescence staining. (C) Fluorescence control for panel B.
MIF protein expression and release upon influenza A virus infection.
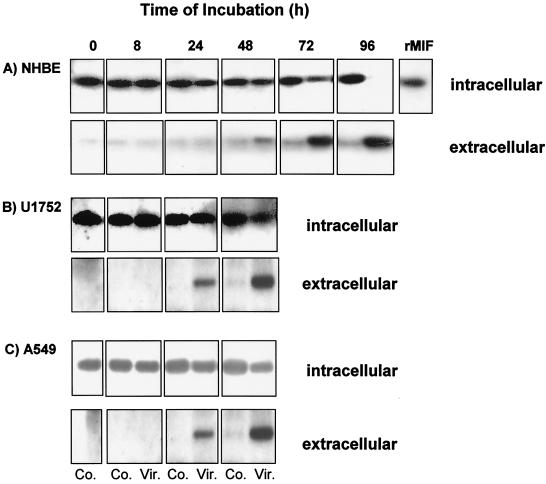
Confluent primary NHBE, bronchiolar U1752, and alveolar A549 cell monolayers were infected with influenza virus A/PR/8/34 at an MOI of 2. After different time intervals, the culture supernatants were collected, the cells were harvested, lysed by repeated freeze and thaw cycles, and centrifuged, and the cell-free supernatant was used for MIF determination.
Analysis of the intracellular MIF content of infected and noninfected primary NHBE cells by Western blotting revealed no difference between control and infected cells after 8 and 24 h of infection (Fig. 2A). Significantly lower MIF levels were detected in infected cells at 48 to 72 h compared to those in control cells. At 96 h after infection, no intracellular MIF was found in infected cells. The decline of intracellular MIF was paralleled by an increase of extracellular MIF, starting at 48 h after infection and reaching peak levels at 96 h.
FIG. 2.
Constitutive MIF expression and release by primary NHBE cells (A), U1752 cells (B), and A549 cells (C) upon A/PR/8/34 infection (MOI, 2). Cells (5 × 105/ml) were incubated for 8, 24, 48, 72, or 96 h after virus infection. The intracellular and extracellular contents of MIF were analyzed by Western blotting (2 μg of protein/lane). Recombinant MIF (rMIF) (10 ng) served as the standard. Co., uninfected controls; Vir., A/PR/8/34-infected cells.
Similar to the release of MIF from primary human bronchiolar cells, the intracellular MIF content of bronchiolar cell line U1752 (Fig. 2B) and alveolar A549 cells (Fig. 2C) decreased intracellularly and increased extracellularly after influenza A virus infection, with the notable difference that these cell lines released MIF 24 h earlier.
Release of MIF and induction of chemokines.
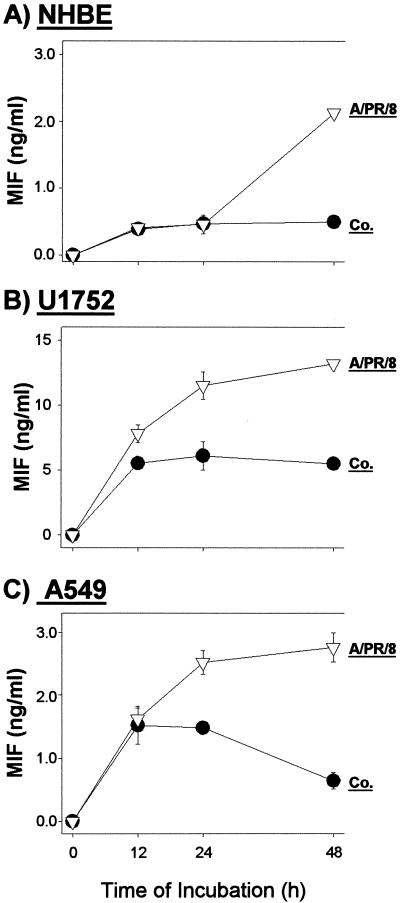
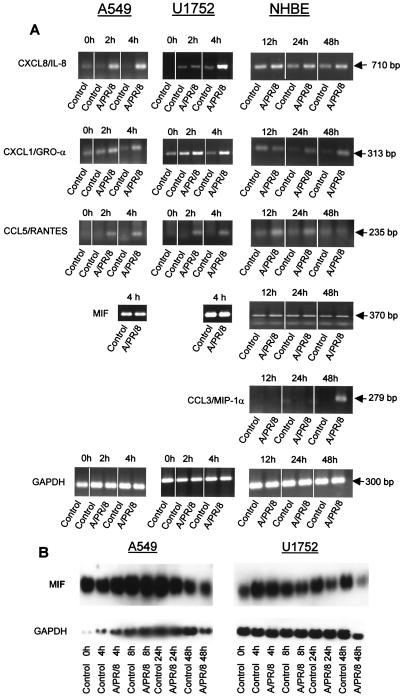
Similar to Western blot analysis (Fig. 2), MIF determination by ELISA showed that in primary NHBE cells there was a clearly detectable release 48 h after influenza A virus infection (Fig. 3A). This was rather delayed compared to releases in cell lines U1752 and A549, where a significant MIF release was already found 24 h after infection (Fig. 3B and C). Note that in cell lines U1752 and A549 the spontaneous MIF releases from uninfected cells were higher than that in primary NHBE cells, which could also have facilitated the more rapid liberation of MIF following infection. Infection with UV- or heat-inactivated virus failed to induce release of MIF.
FIG. 3.
Kinetics of MIF release from NHBE (A), U1752 (B), and A549 (C) lung epithelial cells after influenza A virus infection. Supernatants of noninfected control cells (Co.) and A/PR/8/34-infected (MOI, 2) cells were collected at different time points and analyzed by ELISA. Values are the means ± standard deviations of triplicate incubations.
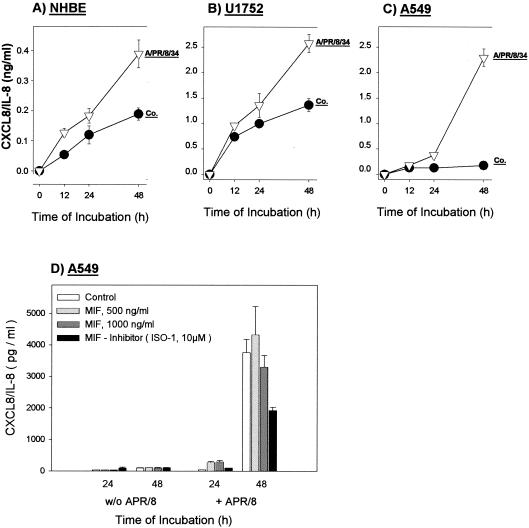
The secretion of CXCL8/IL-8 from primary NHBE cells could be detected at 12 h after infection and increased during incubation for up to 48 h (Fig. 4A). Releases of CXCL8/IL-8 from U1752 bronchiolar cells (Fig. 4B) and A549 alveolar cells (Fig. 4C) in the supernatants were six-fold higher (∼2.5 ng/ml) than that from NHBE cells.
FIG. 4.
Kinetics of CXCL8/IL-8 secretion from NHBE (A), U1752 (B), and A549 (C) lung epithelial cells after influenza A virus infection. Supernatants of noninfected control cells (Co.) and A/PR/8/34-infected (MOI, 2) cells were collected at the indicated time points and analyzed by ELISA. Values are the means ± standard deviations of triplicate incubations. (D) Influence of recombinant MIF (500 and 1,000 ng/ml) and MIF inhibitor (ISO-1; 10 μM) on the release of CXCL8/IL-8 in control and virus-infected A549 cells. Recombinant MIF (500 and 1,000 ng/ml) or MIF inhibitor ISO-1 (2,350 ng/ml [10 μM]) was added 1 h after infection. The supernatants were measured for CXCL8/IL-8 at 24 and 48 h.
To address the question whether MIF influences CXCL8/IL-8 secretion in bronchiolar epithelial cells, we set up infections in the presence of recombinant MIF and a new chemical inhibitor of MIF (ISO-1). The data showed that the addition of recombinant MIF did not affect the release of CXCL8/IL-8 in virus-infected A549 cells. However, the virus-dependent release of CXCL8/IL-8 was significantly reduced in the presence of MIF inhibitor ISO-1 (Fig. 4D).
Chemokines CXCL1/GRO-α, CCL5/RANTES, and CCL2/MCP-1 were not detected in the medium of infected primary NHBE cells 48 h postinfection (p.i.) (Table 2). In contrast to these normal cells, cell lines U1752 and A549 showed a release of CXCL1/GRO-α and CCL5/RANTES 48 h after infection (Table 2). The low release of CCL2/MCP-1 from U1752 cells was not affected by infection, but A549 alveolar cells showed a threefold increase. Chemokine CCL3/MIP-1α was not detected in any of the cell types.
TABLE 2.
Chemokine release from influenza A virus-infected cellsa
| Cells | Stimulus | Chemokine concn (ng/ml)
|
|||
|---|---|---|---|---|---|
| CXCL1 (GRO-α) | CCL5 (RANTES) | CCL3 (MIP-1α) | CCL2 (MCP-1) | ||
| NHBE | Control | ND | ND | ND | ND |
| A/PR/8/34 | ND | ND | ND | ND | |
| U1752 | Control | 0.03 ± 0.02 | 0.07 ± 0.01 | ND | 0.09 ± 0.01 |
| A/PR/8/34 | 0.50 ± 0.03 | 0.29 ± 0.01 | ND | 0.09 ± 0.02 | |
| A549 | Control | 0.70 ± 0.07 | 0.07 ± 0.01 | ND | 1.60 ± 0.14 |
| A/PR/8/34 | 5.00 ± 0.07 | 8.20 ± 0.40 | ND | 4.92 ± 0.41 | |
NHBE, U1752, and A549 cells (5 × 105/ml) were incubated with or without A/PR/8/34 (MOI, 2), and after 48 h the cell culture supernatants were harvested and the content of chemokines was determined by specific ELISAs. Values represent the mean ± standard deviations of triplicate incubations. ND, not detectable.
To elucidate the molecular background of MIF release and chemokine production after infection with influenza A virus, we analyzed the mRNA levels for chemokines and MIF by reverse transcription-PCR and Northern blotting. In A549 and U1752 cells, chemokine mRNAs for CXCL8/IL-8, CXCL1/GRO-α, and CCL5/RANTES were induced 2 h after infection, reached maximum levels at 4 h (Fig. 5A), remained elevated after 12 h, and declined at 24 h p.i. (data not shown). No transcripts for CCL2/MCP-1 could be detected in these cell lines. Consistently, a constitutive and high MIF gene expression was found not only in untreated cells but also in virus-infected cells, which suggests that the rise in extracellular MIF production from virus-infected cells cannot be explained by an enhanced transcription of the MIF gene. In comparison to cell lines, induction of chemokine mRNA was delayed in the primary NHBE cells. We obtained a transient increase of CCL5/RANTES at 12 h, which disappeared at a later time point of infection (Fig. 5A). mRNAs for CXCL/IL-8 and CXCL1/GRO-α were also enhanced at 12 h and sustained until the end of the infection course (Fig. 5A). Also in contrast to what was found for the cell lines, transcripts for CCL3/MIP-1α were observed as a late event of viral infection (Fig. 5A). Constitutive MIF mRNA expression not altered by virus infection was found by PCR analysis of NHBE cells.
FIG.5.
Chemokine and MIF gene expression in A549, U1752, and NHBE cells after infection with A/PR/8/34 at an MOI of 2. (A) At 0, 2, and 4 h for A549 and U1752 cells and at 12, 24, and 48 h for NHBE cells, 2 μg of total RNA was reverse transcribed into cDNA. The PCRs were performed with 1 μl of cDNA for 30 cycles. GAPDH served as the control. (B) MIF Northern blot of A549 and U1752 cells after infection with influenza A virus. Total RNA was isolated 0, 4, 8, 24, and 48 h after infection. Five micrograms of RNA per sample was transferred onto nylon membranes and hybridized with a specific DIG-labeled cRNA probe as outlined in Materials and Methods. Equal amounts of RNA were hybridized with a cRNA probe for GAPDH. A similar pattern of consistent, virus-independent MIF expression was obtained in NHBE cells by PCR analysis (A).
Northern blot analysis of A549 and U1752 cells revealed consistent and high steady-state levels of MIF mRNA during the initial 8 h of infection, which for U1752 cells declined thereafter (Fig. 5B).
Induction of necrosis and not apoptosis by A/PR/8/34 infection.
At 24 h after influenza A virus exposure, dramatic cytopathic changes occurred in U1752 and A549 cells compared to uninfected cells. In the NHBE cells, this cytopathic effect was delayed to 48 h p.i. When viral titers were measured by plaque test using the cell supernatants of 5 × 105 cells, the replication rate was found to be higher in bronchiolar cells (9 × 106 PFU/ml) than in alveolar cells (1.3 × 106 PFU/ml).
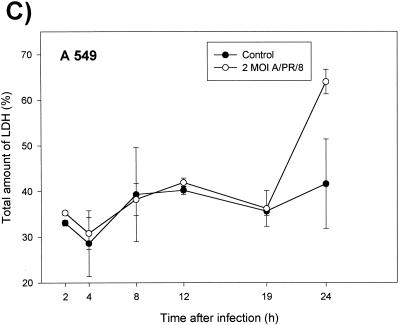
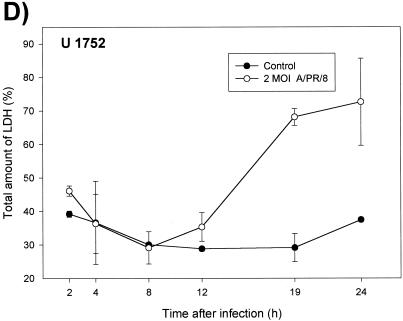
Because of the clearly apparent cytopathic effects, it was of particular interest to examine the type of virus-induced cell death. To differentiate apoptosis from necrosis, NHBE cells were stained 24 h after infection by the annexin V-fluorescein method. By this method, apoptotic cells display fluorescein isothiocyanate fluorescence of the cell membranes only whereas necrotic cells, in addition, show a red staining of the nuclei. Figure 6 shows membrane fluorescence as well as red DNA staining of the nuclei, which clearly documents that all cells became necrotic and not apoptotic 24 h after infection with A/PR/8/34. In addition, high levels of LDH release (Fig. 6C and D) and no DNA degradation ladder (data not shown) were detected, which exclude apoptosis.
FIG.6.
Necrosis of bronchiolar epithelial cells after A/PR/8/34 infection. After 24 h, infected (A) and noninfected NHBE cells (B) were stained with annexin V and propidium iodide and visualized under a fluorescence microscope. (C and D) Kinetics of LDH release in A549 and U1752 cells after infection with influenza A virus. The supernatants of control and virus infected cells were tested at the indicated time points for LDH as described in Materials and Methods. Depicted are the means ± standard deviations of triplicate samples of one representative experiment.
Bioactivity of released MIF.
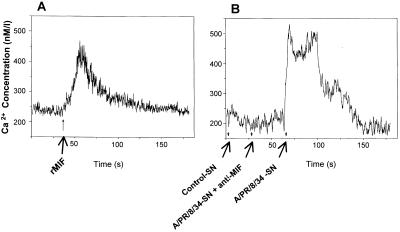
To investigate whether MIF released from influenza A virus-infected cells maintained its bioactivity, a newly established bioassay to measure calcium flux into rat peritubular cells was employed (37). As a positive control, recombinant MIF was also tested in the assay. Figure 7A shows a rapid calcium influx not only after treatment with recombinant MIF but also in cell supernatants from influenza A virus-infected A549 cells. This MIF-triggered calcium flux was specifically blocked with a neutralizing anti-MIF antibody (Fig. 7B). A similar pattern of calcium influx was obtained with supernatants from virus-infected U1752 cells (data not shown).
FIG. 7.
Bioactivity of released MIF. Recombinant MIF (rMIF) (A) and MIF released from virus-infected A549 cells (B) led to calcium flux in rat peritubular cells. The effect of MIF to induce a calcium influx was blocked by a specific anti-MIF monoclonal antibody (B). SN, supernatant. A similar pattern of calcium influx was obtained with the supernatants from virus-infected U1752 cells (data not shown).
DISCUSSION
In the initial stage of an influenza A virus infection, the primary target cells are tracheobronchiolar epithelial cells (17, 18, 19). It is as yet unknown which signals are generated to call the immune system into action. The most likely candidates are cytokines and particularly those which initiate an immigration and activation of leukocytes. For these reasons, we focused in this study on chemokines and on MIF as a representative leukocyte-activating cytokine.
Among chemokines, influenza A virus-infected primary NHBE cells released only CXCL8/IL-8 (Fig. 4). In contrast, cell lines U1752 and A549 produced additionally CXCL1/GRO-α, CCL2/MCP-1, and CCL5/RANTES (Table 2). In all cases, release of chemokines was preceded by an accumulation of de novo-generated chemokine transcripts (Fig. 5), which clearly indicates that, during the process of virus infection, as yet unknown mechanisms that induced gene transcription were triggered. It was particularly noteworthy that, despite chemokine mRNA generation, the primary NHBE cells produced only CXCL8/IL-8 whereas the cell lines released a broader spectrum of chemokines. This finding emphasizes the need for using primary cells and not solely relying on cell lines when studying effects of infectious agents which occur in vivo. Although our cell lines maintained most of their characteristic properties, they might have lost response restrictions that are physiologically present in regulated tissue compartments.
The production of CXCL8/IL-8 was an astonishing finding since we have previously shown that influenza A virus selectively induces only mononuclear leukocyte-attracting chemokines and even suppresses neutrophil-attracting chemokines (31). We even postulated that the pathognomonically typical infiltration of mononuclear leukocytes into virus-infected tissue is due to a selective production of the mononuclear leukocyte-attracting chemokines. The divergence of our new findings for cells of the respiratory tract from the previous data for monocytes/macrophages is most likely the result of tissue differences. It may be advantageous for infected respiratory epithelial cells to release primarily the neutrophil-attracting CXCL8/IL-8 since neutrophils rapidly remove necrotic debris and are the first defense line against bacterial superinfections. In addition, CXCL8/IL-8 has been shown to attract and activate T lymphocytes (22, 23, 38), which would aid in raising an immunologically specific response against influenza A virus. When virus infection progresses beyond the epithelial cell barrier, mononuclear cell-attracting leukocytes may come into play and then dominate the scene as previously described (31).
Of note, our in vitro experiments using a new chemical inhibitor for MIF (25) provided the first evidence that endogenous MIF, passively released from virus-infected lung epithelial cells, assists in the release of CXCL8/IL-8 (Fig. 4D). The underlying mechanism is as yet unknown and is the topic of further studies.
In close analogy to the in vivo situation, where lung epithelial cells were found to be a major source of MIF production (1), our in vitro-maintained lung cells constitutively expressed MIF (Fig. 1). Upon an influenza A virus infection, MIF was released from primary NHBE cells and was released more rapidly from cell lines (Fig. 3). The different timings of MIF release from normal versus tumor-derived cell lines may be explained by their different susceptibilities to influenza A virus, which in the latter caused massive cell death after 48 h compared to 96 h in NHBE cells. Both cell lines are also characterized by higher basal MIF levels than normal cells. This phenomenon of enhanced MIF expression in tumor cells has recently been described in a series of studies and has been correlated with MIF's property of promoting tumor progression and angiogenesis (10, 20, 30, 33).
Quite clearly, virus-induced MIF release was a discharge from preformed and stored products as documented by decreasing intracellular deposits and increasing extracellular content (Fig. 2). The emptying of intracellular stores occurred faster in cell lines than in primary NHBE cells, which again underlines the fact that cell lines may display peculiarities that are normally not encountered in normal, primary cells. The finding of a massive release of MIF from preformed stores was further supported by the finding that an influenza A virus infection did not induce MIF gene transcription, at least not to a large extent, since MIF mRNA levels remained unchanged whether the cells were infected or not (Fig. 5). In this respect, MIF expression followed a pattern entirely different from that of chemokines, which were clearly induced on the transcriptional level by virus infection.
In striking contrast to our and other previously obtained results with monocytes and macrophages, where influenza A virus infection induced apoptosis (15, 28, 34), the lung cells responded with necrosis (Fig. 6). Thus, the cell type rather than the virus determines which death pathway will be chosen. Normally, a nonprogrammed cell death by necrosis might be deleterious to the surrounding tissue, but the present results show that this process was slow enough to permit a de novo production of CXCL8/IL-8 for up to 24 to 48 h, which is most likely sufficient to induce an immigration of neutrophils that are known scavengers of cell debris. In addition and in contrast to what is found for most other tissues, necrotic cellular debris and scavenging leukocytes can be expectorated from the lung, which may render apoptosis a superfluous disposal mechanism.
Despite virus-induced necrosis, the passively liberated MIF remained bioactive (Fig. 7) as shown by a newly developed bioassay using intracellular Ca2+ mobilization in primary peritubular cells of the rat epididymis (37). It was of particular note that MIF appeared to be the only Ca2+-mobilizing factor in the supernatants of virus-infected lung epithelial cells as the activity could be entirely blocked by specific MIF antibodies.
The increase of extracellular bioactive MIF as a consequence of an airway infection with influenza A virus is very likely to positively affect the host immune response. In view of the well-established immunostimulatory effects of MIF on macrophages (9), T lymphocytes (2), and neutrophils (32), a local leukocyte activation which could limit further virus spread and tissue damage may occur. This assumption is supported by the observation that, in cells from alveolar lavages obtained from acute respiratory distress syndrome patients, blockade of MIF by antibodies led to a marked decrease of proinflammatory cytokine production (13). However, MIF overflow as a result of massive epithelial cell death may also take place and could be accompanied by overwhelming activation of invading mononuclear and polynuclear leukocytes, which would result in tissue damage. Thus, in severe infections the balance between virus-induced and cytokine-induced tissue damage may determine the outcome of an influenza A virus infection.
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (Ge 354/12-1)
REFERENCES
- 1.Bacher, M., A. Meinhardt, H. Y. Lan, W. Mu, C. N. Metz, J. A. Chesney, T. Calandra, D. Gemsa, T. Donnelly, R. C. Atkins, and R. Bucala. 1997. Migration inhibitory factor expression in experimentally induced endotoxemia. Am. J. Pathol. 150:235-246. [PMC free article] [PubMed] [Google Scholar]
- 2.Bacher, M., C. N. Metz, T. Calandra, K. Mayer, J. Chesney, M. Lohoff, D. Gemsa, T. Donnelly, and R. Bucala. 1996. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. USA 93:7849-7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergh, J., K. Nilsson, L. Zech, and B. Giovanella. 1985. Establishment and characterization of a continuous lung squamous carcinoma cell line (U 1752). Anticancer Res. 1:317-322. [PubMed] [Google Scholar]
- 4.Bernhagen, J., M. Bacher, T. Calandra, C. N. Metz, S. Doty, T. Donnelly, and R. Bucala. 1996. An essential role for macrophage migration inhibitory factor (MIF) in the tuberculin delayed type hypersensitivity reaction. J. Exp. Med. 183:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernhagen, J., T. Calandra, A. Cerami, and R. Bucala. 1994. Macrophage migration inhibitory factor is a neuroendocrine mediator of endotoxaemia. Trends Microbiol. 2:198-201. [DOI] [PubMed] [Google Scholar]
- 6.Bernhagen, J., T. Calandra, R. A. Mitchell, S. B. Martin, K. J. Tracey, W. Voelter, K. R. Manogue, A. Cerami, and R. Bucala. 1993. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365:756-759. [DOI] [PubMed] [Google Scholar]
- 7.Bloom, B. R., and B. Bennet. 1966. Mechanisms for reaction in vitro associated with delayed-type hypersensitivity. Science 153:80-82. [DOI] [PubMed] [Google Scholar]
- 8.Bozza, M., A. R. Satoskar, G. Lin, B. Lu, A. A. Humbles, C. Gerard, and J. R. David. 1999. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calandra, T., J. Bernhagen, R. A. Mitchell, and R. Bucala. 1994. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 179:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesney, J., C. Metz, M. Bacher, T. Peng, A. Meinhardt, and R. Bucala. 1999. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol. Med. 5:181-191. [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, A. M., and D. B. Jacoby. 1992. Influenza A virus infection induces interleukin-8 gene expression in human airway epithelial cells. FEBS Lett. 309:327-329. [DOI] [PubMed] [Google Scholar]
- 12.David, J. 1966. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA 56:72-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly, S., C. C. Haslett, P. T. Reid, I. S. Grant, W. A. Wallace, C. N. Metz, L. J. Bruce, and R. Bucala. 1997. Regulatory role for macrophage migration inhibitory factor in acute distress syndrome. Nat. Med. 3:320-323. [DOI] [PubMed] [Google Scholar]
- 14.Fadok, V. A., D. R. Voelker, P. A. Campbell, J. J. Cohen, D. L. Bratton, and P. M. Henson. 1992. Exposure of phoshatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207-2216. [PubMed] [Google Scholar]
- 15.Fesq, H., M. Bacher, M. Nain, and D. Gemsa. 1994. Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology 190:175-182. [DOI] [PubMed] [Google Scholar]
- 16.Glaum, S. R., J. A. Holzwarth, and R. J. Miller. 1990. Glutamate receptors activate Ca2+ mobilization and Ca 2+ influx into astrocytes. Proc. Natl. Acad. Sci. USA 87:3454-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hers, J. F. 1966. Disturbances of the ciliated epithelium due to influenza virus. Am. Rev. Respir. Dis. 93(Suppl.):162-167. [DOI] [PubMed] [Google Scholar]
- 18.Hers, J. F., and J. Mulder. 1961. Broad aspects of the pathology and pathogenesis of human influenza. Am. Rev. Respir. Dis. 83(Suppl.):54-84. [DOI] [PubMed] [Google Scholar]
- 19.Hers, J. F., N. Masurel, and J. Mulder. 1958. Bacteriology and histology of the respiratory tract and lungs in fatal Asian influenza. Lancet ii:1141. [DOI] [PubMed]
- 20.Hudson, J. D., M. A. Shoaibi, R. Maestro, A. Carnero, G. J. Hannon, and D. H. Bach. 1999. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 190:1375-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayser, K., C. Zeilinger, F. Y. Zeng, S. Gabius, H. J. Gabius, and W. Y. Weiser. 1993. Detection of the lymphokine migration inhibitory factor in normal and disease-affected lung by antibody and by its major binding protein, the interferon antagonist sarcolectin. Pathol. Res. Pract. 189:992-995. [DOI] [PubMed] [Google Scholar]
- 22.Kim, J. J., L. K. Nottingham, J. I. Sin, A. Tsai, L. Morrison, K. Dang, Y. Hu, K. Kazahaya, M. Bennett, T. Dentchev, D. M. Wilson, A. A. Chalian, J. D. Boyer, M. G. Agadjanyan, and D. B. Weiner. 1998. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J. Clin. Investig. 102:1112-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen, C. G., A. O. Anderson, E. Appella, J. J. Oppenheim, and K. Matsushima. 1989. The neutrophil-activating protein NAP-1 is also chemotactic for T lymphocytes. Science 243:1464-1466. [DOI] [PubMed] [Google Scholar]
- 24.Lieber, M., B. Smith, A. Szakal, W. Nelson-Rees, and G. Todaro. 1976. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 17:62-70. [DOI] [PubMed] [Google Scholar]
- 25.Lubetsky, J. B., A. Dios, J. Han, B. Aljabari, B. Ruzsicska, R. Mitchell, E. Lolis, and Y. Al-Abed. 2002. The tautomerase activity site of MIF is a potential target for discovery of novel anti-inflammatory agents. J. Biol. Chem. 277:24976-24982. [DOI] [PubMed] [Google Scholar]
- 26.Matsakura, S., F. Kokubu, H. Kubo, T. Tomita, H. Tokunaga, M. Kadokura, T. Yamamoto, Y. Kuroiwa, T. Ohno, H. Suzaki, and M. Adachi. 1998. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am. J. Respir. Cell Mol. Biol. 18:255-264. [DOI] [PubMed] [Google Scholar]
- 27.Matsakura, S., F. Kokubu, H. Noda, H. Tokunaga, and M. Adachi. 1996. Expression of IL-6, IL-8, and RANTES on human bronchiolar epithelial cells, NCI-H292, induced by Influenza virus. Am. J. Allergy Clin. Immunol. 98:1080-1087. [DOI] [PubMed] [Google Scholar]
- 28.Mori, I., T. Komatsu, K. Takeuchi, K. Nakakuki, M. Sudo, and Y. Kimura. 1995. In vivo induction of apoptosis by influenza virus. J. Gen. Virol. 76:2869-2873. [DOI] [PubMed] [Google Scholar]
- 29.Nain, M., F. Hinder, H. J. Gong, A. Schmidt, A. Bender, H. Sprenger, and D. Gemsa. 1990. Tumor necrosis factor-α production of influenza A virus-infected macrophages and potentiating effect of lipopolysaccharides. J. Immunol. 145:1921-1928. [PubMed] [Google Scholar]
- 30.Repp, A. C., E. S. Mayhew, S. Apte, and J. Y. Niederkorn. 2000. Human uveal melanoma cells produce macrophage migration inhibitory factor to prevent lysis by NK cells. J. Immunol. 165:710-715. [DOI] [PubMed] [Google Scholar]
- 31.Sprenger, H., R. G. Meyer, A. Kaufmann, D. Bussfeld, E. Rischkowsky, and D. Gemsa. 1996. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J. Exp. Med. 184:1191-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swope, M., H.-W. Sun, P. R. Blake, and E. Lolis. 1998. Direct link between cytokine activity and a catalytic site for macrophage migration inhibitory factor. EMBO J. 17:3534-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi, N., J. Nishihira, Y. Sato, M. Kondo, H. Ogawa, T. Ohshima, Y. Une, and S. Todo. 1998. Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth. Mol. Med. 4:707-714. [PMC free article] [PubMed] [Google Scholar]
- 34.Takizawa, T., S. Matsukawa, Y. Higuchi, S. Nakamura, Y. Nakanishi, and R. Fukada. 1993. Induction of programmed cell death (apoptosis) by influenza virus infection in cell culture cells. J. Gen. Virol. 74:2347-2355. [DOI] [PubMed] [Google Scholar]
- 35.Van Engeland, M., F. C. Ramaekers, B. Schutte, and C. P. Reutelingsperger. 1996. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 24:131-139. [DOI] [PubMed] [Google Scholar]
- 36.Vermes, I., C. Haanen, H. Steffens-Makken, and C. Reutelingsperger. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 184:39-51. [DOI] [PubMed] [Google Scholar]
- 37.Wennemuth, G., G. Aumüller, M. Bacher, and A. Meinhardt. 2000. Macrophage migration inhibitory factor induced Ca2+ response in rat testicular peritubular cells. Biol. Reprod. 62:1632-1639. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson, P. C., and I. Newman. 1992. Identification of IL-8 as a locomotor attractant for activated human lymphocytes in mononuclear cell cultures with anti-CD3 or purified protein derivative of mycobacterium tuberculosis. J. Immunol. 149:2689-2694. [PubMed] [Google Scholar]