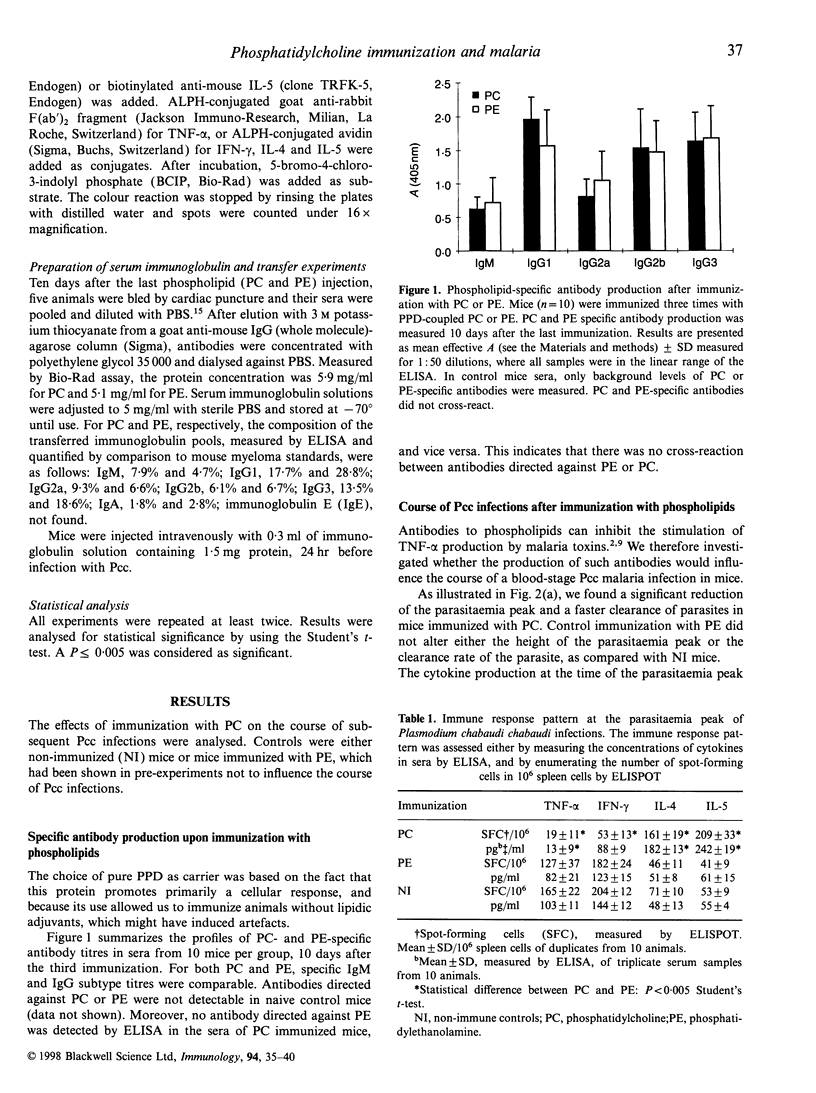
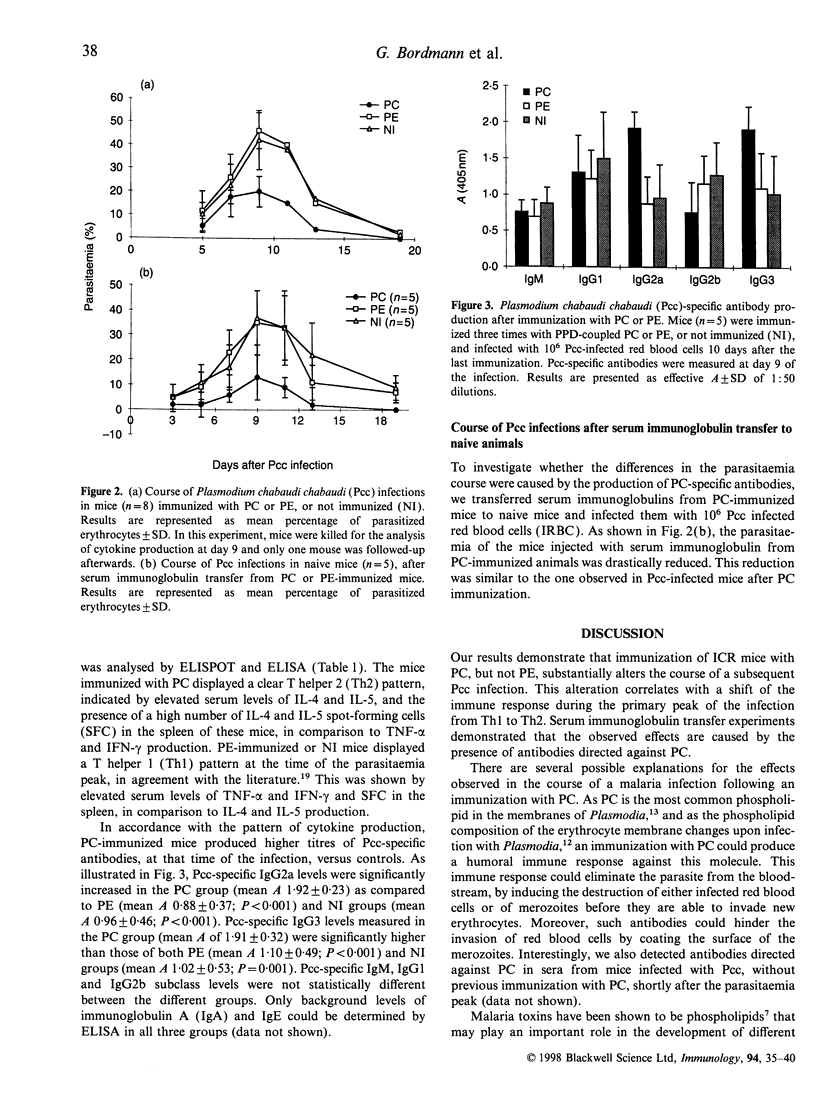
Abstract
It has been suggested that phospholipids and antibodies directed against phospholipids are important in the pathology of malaria. We have investigated the influence of immunizations with phospholipids on the course of subsequent blood-stage Plasmodium chabaudi chabaudi infections in ICR inbred mice. We observed a significant reduction in the parasitaemia following immunization with phosphatidylcholine (PC), but not with phosphatidylethanolamine (PE) immunization. At the peak of the infection, PC-immunized mice displayed a T-helper 2 (Th2)-type cytokine production pattern, whereas PE-immunized or non-treated controls displayed a cytokine production pattern of the T-helper 1 (Th1) type. Serum immunoglobulin transfer from PC-immunized mice protected naive mice in a similar fashion to PC-immunization, demonstrating that the observed reduction of parasitaemia was caused by the presence of PC-specific antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bate C. A., Kwiatkowski D. P. Stimulators of tumour necrosis factor production released by damaged erythrocytes. Immunology. 1994 Oct;83(2):256–261. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Davé A., Playfair J. H. Malaria exoantigens induce T-independent antibody that blocks their ability to induce TNF. Immunology. 1990 Jul;70(3):315–320. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Kwiatkowski D., Playfair J. H. Phospholipids coupled to a carrier induce IgG antibody that blocks tumour necrosis factor induction by toxic malaria antigens. Immunology. 1993 May;79(1):138–145. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Román E., Moreno C., Playfair J. H. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology. 1992 Jan;75(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Bordmann G., Favre N., Rudin W. Malaria toxins: effects on murine spleen and bone marrow cell proliferation and cytokine production in vitro. Parasitology. 1997 Nov;115(Pt 5):475–483. doi: 10.1017/s0031182097001595. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Chaudhri G., Cowden W. B. Roles of tumour necrosis factor in the illness and pathology of malaria. Trans R Soc Trop Med Hyg. 1989 Jul-Aug;83(4):436–440. doi: 10.1016/0035-9203(89)90240-x. [DOI] [PubMed] [Google Scholar]
- D'Império Lima M. R., Alvarez J. M., Furtado G. C., Kipnis T. L., Coutinho A., Minóprio P. Ig-isotype patterns of primary and secondary B cell responses to Plasmodium chabaudi chabaudi correlate with IFN-gamma and IL-4 cytokine production with CD45RB expression by CD4+ spleen cells. Scand J Immunol. 1996 Mar;43(3):263–270. doi: 10.1046/j.1365-3083.1996.d01-35.x. [DOI] [PubMed] [Google Scholar]
- Das B. K., Parida S., Ravindran B. A prognostic role for anti-phosphatidyl choline antibodies in human cerebral malaria. Clin Exp Immunol. 1996 Mar;103(3):442–445. doi: 10.1111/j.1365-2249.1996.tb08300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facer C. A., Agiostratidou G. High levels of anti-phospholipid antibodies in uncomplicated and severe Plasmodium falciparum and in P. vivax malaria. Clin Exp Immunol. 1994 Feb;95(2):304–309. doi: 10.1111/j.1365-2249.1994.tb06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre N., Bordmann G., Rudin W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods. 1997 May 12;204(1):57–66. doi: 10.1016/s0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- Favre N., Ryffel B., Bordmann G., Rudin W. The course of Plasmodium chabaudi chabaudi infections in interferon-gamma receptor deficient mice. Parasite Immunol. 1997 Aug;19(8):375–383. doi: 10.1046/j.1365-3024.1997.d01-227.x. [DOI] [PubMed] [Google Scholar]
- Metzger D. W., McNutt R. M., Collins J. T., Buchanan J. M., Van Cleave V. H., Dunnick W. A. Interleukin-12 acts as an adjuvant for humoral immunity through interferon-gamma-dependent and -independent mechanisms. Eur J Immunol. 1997 Aug;27(8):1958–1965. doi: 10.1002/eji.1830270820. [DOI] [PubMed] [Google Scholar]
- Rudin W., Quesniaux V., Favre N., Bordmann G. Malaria toxins from P. chabaudi chabaudi AS and P. berghei ANKA cause dyserythropoiesis in C57BL/6 mice. Parasitology. 1997 Nov;115(Pt 5):467–474. doi: 10.1017/s0031182097001583. [DOI] [PubMed] [Google Scholar]
- Schofield L., Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993 Jan 1;177(1):145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Vivas L., Hackett F., Gerold P., Schwarz R. T., Tachado S. Neutralizing monoclonal antibodies to glycosylphosphatidylinositol, the dominant TNF-alpha-inducing toxin of Plasmodium falciparum: prospects for the immunotherapy of severe malaria. Ann Trop Med Parasitol. 1993 Dec;87(6):617–626. doi: 10.1080/00034983.1993.11812820. [DOI] [PubMed] [Google Scholar]
- Simões A. P., Fiebig S., Wunderlich F., Vial H., Roelofsen B., Op den Kamp J. A. Plasmodium chabaudi-parasitized erythrocytes: phosphatidylcholine species of parasites and host cell membranes. Mol Biochem Parasitol. 1993 Feb;57(2):345–348. doi: 10.1016/0166-6851(93)90211-f. [DOI] [PubMed] [Google Scholar]
- von der Weid T., Langhorne J. The roles of cytokines produced in the immune response to the erythrocytic stages of mouse malarias. Immunobiology. 1993 Nov;189(3-4):397–418. doi: 10.1016/s0171-2985(11)80367-0. [DOI] [PubMed] [Google Scholar]


