Abstract
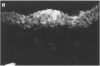
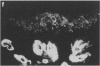
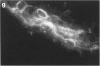
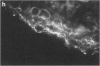
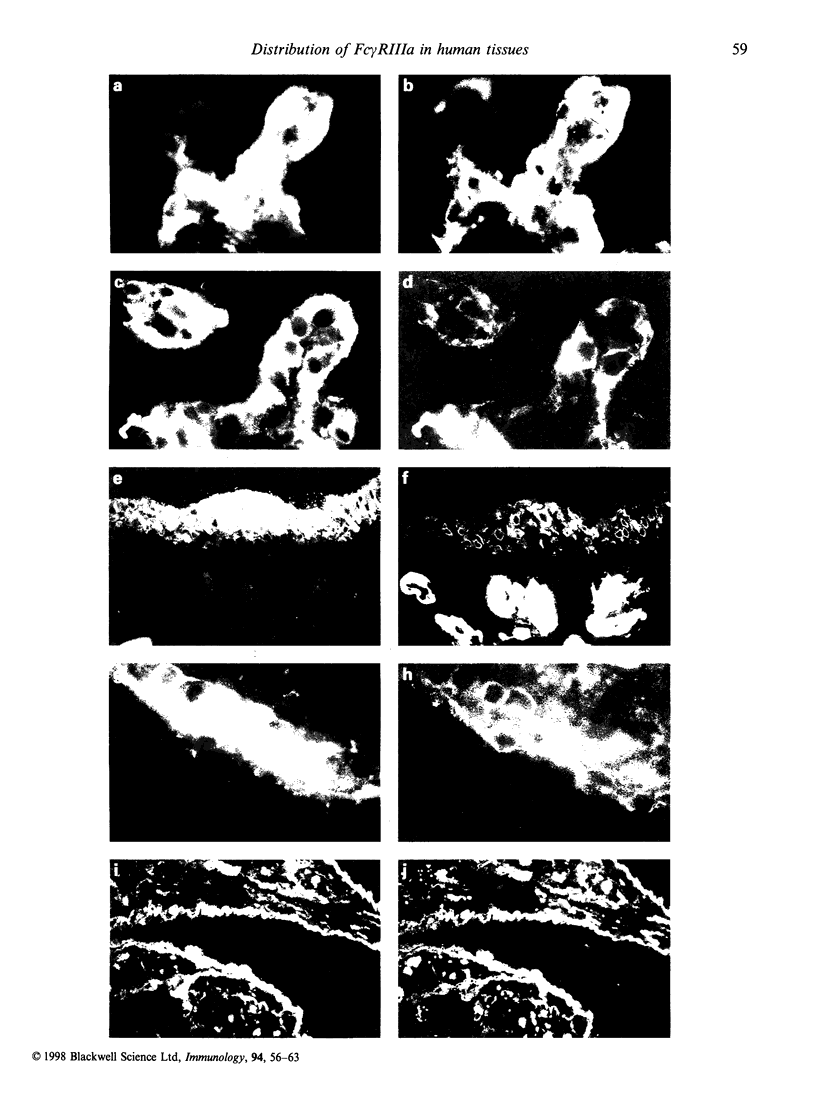
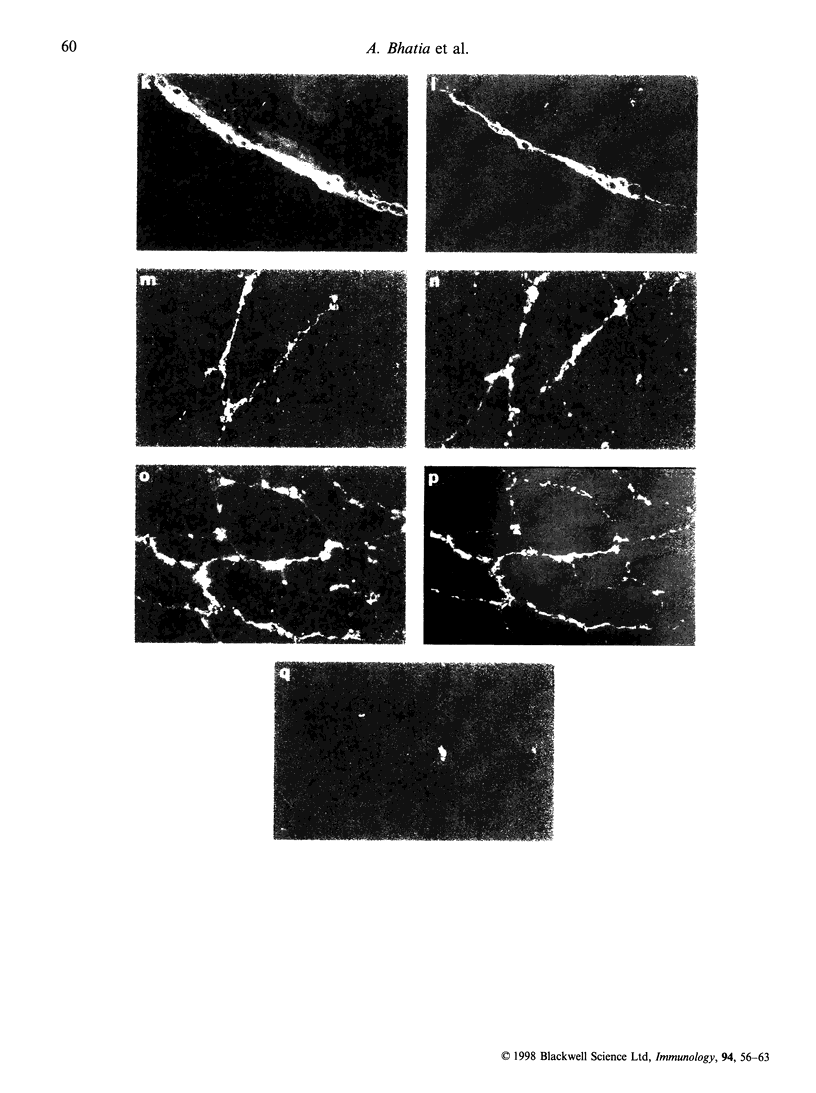
Fc gamma RIIIa is a cytokine-inducible IgG Fc receptor implicated in the activation of macrophages by immune complexes. Differential expression of Fc gamma RIIIa by macrophages in different tissues may therefore modulate local immune responsiveness. Fc gamma RIIIa expression in normal human tissues was assessed semiquantitatively using microdensitometry. Synovial intimal, serosal, alveolar, salivary gland and placental macrophages, Kupffer cells, and macrophages in mechanically stressed dermis expressed high levels of Fc gamma RIIIa. Less consistent expression was seen in skeletal muscle and lymphoid organs. No significant expression was observed in brain, thyroid, spine, intestine, myocardium, prostate, uterus, flexor forearm dermis, uterus, or kidney. Staining for Fc gamma RIII was also observed on extracellular matrix, and co-localized with both complement decay-accelerating factor and fibrillin-1. It is proposed that differential levels of both cellular and extracellular Fc gamma RIIIa, by modulating the response to immune complexes, may contribute to relative tissue susceptibility to infection and autoimmune disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen R., Brugger W., Scheibenbogen C., Kreutz M., Leser H. G., Rehm A., Löhr G. W. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990 Jun;47(6):490–497. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- Bashir M. M., Han M. D., Abrams W. R., Tucker T., Ma R. I., Gibson M., Ritty T., Mecham R., Rosenbloom J. Analysis of the human gene encoding latent transforming growth factor-beta-binding protein-2. Int J Biochem Cell Biol. 1996 May;28(5):531–542. doi: 10.1016/1357-2725(95)00167-0. [DOI] [PubMed] [Google Scholar]
- Bordessoule D., Jones M., Gatter K. C., Mason D. Y. Immunohistological patterns of myeloid antigens: tissue distribution of CD13, CD14, CD16, CD31, CD36, CD65, CD66 and CD67. Br J Haematol. 1993 Mar;83(3):370–383. doi: 10.1111/j.1365-2141.1993.tb04659.x. [DOI] [PubMed] [Google Scholar]
- Brodbeck W. G., Liu D., Sperry J., Mold C., Medof M. E. Localization of classical and alternative pathway regulatory activity within the decay-accelerating factor. J Immunol. 1996 Apr 1;156(7):2528–2533. [PubMed] [Google Scholar]
- Bröker B. M., Edwards J. C., Fanger M. W., Lydyard P. M. The prevalence and distribution of macrophages bearing Fc gamma R I, Fc gamma R II, and Fc gamma R III in synovium. Scand J Rheumatol. 1990;19(2):123–135. doi: 10.3109/03009749009102116. [DOI] [PubMed] [Google Scholar]
- Davies K. A., Hird V., Stewart S., Sivolapenko G. B., Jose P., Epenetos A. A., Walport M. J. A study of in vivo immune complex formation and clearance in man. J Immunol. 1990 Jun 15;144(12):4613–4620. [PubMed] [Google Scholar]
- Edwards J. C., Blades S., Cambridge G. Restricted expression of Fc gammaRIII (CD16) in synovium and dermis: implications for tissue targeting in rheumatoid arthritis (RA). Clin Exp Immunol. 1997 Jun;108(3):401–406. doi: 10.1046/j.1365-2249.1997.3941286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg R. W., White W., Nicholson-Weller A. Decay-accelerating factor expression on either effector or target cells inhibits cytotoxicity by human natural killer cells. J Immunol. 1992 Sep 15;149(6):2055–2060. [PubMed] [Google Scholar]
- Gessner J. E., Grussenmeyer T., Kolanus W., Schmidt R. E. The human low affinity immunoglobulin G Fc receptor III-A and III-B genes. Molecular characterization of the promoter regions. J Biol Chem. 1995 Jan 20;270(3):1350–1361. doi: 10.1074/jbc.270.3.1350. [DOI] [PubMed] [Google Scholar]
- Gibson M. A., Hatzinikolas G., Davis E. C., Baker E., Sutherland G. R., Mecham R. P. Bovine latent transforming growth factor beta 1-binding protein 2: molecular cloning, identification of tissue isoforms, and immunolocalization to elastin-associated microfibrils. Mol Cell Biol. 1995 Dec;15(12):6932–6942. doi: 10.1128/mcb.15.12.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J. Aspects of scanning microdensitometry. III. The monochromator system. J Microsc. 1975 Sep;105(1):33–56. doi: 10.1111/j.1365-2818.1975.tb04035.x. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., Ayles H. M., McKeating J. A., Butcher R. G., Griffiths P. D., Poulter L. W. Enhancement of class I HLA antigen expression by cytomegalovirus: role in amplification of virus infection. J Med Virol. 1988 Aug;25(4):483–495. doi: 10.1002/jmv.1890250412. [DOI] [PubMed] [Google Scholar]
- Huizinga T. W., van Kemenade F., Koenderman L., Dolman K. M., von dem Borne A. E., Tetteroo P. A., Roos D. The 40-kDa Fc gamma receptor (FcRII) on human neutrophils is essential for the IgG-induced respiratory burst and IgG-induced phagocytosis. J Immunol. 1989 Apr 1;142(7):2365–2369. [PubMed] [Google Scholar]
- Mannik M. Rheumatoid factors in the pathogenesis of rheumatoid arthritis. J Rheumatol Suppl. 1992 Jan;32:46–49. [PubMed] [Google Scholar]
- McMenamin P. G., Holthouse I., Holt P. G. Class II major histocompatibility complex (Ia) antigen-bearing dendritic cells within the iris and ciliary body of the rat eye: distribution, phenotype and relation to retinal microglia. Immunology. 1992 Nov;77(3):385–393. [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. G., Selvendran Y., Allen C., Revell P. A., Hogg N. Features of synovial membrane identified with monoclonal antibodies. Clin Exp Immunol. 1985 Mar;59(3):529–538. [PMC free article] [PubMed] [Google Scholar]
- Pfaff M., Reinhardt D. P., Sakai L. Y., Timpl R. Cell adhesion and integrin binding to recombinant human fibrillin-1. FEBS Lett. 1996 Apr 22;384(3):247–250. doi: 10.1016/0014-5793(96)00325-0. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Reinhardt D. P., Chalberg S. C., Sakai L. Y. The structure and function of fibrillin. Ciba Found Symp. 1995;192:128–147. doi: 10.1002/9780470514771.ch7. [DOI] [PubMed] [Google Scholar]
- Riser B. L., Cortes P., Heilig C., Grondin J., Ladson-Wofford S., Patterson D., Narins R. G. Cyclic stretching force selectively up-regulates transforming growth factor-beta isoforms in cultured rat mesangial cells. Am J Pathol. 1996 Jun;148(6):1915–1923. [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Broekelmann T., Cheresh D. A., Ramirez F., Rosenbloom J., Mecham R. P. Cell-type specific recognition of RGD- and non-RGD-containing cell binding domains in fibrillin-1. J Biol Chem. 1996 Mar 1;271(9):4916–4922. [PubMed] [Google Scholar]
- Schumann G., Dasgupta J. D. Specificity of signal transduction through CD16, TCR-CD3 and BCR receptor chains containing the tyrosine-associated activation motif. Int Immunol. 1994 Sep;6(9):1383–1392. doi: 10.1093/intimm/6.9.1383. [DOI] [PubMed] [Google Scholar]
- Shaw S. K., Brenner M. B. The beta 7 integrins in mucosal homing and retention. Semin Immunol. 1995 Oct;7(5):335–342. doi: 10.1016/1044-5323(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Shintani S., Tsuruoka S., Tamaki M., Mihara N., Shiigai T., Kikuchi M. Immunofluorescence study of immune complexes in polymyalgia rheumatica. J Neurol Sci. 1995 Jan;128(1):103–106. doi: 10.1016/0022-510x(94)00214-9. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Kyle V., Cawston T. E., Hazleman B. L. Isolation and analysis of immune complexes from sera of patients with polymyalgia rheumatica and giant cell arteritis. Ann Rheum Dis. 1987 Jun;46(6):468–474. doi: 10.1136/ard.46.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J. W. Anterior chamber associated immune deviation: the privilege of immunity in the eye. Surv Ophthalmol. 1990 Jul-Aug;35(1):67–73. doi: 10.1016/0039-6257(90)90048-z. [DOI] [PubMed] [Google Scholar]
- Tuijnman W. B., Van Wichen D. F., Schuurman H. J. Tissue distribution of human IgG Fc receptors CD16, CD32 and CD64: an immunohistochemical study. APMIS. 1993 Apr;101(4):319–329. doi: 10.1111/j.1699-0463.1993.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Waggett A. D., Kielty C. M., Shuttleworth C. A. Microfibrillar elements in the synovial joint: presence of type VI collagen and fibrillin-containing microfibrils. Ann Rheum Dis. 1993 Jun;52(6):449–453. doi: 10.1136/ard.52.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., Welch G. R., Wong H. L. Transforming growth factor-beta in synovial fluids modulates Fc gamma RII (CD16) expression on mononuclear phagocytes. J Immunol. 1992 Jan 15;148(2):485–490. [PubMed] [Google Scholar]
- Wong H. L., Welch G. R., Brandes M. E., Wahl S. M. IL-4 antagonizes induction of Fc gamma RIII (CD16) expression by transforming growth factor-beta on human monocytes. J Immunol. 1991 Sep 15;147(6):1843–1848. [PubMed] [Google Scholar]
- van Ravenswaay Claasen H. H., Kluin P. M., Fleuren G. J. Tumor infiltrating cells in human cancer. On the possible role of CD16+ macrophages in antitumor cytotoxicity. Lab Invest. 1992 Aug;67(2):166–174. [PubMed] [Google Scholar]
- van de Winkel J. G., Capel P. J. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993 May;14(5):215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]