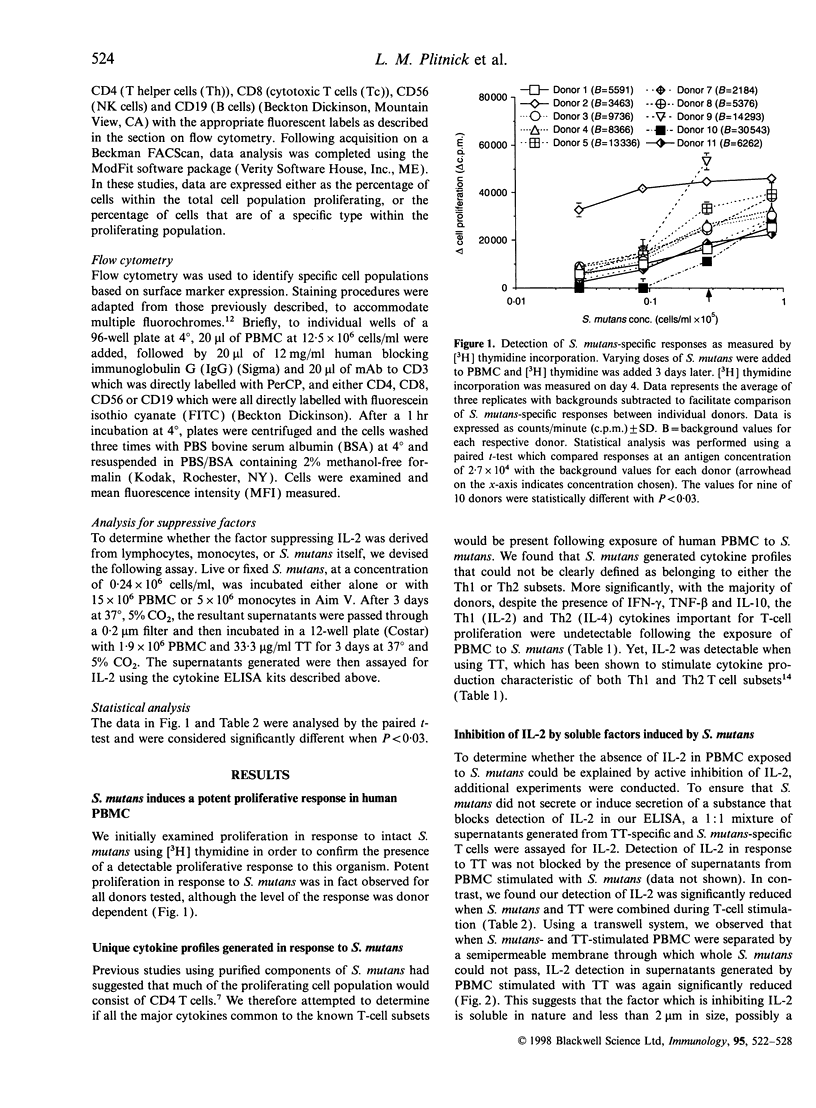
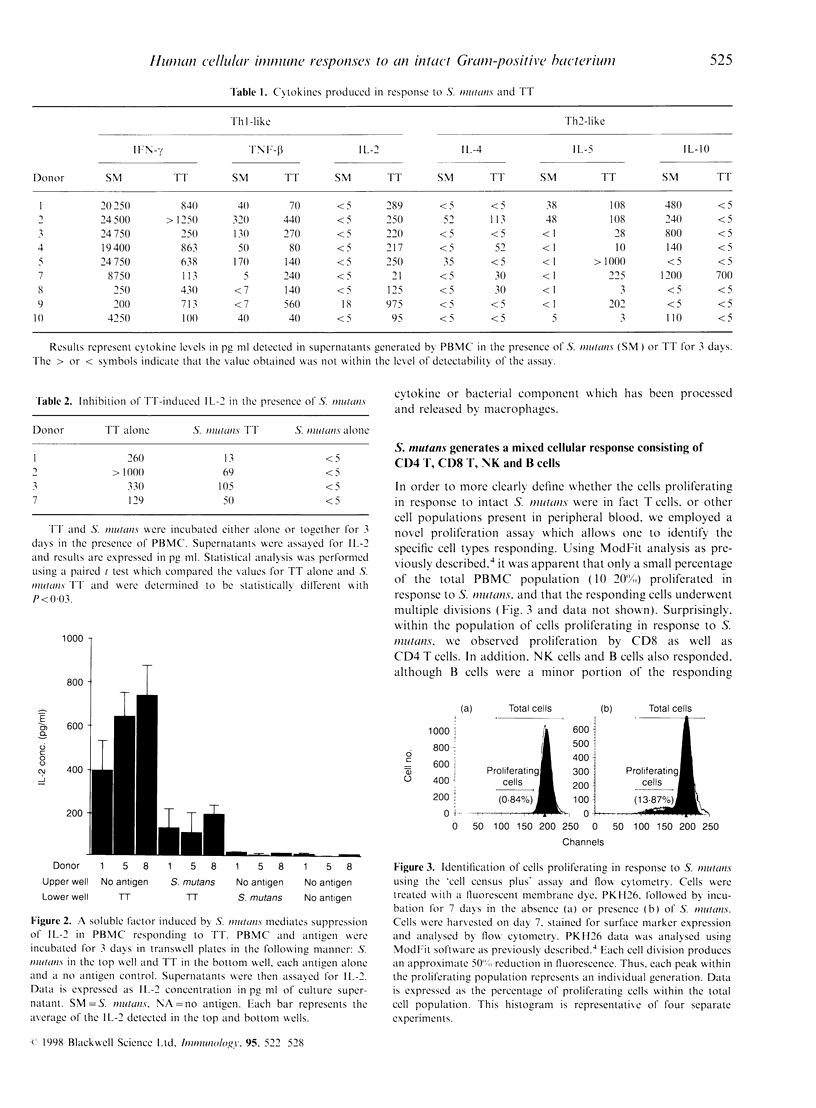
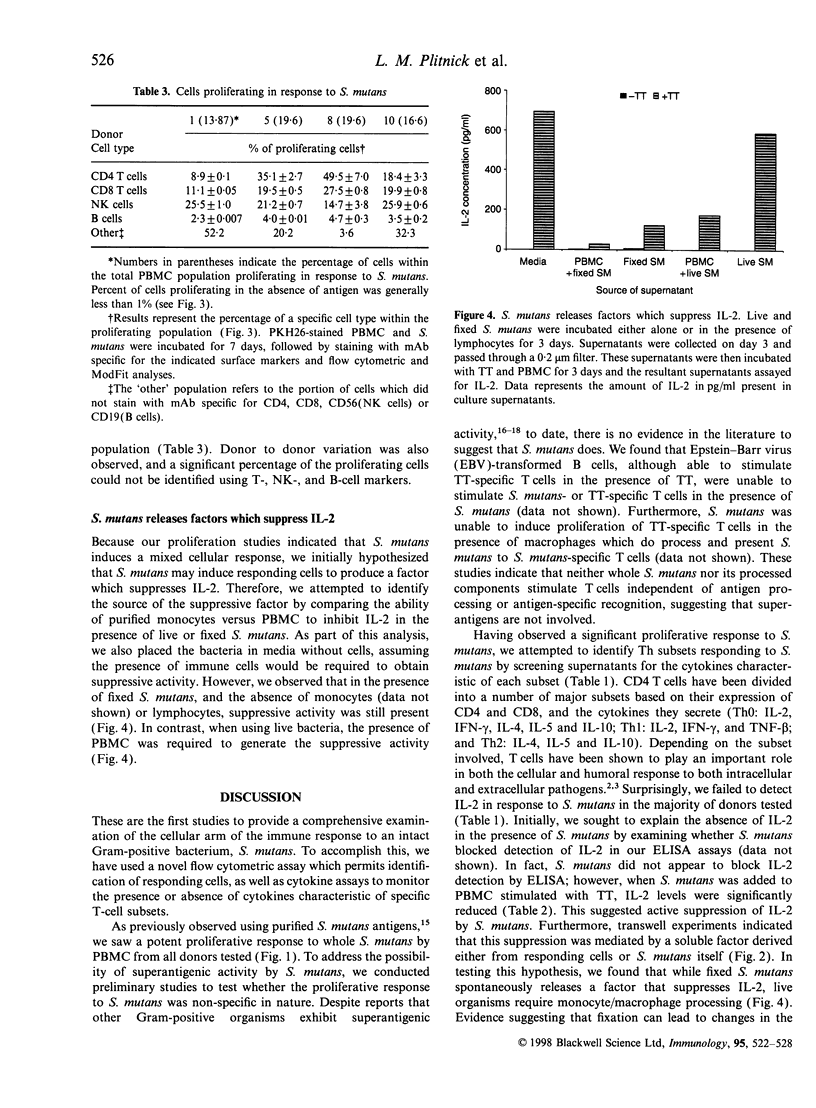
Abstract
Generation of an effective cellular immune response is key to the successful development of both humoral and cellular immune defences against most pathogens. However, while the type of cellular immune response elicited by any given pathogen is dictated by the entire array of antigens and molecules which comprise that pathogen, most studies of human immune responses to bacterial pathogens tend to focus on selected antigens. This is a result, in part, of a desire to find those antigens that will generate a desired immune response, as well as limited technology for monitoring the complex array of responses generated by an intact organism. Utilizing Streptococcus mutans as a model Gram-positive organism, a novel flow cytometric assay that permits the identification of individual cells within a responding population, and highly sensitive cytokine assays, we show for the first time that CD8 T cells and natural killer (NK) cells comprise a significant component of the response to this organism in humans. This is despite the fact that CD8 T cells are traditionally thought to respond to endogenously derived antigens only. In addition, we provide the first evidence that a Gram-positive organism can actively inhibit interleukin-2 (IL-2), an important autocrine growth factor for T cells. The latter observation could represent an additional mechanism by which Gram-positive organisms evade host defences.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton J. D., Bamford R. N., Peters C., Grant A. J., Kurys G., Goldman C. K., Brennan J., Roessler E., Waldmann T. A. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childerstone A., Haron J., Lehner T. The reactivity of naturally sensitized human CD4 cells and IgG antibodies to synthetic peptides derived from the amino terminal sequences of a 3800 MW Streptococcus mutans antigen. Immunology. 1990 Feb;69(2):177–183. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Franzl R. E., McMaster P. D. The primary immune response in mice. I. The enhancement and suppression of hemolysin production by a bacterial endotoxin. J Exp Med. 1968 Jun 1;127(6):1087–1107. doi: 10.1084/jem.127.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A., al-Sabbagh A., Santos L. M., Fishman-Lobell J., Polanski M., Das M. P., Khoury S. J., Weiner H. L. Oral tolerance: a biologically relevant pathway to generate peripheral tolerance against external and self antigens. Chem Immunol. 1994;58:259–290. [PubMed] [Google Scholar]
- Gosselin E. J., Brown M. F., Anderson C. L., Zipf T. F., Guyre P. M. The monoclonal antibody 41H16 detects the Leu 4 responder form of human Fc gamma RII. J Immunol. 1990 Mar 1;144(5):1817–1822. [PubMed] [Google Scholar]
- Gray J. D., Hirokawa M., Horwitz D. A. The role of transforming growth factor beta in the generation of suppression: an interaction between CD8+ T and NK cells. J Exp Med. 1994 Nov 1;180(5):1937–1942. doi: 10.1084/jem.180.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen L. Biological properties of bacterial peptidoglycan. APMIS. 1993 May;101(5):337–344. doi: 10.1111/j.1699-0463.1993.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Jutel M., Wyss-Coray T., Carballido J. M., Blaser K., Müller U. R., Pichler W. J. Selective restimulation of antigen or allergen preactivated T cells using OKT3 F(ab)2 results in the secretion of TH-1 or TH-2-like cytokine patterns. Clin Exp Allergy. 1995 Nov;25(11):1108–1117. doi: 10.1111/j.1365-2222.1995.tb03258.x. [DOI] [PubMed] [Google Scholar]
- Lane P. Development of B-cell memory and effector function. Curr Opin Immunol. 1996 Jun;8(3):331–335. doi: 10.1016/s0952-7915(96)80121-x. [DOI] [PubMed] [Google Scholar]
- Lehner T., Caldwell J., Avery J. Sequential development of helper and suppressor functions, antibody titers and functional avidities to a streptococcal antigen in rhesus monkeys. Eur J Immunol. 1984 Sep;14(9):814–819. doi: 10.1002/eji.1830140909. [DOI] [PubMed] [Google Scholar]
- Lehner T., Walker P., Smerdon R., Childerstone A., Bergmeier L. A., Haron J. Identification of T- and B-cell epitopes in synthetic peptides derived from a Streptococcus mutans protein and characterization of their antigenicity and immunogenicity. Arch Oral Biol. 1990;35 (Suppl):39S–45S. doi: 10.1016/0003-9969(90)90129-x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzer S. J., Guyre P. M., Burakoff S. J., Faller D. V. Spontaneous aggregation as a mechanism for human monocyte purification. Cell Immunol. 1986 Sep;101(2):312–319. doi: 10.1016/0008-8749(86)90144-9. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Arnold R. R., Michalek S. M., Prince S. J., Babb J. L. Selective induction of an immune response in human external secretions by ingestion of bacterial antigen. J Clin Invest. 1978 Mar;61(3):731–737. doi: 10.1172/JCI108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. A., Jackson R. W. The effect of a streptococcus pyogenes teichoic acid on the immune response of mice. J Immunol. 1973 Jan;110(1):148–156. [PubMed] [Google Scholar]
- Molloy A., Gaudernack G., Levis W. R., Cohn Z. A., Kaplan G. Suppression of T-cell proliferation by Mycobacterium leprae and its products: the role of lipopolysaccharide. Proc Natl Acad Sci U S A. 1990 Feb;87(3):973–977. doi: 10.1073/pnas.87.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjan A. A., Collector M. I. Stress-induced modulation of the immune response. Science. 1977 Apr 15;196(4287):307–308. doi: 10.1126/science.557841. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R. Regulation of immune responses by T cells with different cytokine secretion phenotypes: role of a new cytokine, cytokine synthesis inhibitory factor (IL10). Int Arch Allergy Appl Immunol. 1991;94(1-4):110–115. doi: 10.1159/000235340. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996 Mar;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Munro C., Michalek S. M., Macrina F. L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991 Jul;59(7):2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Alouf H., Alouf J. E., Gerlach D., Ozegowski J. H., Fitting C., Cavaillon J. M. Human pro- and anti-inflammatory cytokine patterns induced by Streptococcus pyogenes erythrogenic (pyrogenic) exotoxin A and C superantigens. Infect Immun. 1996 Apr;64(4):1450–1453. doi: 10.1128/iai.64.4.1450-1453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A., Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994 Jun;6(3):458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Rock K. L. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996 Mar;17(3):131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- Räsänen L., Arvilommi H. Cell walls, peptidoglycans, and teichoic acids of Gram-positive bacteria as polyclonal inducers and immunomodulators of proliferative and lymphokine responses of human B and T lymphocytes. Infect Immun. 1982 Feb;35(2):523–527. doi: 10.1128/iai.35.2.523-527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räsänen L., Karhumäki E., Majuri R., Arvilommi H. Polyclonal activation of human lymphocytes by bacteria. Infect Immun. 1980 May;28(2):368–372. doi: 10.1128/iai.28.2.368-372.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri L. J., Knox K. W., Wicken A. J., Hoffmann E. M. Inhibition of complement-mediated lysis of sheep erythrocytes by cell-free preparations from Streptococcus mutans BHT. J Immunol. 1979 Jan;122(1):54–60. [PubMed] [Google Scholar]
- Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. 1996 Apr;8(2):199–205. doi: 10.1016/s0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Lehtonen O. P., Aaltonen A. S. Caries development in children in relation to the presence of mutans streptococci in dental plaque and of serum antibodies against whole cells and protein antigen I/II of Streptococcus mutans. Caries Res. 1990;24(1):59–64. doi: 10.1159/000261240. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Wennerholm K., Emilson C. G. Sucrose retention and colonization by mutans streptococci at different sites of the dentition. Caries Res. 1995;29(5):396–401. doi: 10.1159/000262098. [DOI] [PubMed] [Google Scholar]
- Wilson M., Seymour R., Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998 Jun;66(6):2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y., Rodriguez N., Schwartz A., Eylar E., Bagwell B., Yano N. A new flow cytometric method for quantitative assessment of lymphocyte mitogenic potentials. Cell Mol Biol (Noisy-le-grand) 1995;41 (Suppl 1):S121–S132. [PubMed] [Google Scholar]


