Abstract
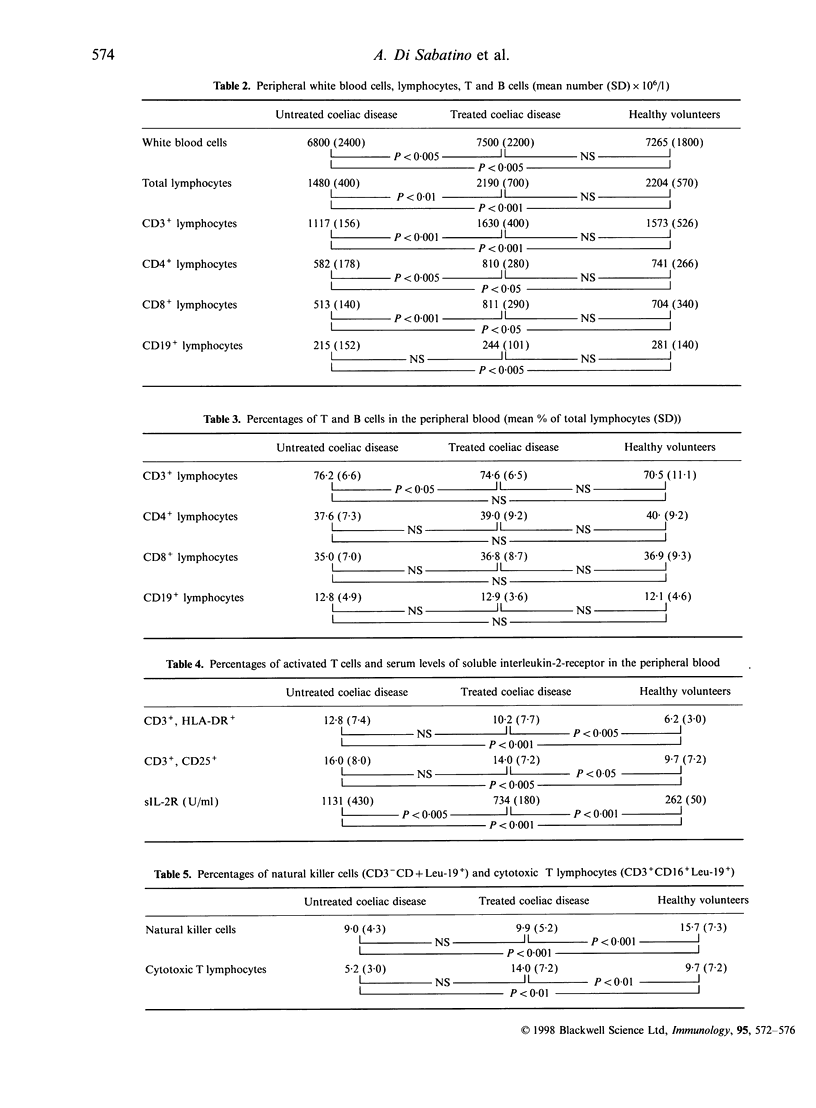
In coeliac disease immunological abnormalities are not confined to the small bowel and it has been suggested that changes in peripheral blood lymphocytes may predispose to autoimmune or malignant complications. Using dual-colour immunofluorescence with labelled monoclonal antibodies, multiparameter flow cytometry was used to analyse peripheral blood lymphocytes in 32 untreated coeliacs, 29 treated coeliacs and 20 healthy volunteers. When the absolute numbers were considered, a decrease of CD3+, CD4+, CD8+ and CD19+ lymphocytes was found in untreated coeliacs compared with treated coeliacs and healthy volunteers. The proportion of CD3+ was significantly higher in untreated coeliacs (P<0.05) than in healthy volunteers. No differences were observed in CD4+, CD8+ and CD19+ subsets between the three groups studied. The proportion of CD3+ CD25+ and CD3+ HLA-DR+ cells were higher in untreated coeliacs (P<0. 001 and P>0.005) and in treated coeliacs (P<0.005 and P<0.05) than in healthy volunteers. On the contrary, natural killer cells and cytotoxic cells were lower in untreated and treated coeliacs than in healthy volunteers. As regards B-cell subsets, the only difference was the increase in FcepsilonR+ B cells in untreated coeliacs. The absolute reduction of peripheral lymphocytes in coeliac disease probably reflects their compartimentalization in intestinal mucosa. The decrease of natural killer cells and cytotoxic cells may be in keeping with the increased prevalence of malignancy in this condition. Finally, the phenotypic changes found in untreated coeliacs indicate T-cell activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Borthwick N., Salmon M., Gombert W., Bofill M., Shamsadeen N., Pilling D., Pett S., Grundy J. E., Janossy G. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993 Aug 1;178(2):427–438. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt L., Stenstam M. Letter: Subnormal lymphocyte-counts in adult coeliac disease. Lancet. 1975 Apr 26;1(7913):978–979. doi: 10.1016/s0140-6736(75)92041-3. [DOI] [PubMed] [Google Scholar]
- Bullen A. W., Losowsky M. S. Lymphocyte subpopulations in adult coeliac disease. Gut. 1978 Oct;19(10):892–897. doi: 10.1136/gut.19.10.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper B. T., Holmes G. K., Cooke W. T. Coeliac disease and immunological disorders. Br Med J. 1978 Mar 4;1(6112):537–539. doi: 10.1136/bmj.1.6112.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazza G. R., Sarchielli P., Londei M., Frisoni M., Gasbarrini G. Gluten specific suppressor T cell dysfunction in coeliac disease. Gut. 1986 Apr;27(4):392–398. doi: 10.1136/gut.27.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Heatley R. V., Juby L. D., Howdle P. D., Losowsky M. S. Serum interleukin-2-receptor in coeliac disease: response to treatment and gluten challenge. Clin Exp Immunol. 1989 Sep;77(3):345–348. [PMC free article] [PubMed] [Google Scholar]
- Douglas A. P., Weetman A. P., Haggith J. W. The distribution and enteric loss of 51Cr-labelled lymphocytes in normal subjects and in patients with coeliac disease and other disorders of the small intestine. Digestion. 1976;14(1):29–43. doi: 10.1159/000197797. [DOI] [PubMed] [Google Scholar]
- Dowd P. S., Heatley R. V. The influence of undernutrition on immunity. Clin Sci (Lond) 1984 Mar;66(3):241–248. doi: 10.1042/cs0660241. [DOI] [PubMed] [Google Scholar]
- Foster P. N., Heatley R. V., Losowsky M. S. Natural killer cells in coeliac disease. J Clin Lab Immunol. 1985 Aug;17(4):173–176. [PubMed] [Google Scholar]
- Gougeon M. L., Lecoeur H., Dulioust A., Enouf M. G., Crouvoiser M., Goujard C., Debord T., Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996 May 1;156(9):3509–3520. [PubMed] [Google Scholar]
- Gray J. D., Hirokawa M., Horwitz D. A. The role of transforming growth factor beta in the generation of suppression: an interaction between CD8+ T and NK cells. J Exp Med. 1994 Nov 1;180(5):1937–1942. doi: 10.1084/jem.180.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson H. J., Davies R. J., Gent A. E. Atopic disorders and adult coeliac disease. Lancet. 1976 Jan 17;1(7951):115–117. doi: 10.1016/s0140-6736(76)93155-x. [DOI] [PubMed] [Google Scholar]
- Holmes G. K., Prior P., Lane M. R., Pope D., Allan R. N. Malignancy in coeliac disease--effect of a gluten free diet. Gut. 1989 Mar;30(3):333–338. doi: 10.1136/gut.30.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A., Gray J. D., Ohtsuka K., Hirokawa M., Takahashi T. The immunoregulatory effects of NK cells: the role of TGF-beta and implications for autoimmunity. Immunol Today. 1997 Nov;18(11):538–542. doi: 10.1016/s0167-5699(97)01149-3. [DOI] [PubMed] [Google Scholar]
- Howdle P. D., Simpson F. G., Losowsky M. S. The distribution of gluten-sensitive lymphocytes in coeliac patients--is it related to dietary gluten? Clin Exp Immunol. 1986 Nov;66(2):393–398. [PMC free article] [PubMed] [Google Scholar]
- Katz P., Mitchell S. R., Cupps T. R., Evans M., Whalen G. Suppression of B cell responses by natural killer cells is mediated through direct effects on T cells. Cell Immunol. 1989 Mar;119(1):130–142. doi: 10.1016/0008-8749(89)90229-3. [DOI] [PubMed] [Google Scholar]
- Kerttula T. O., Hällström O., Mäki M. Phenotypical characterization of peripheral blood T cells in patients with coeliac disease: elevation of antigen-primed CD45RO+ T lymphocytes. Immunology. 1995 Sep;86(1):104–109. [PMC free article] [PubMed] [Google Scholar]
- Kos F. J., Engleman E. G. Immune regulation: a critical link between NK cells and CTLs. Immunol Today. 1996 Apr;17(4):174–176. doi: 10.1016/0167-5699(96)80616-5. [DOI] [PubMed] [Google Scholar]
- Letellier M., Nakajima T., Delespesse G. IgE receptor on human lymphocytes. IV. Further analysis of its structure and of the role of N-linked carbohydrates. J Immunol. 1988 Oct 1;141(7):2374–2381. [PubMed] [Google Scholar]
- Marsh M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992 Jan;102(1):330–354. [PubMed] [Google Scholar]
- O'Donoghue D. P., Lancaster-Smith M., Laviniere P., Kumar P. J. T cell depletion in untreated adult coeliac disease. Gut. 1976 May;17(5):328–331. doi: 10.1136/gut.17.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. B., Losowsky M. S. Depressed cell-mediated immunity in coeliac disease. Gut. 1976 Nov;17(11):900–905. doi: 10.1136/gut.17.11.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L., Bruserud O., Gaudernack G., Thorsby E. The role of the CD8-positive subset of T cells in proliferative responses to soluble antigens. I. Studies of healthy subjects, type 1 diabetics, and coeliac disease patients. Scand J Immunol. 1986 Apr;23(4):461–467. doi: 10.1111/j.1365-3083.1986.tb03077.x. [DOI] [PubMed] [Google Scholar]
- Suda T., Hashimoto H., Tanaka M., Ochi T., Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997 Dec 15;186(12):2045–2050. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa M., Tamamori T., Horiguchi D., Sakamoto T., Yamada M., Yoshioka A., Toda K., Imamura S., Yodoi J. Fc epsilon receptor II/CD23-positive lymphocytes in atopic dermatitis. I. The proportion of Fc epsilon RII+ lymphocytes correlates with the extent of skin lesion. Clin Exp Immunol. 1991 May;84(2):275–282. [PMC free article] [PubMed] [Google Scholar]
- Tew J. G., Phipps R. P., Mandel T. E. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Howdle P. D. T-cell responses and cellular immunity in coeliac disease. Baillieres Clin Gastroenterol. 1995 Jun;9(2):251–272. doi: 10.1016/0950-3528(95)90031-4. [DOI] [PubMed] [Google Scholar]
- Verkasalo M. A., Arató A., Savilahti E., Tainio V. M. Effect of diet and age on jejunal and circulating lymphocyte subsets in children with coeliac disease: persistence of CD4-8-intraepithelial T cells through treatment. Gut. 1990 Apr;31(4):422–425. doi: 10.1136/gut.31.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]


