Abstract
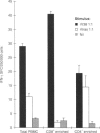
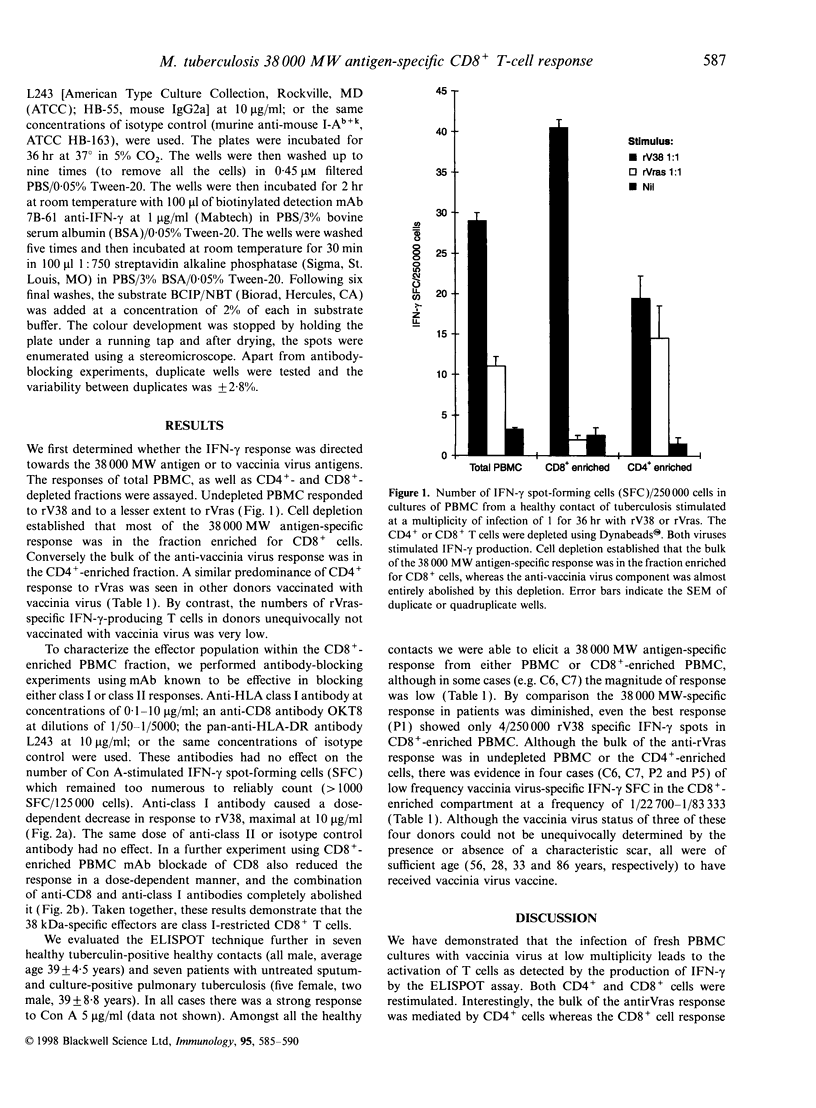
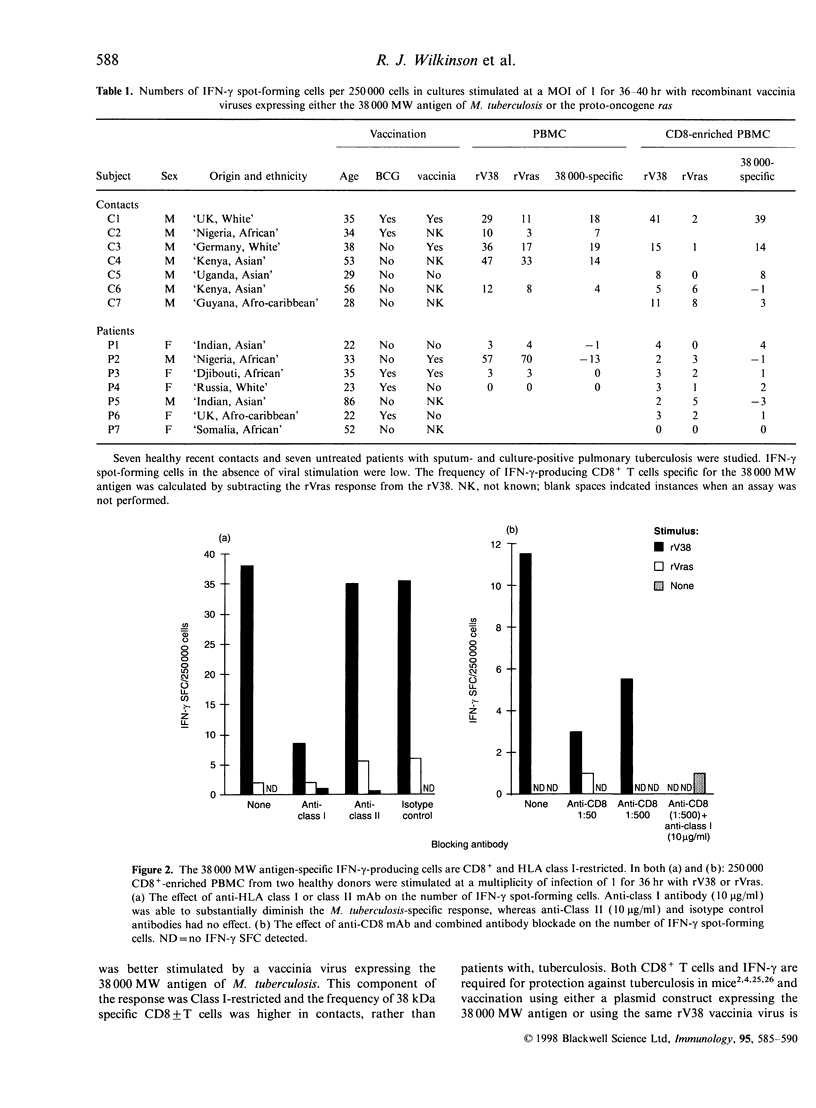
CD8+ T lymphocytes are required to protect mice against Mycobacterium tuberculosis, although in early infection the mechanism appears not to be via perforin or granzyme-mediated lysis of the infected target, and may be via interferon-gamma (IFN-gamma) production. We therefore investigated whether CD8+ T cells specific for the immunoprotective 38 000 MW antigen of M. tuberculosis could be detected in infected humans. Using a recombinant vaccinia virus expressing the 38 000 MW antigen of M. tuberculosis (rV38) and a control vaccinia virus (rVras) we demonstrated that both viruses stimulated IFN-gamma production from freshly isolated peripheral blood mononuclear cells (PBMC) in a 36-hr enzyme-linked immunospot assay. Cell depletion and antibody blockade established that the bulk of the 38 000 MW antigen-specific IFN-gamma response was mediated by CD8+, major histocompatibility complex class I-restricted T cells, whereas the anti-vaccinia virus response was predominantly mediated by CD4+ T cells. In further evaluations PBMC from all seven healthy tuberculosis-exposed contacts had a 38 000 MW antigen-specific IFN-gamma response, whereas seven patients with untreated sputum-positive pulmonary tuberculosis had very low levels of 38 000 antigen-specific IFN-gamma-producing cells. These preliminary observations demonstrate the utility of recombinant vaccinia viruses in restimulating freshly isolated CD4+ and CD8+ T cells. The bias towards a higher frequency of IFN-gamma-producing CD8+ T cells in contacts rather than patients may indicate a protective role for CD8+ cells in human tuberculosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainslie G. M., Solomon J. A., Bateman E. D. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of human pulmonary tuberculosis at presentation and during recovery. Thorax. 1992 Jul;47(7):513–518. doi: 10.1136/thx.47.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothamley G. H., Festenstein F., Newland A. Protective role for CD8 cells in tuberculosis. Lancet. 1992 Feb 1;339(8788):315–316. doi: 10.1016/0140-6736(92)91397-q. [DOI] [PubMed] [Google Scholar]
- Chang Z., Choudhary A., Lathigra R., Quiocho F. A. The immunodominant 38-kDa lipoprotein antigen of Mycobacterium tuberculosis is a phosphate-binding protein. J Biol Chem. 1994 Jan 21;269(3):1956–1958. [PubMed] [Google Scholar]
- Clemens D. L., Horwitz M. A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995 Jan 1;181(1):257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz G. A., Brewer T. F., Berkey C. S., Wilson M. E., Burdick E., Fineberg H. V., Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994 Mar 2;271(9):698–702. [PubMed] [Google Scholar]
- Cooper A. M., D'Souza C., Frank A. A., Orme I. M. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997 Apr;65(4):1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. M., Dalton D. K., Stewart T. A., Griffin J. P., Russell D. G., Orme I. M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993 Dec 1;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demkowicz W. E., Jr, Littaua R. A., Wang J., Ennis F. A. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996 Apr;70(4):2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith A., Schellenberg D. M., Rees A. D., Mitchell D. M. Antigenic specificity and subset analysis of T cells isolated from the bronchoalveolar lavage and pleural effusion of patients with lung disease. Clin Exp Immunol. 1992 Feb;87(2):272–278. doi: 10.1111/j.1365-2249.1992.tb02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993 Dec 1;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Goldstein M. M., Triebold K. J., Koller B., Bloom B. R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C. S., Hussain R., Toossi Z., Dawood G., Shahid F., Ellner J. J. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalvani A., Brookes R., Hambleton S., Britton W. J., Hill A. V., McMichael A. J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997 Sep 15;186(6):859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre P., Braibant M., de Wit L., Kalai M., Röeper D., Grötzinger J., Delville J. P., Peirs P., Ooms J., Huygen K. Three different putative phosphate transport receptors are encoded by the Mycobacterium tuberculosis genome and are present at the surface of Mycobacterium bovis BCG. J Bacteriol. 1997 May;179(9):2900–2906. doi: 10.1128/jb.179.9.2900-2906.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport M. J., Huxley C. M., Huston S., Hawrylowicz C. M., Oostra B. A., Williamson R., Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996 Dec 26;335(26):1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Mutis T. Role of cytotoxic cells in the protective immunity against and immunopathology of intracellular infections. Eur J Clin Invest. 1995 Jun;25(6):371–377. doi: 10.1111/j.1365-2362.1995.tb01716.x. [DOI] [PubMed] [Google Scholar]
- Randhawa P. S. Lymphocyte subsets in granulomas of human tuberculosis: an in situ immunofluorescence study using monoclonal antibodies. Pathology. 1990 Jul;22(3):153–155. doi: 10.3109/00313029009063555. [DOI] [PubMed] [Google Scholar]
- Rees A., Scoging A., Mehlert A., Young D. B., Ivanyi J. Specificity of proliferative response of human CD8 clones to mycobacterial antigens. Eur J Immunol. 1988 Dec;18(12):1881–1887. doi: 10.1002/eji.1830181203. [DOI] [PubMed] [Google Scholar]
- Singh M., Andersen A. B., McCarthy J. E., Rohde M., Schütte H., Sanders E., Timmis K. N. The Mycobacterium tuberculosis 38-kDa antigen: overproduction in Escherichia coli, purification and characterization. Gene. 1992 Aug 1;117(1):53–60. doi: 10.1016/0378-1119(92)90489-c. [DOI] [PubMed] [Google Scholar]
- Skipper J., Stauss H. J. Identification of two cytotoxic T lymphocyte-recognized epitopes in the Ras protein. J Exp Med. 1993 May 1;177(5):1493–1498. doi: 10.1084/jem.177.5.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger S., Mazzaccaro R. J., Uyemura K., Cho S., Barnes P. F., Rosat J. P., Sette A., Brenner M. B., Porcelli S. A., Bloom B. R. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997 Jun 13;276(5319):1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- Surcel H. M., Troye-Blomberg M., Paulie S., Andersson G., Moreno C., Pasvol G., Ivanyi J. Th1/Th2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994 Feb;81(2):171–176. [PMC free article] [PubMed] [Google Scholar]
- Tan J. S., Canaday D. H., Boom W. H., Balaji K. N., Schwander S. K., Rich E. A. Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J Immunol. 1997 Jul 1;159(1):290–297. [PubMed] [Google Scholar]
- Tascon R. E., Stavropoulos E., Lukacs K. V., Colston M. J. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998 Feb;66(2):830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J., Dockrell H. M. Stimulation of human peripheral blood mononuclear cells with live Mycobacterium bovis BCG activates cytolytic CD8+ T cells in vitro. Immunology. 1996 Mar;87(3):339–342. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Stauss H. J., Ivanyi J., Vordermeier H. M. Specificity of CD8+ T cells from subunit-vaccinated and infected H-2b mice recognizing the 38 kDa antigen of Mycobacterium tuberculosis. Int Immunol. 1997 Nov;9(11):1669–1676. doi: 10.1093/intimm/9.11.1669. [DOI] [PubMed] [Google Scholar]
- Zhu X., Venkataprasad N., Ivanyi J., Vordermeier H. M. Vaccination with recombinant vaccinia viruses protects mice against Mycobacterium tuberculosis infection. Immunology. 1997 Sep;92(1):6–9. doi: 10.1046/j.1365-2567.1997.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Venkataprasad N., Thangaraj H. S., Hill M., Singh M., Ivanyi J., Vordermeier H. M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997 Jun 15;158(12):5921–5926. [PubMed] [Google Scholar]