Abstract
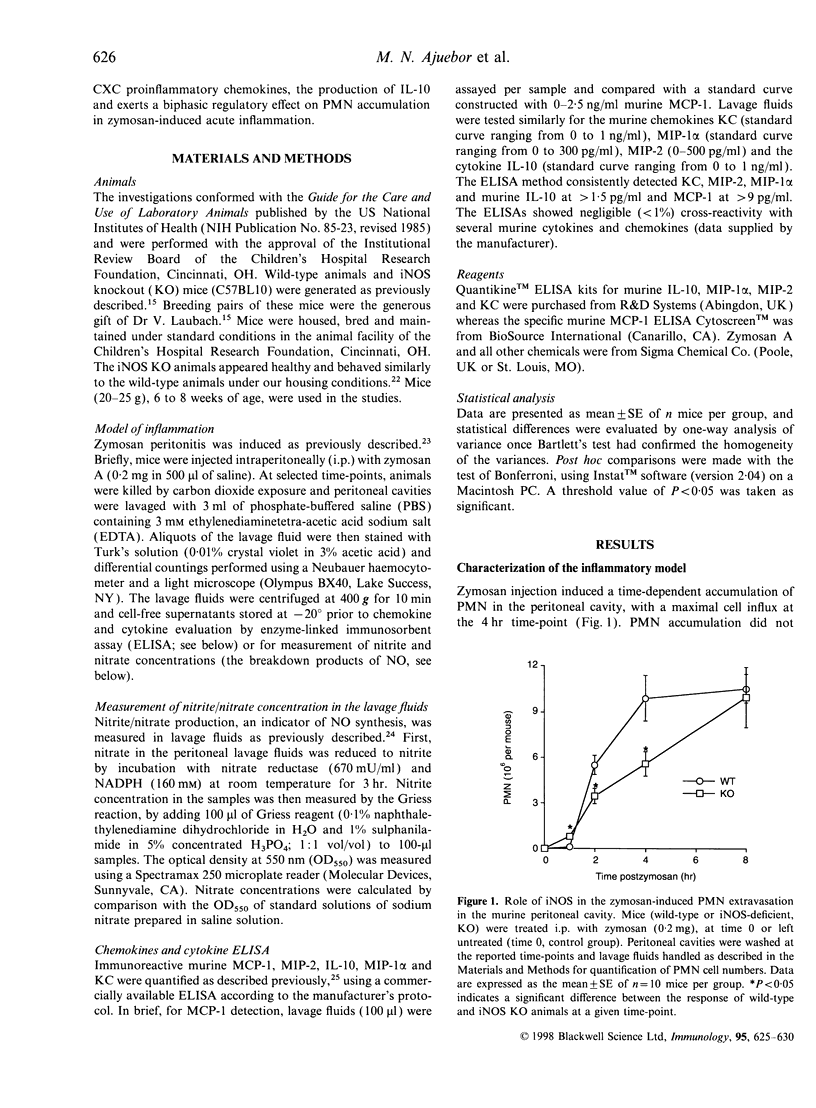
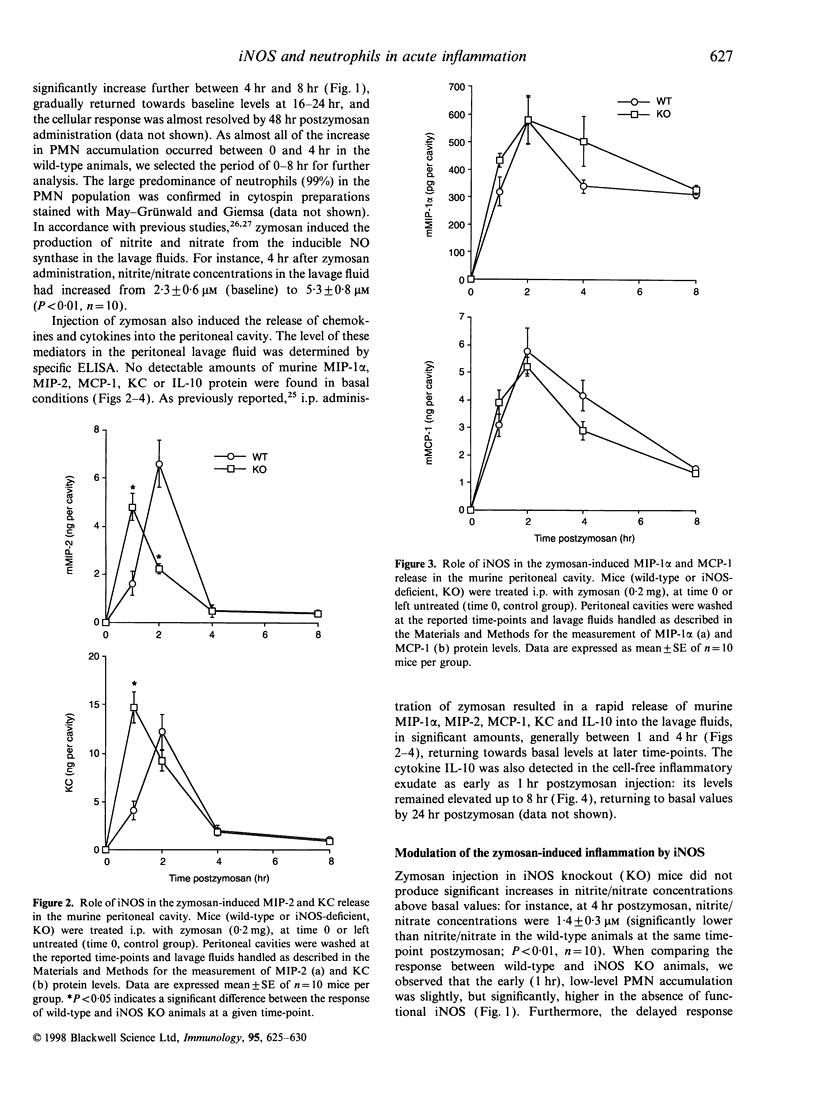
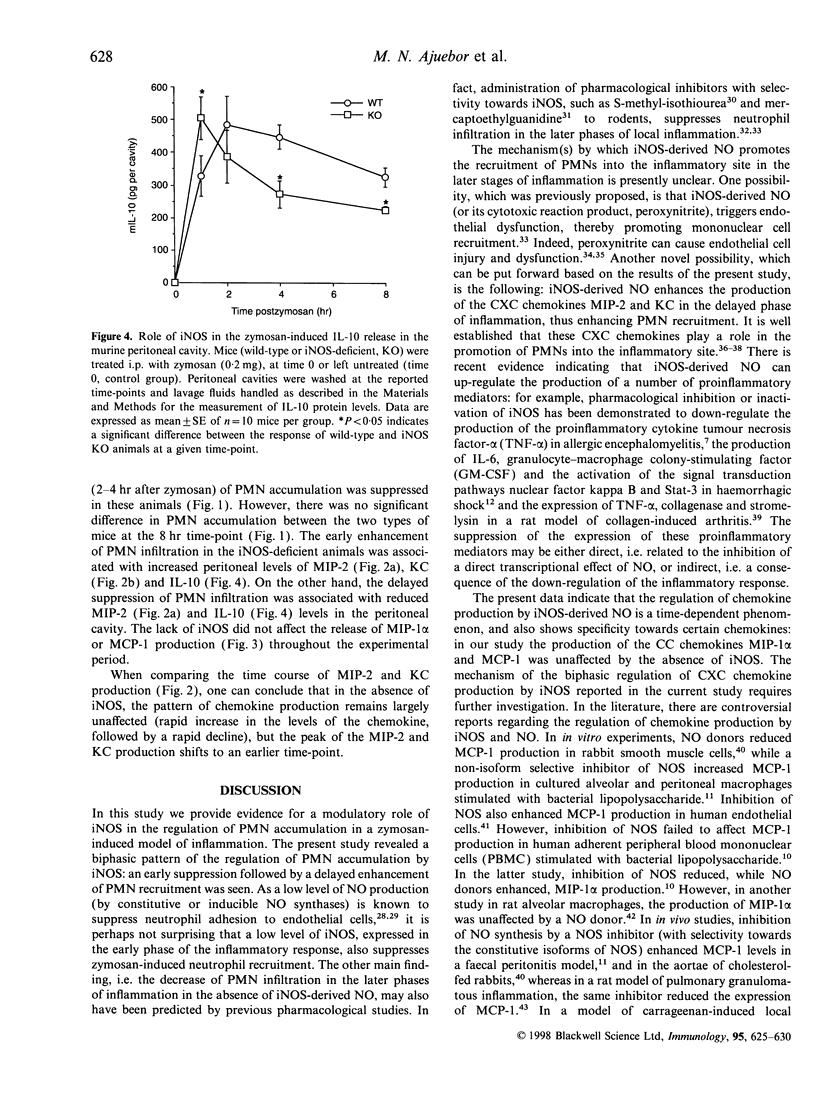
In the present study, by comparing the responses in wild-type mice and mice lacking the inducible (or type 2) nitric oxide synthase (iNOS), we investigated the role played by iNOS in the regulation of polymorphonuclear granulocyte (PMN) accumulation and chemokine production in the mouse peritoneal cavity in response to administration of zymosan (0.2 mg). Zymosan injection induced the production of nitric oxide, and triggered a time-dependent PMN immigration into the peritoneal cavity. This response was associated with increases in the level of the chemokines macrophage inflammatory protein (MIP)-1alpha, MIP-2, monocyte chemo-attractant protein (MCP)-1 and cytokine-induced neutrophil chemo-attractant (KC), as measured in the peritoneal cavities. Injection of zymosan also induced a time-dependent increase in the production of the anti-inflammatory cytokine interleukin-10 (IL-10) in the peritoneal cavity. When comparing the response between wild-type and iNOS knockout (KO) mice, we observed that the low-level PMN accumulation measured at 1 hr was slightly but significantly increased in the absence of functional iNOS. On the other hand, the delayed response (2-4 hr after zymosan) of PMN accumulation was suppressed in the iNOS KO mice. The early enhancement of PMN infiltration in the iNOS-deficient mice was associated with increased peritoneal levels of MIP-2, KC and IL-10 proteins. The delayed suppression of PMN infiltration was associated with reduced MIP-2 and IL-10 levels in the peritoneal cavity. The lack of iNOS did not affect the release of MIP-1alpha and MCP-1 at any of the time-points studied. The current data demonstrate that iNOS regulates the production of certain CXC (but not CC) proinflammatory chemokines, the production of IL-10 and exerts a biphasic regulatory effect on PMN accumulation in zymosan-induced acute inflammation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajuebor M. N., Flower R. J., Hannon R., Christie M., Bowers K., Verity A., Perretti M. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol. 1998 Jan;63(1):108–116. doi: 10.1002/jlb.63.1.108. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Ghelani A. Role of induced nitric oxide synthase and increased NO levels in zymosan peritonitis in the rat. Inflamm Res. 1995 Aug;44 (Suppl 2):S149–S150. doi: 10.1007/BF01778306. [DOI] [PubMed] [Google Scholar]
- Brahn E., Banquerigo M. L., Firestein G. S., Boyle D. L., Salzman A. L., Szabó C. Collagen induced arthritis: reversal by mercaptoethylguanidine, a novel antiinflammatory agent with a combined mechanism of action. J Rheumatol. 1998 Sep;25(9):1785–1793. [PubMed] [Google Scholar]
- Brenner T., Brocke S., Szafer F., Sobel R. A., Parkinson J. F., Perez D. H., Steinman L. Inhibition of nitric oxide synthase for treatment of experimental autoimmune encephalomyelitis. J Immunol. 1997 Mar 15;158(6):2940–2946. [PubMed] [Google Scholar]
- Cassatella M. A., Meda L., Bonora S., Ceska M., Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993 Dec 1;178(6):2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell V., Cook H. T., Ebrahim H., Waddington S. N., Wei X. Q., Assmann K. J., Liew F. Y. Anti-GBM glomerulonephritis in mice lacking nitric oxide synthase type 2. Kidney Int. 1998 Apr;53(4):932–936. doi: 10.1111/j.1523-1755.1998.00892.x. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S., Zingarelli B., Hake P., Salzman A. L., Szabó C. Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic Biol Med. 1998 Feb;24(3):450–459. doi: 10.1016/s0891-5849(97)00280-3. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S., Zingarelli B., Sautebin L., Rizzo A., Crisafulli C., Campo G. M., Costantino G., Calapai G., Nava F., Di Rosa M. Multiple organ failure following zymosan-induced peritonitis is mediated by nitric oxide. Shock. 1997 Oct;8(4):268–275. doi: 10.1097/00024382-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Ding M., Zhang M., Wong J. L., Rogers N. E., Ignarro L. J., Voskuhl R. R. Antisense knockdown of inducible nitric oxide synthase inhibits induction of experimental autoimmune encephalomyelitis in SJL/J mice. J Immunol. 1998 Mar 15;160(6):2560–2564. [PubMed] [Google Scholar]
- Fenyk-Melody J. E., Garrison A. E., Brunnert S. R., Weidner J. R., Shen F., Shelton B. A., Mudgett J. S. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998 Mar 15;160(6):2940–2946. [PubMed] [Google Scholar]
- Getting S. J., Flower R. J., Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997 Mar;120(6):1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkeson G. S., Mudgett J. S., Seldin M. F., Ruiz P., Alexander A. A., Misukonis M. A., Pisetsky D. S., Weinberg J. B. Clinical and serologic manifestations of autoimmune disease in MRL-lpr/lpr mice lacking nitric oxide synthase type 2. J Exp Med. 1997 Aug 4;186(3):365–373. doi: 10.1084/jem.186.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haelens A., Wuyts A., Proost P., Struyf S., Opdenakker G., van Damme J. Leukocyte migration and activation by murine chemokines. Immunobiology. 1996 Oct;195(4-5):499–521. doi: 10.1016/S0171-2985(96)80019-2. [DOI] [PubMed] [Google Scholar]
- Haskó G., Szabó C., Németh Z. H., Salzman A. L., Vizi E. S. Suppression of IL-12 production by phosphodiesterase inhibition in murine endotoxemia is IL-10 independent. Eur J Immunol. 1998 Feb;28(2):468–472. doi: 10.1002/(SICI)1521-4141(199802)28:02<468::AID-IMMU468>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Haskó G., Virág L., Egnaczyk G., Salzman A. L., Szabó C. The crucial role of IL-10 in the suppression of the immunological response in mice exposed to staphylococcal enterotoxin B. Eur J Immunol. 1998 Apr;28(4):1417–1425. doi: 10.1002/(SICI)1521-4141(199804)28:04<1417::AID-IMMU1417>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hickey M. J., Kubes P. Role of nitric oxide in regulation of leucocyte-endothelial cell interactions. Exp Physiol. 1997 Mar;82(2):339–348. doi: 10.1113/expphysiol.1997.sp004029. [DOI] [PubMed] [Google Scholar]
- Hickey M. J., Sharkey K. A., Sihota E. G., Reinhardt P. H., Macmicking J. D., Nathan C., Kubes P. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. FASEB J. 1997 Oct;11(12):955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- Hierholzer C., Harbrecht B., Menezes J. M., Kane J., MacMicking J., Nathan C. F., Peitzman A. B., Billiar T. R., Tweardy D. J. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998 Mar 16;187(6):917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam C. M., Steinhauser M. L., Schock H., Lukacs N., Strieter R. M., Standiford T., Kunkel S. L. Therapeutic effects of nitric oxide inhibition during experimental fecal peritonitis: role of interleukin-10 and monocyte chemoattractant protein 1. Infect Immun. 1998 Feb;66(2):650–655. doi: 10.1128/iai.66.2.650-655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone T., Van Osselaer N., D'Acquisto F., Carnuccio R., Herman A. G. Differential effect of L-NAME and S-methyl-isothiourea on leukocyte emigration in carrageenin-soaked sponge implants in rat. Br J Pharmacol. 1997 Aug;121(8):1637–1644. doi: 10.1038/sj.bjp.0701317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn R. G., Szabó C., Traber D. L. Beneficial versus detrimental effects of nitric oxide synthase inhibitors in circulatory shock: lessons learned from experimental and clinical studies. Shock. 1997 Apr;7(4):235–246. doi: 10.1097/00024382-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Laubach V. E., Shesely E. G., Smithies O., Sherman P. A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A. D. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998 Feb 12;338(7):436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- MacMicking J. D., Nathan C., Hom G., Chartrain N., Fletcher D. S., Trumbauer M., Stevens K., Xie Q. W., Sokol K., Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995 May 19;81(4):641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- McCafferty D. M., Mudgett J. S., Swain M. G., Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997 Mar;112(3):1022–1027. doi: 10.1053/gast.1997.v112.pm9041266. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R. Properties and functions of interleukin-10. Adv Immunol. 1994;56:1–26. [PubMed] [Google Scholar]
- Mühl H., Dinarello C. A. Macrophage inflammatory protein-1 alpha production in lipopolysaccharide-stimulated human adherent blood mononuclear cells is inhibited by the nitric oxide synthase inhibitor N(G)-monomethyl-L-arginine. J Immunol. 1997 Nov 15;159(10):5063–5069. [PubMed] [Google Scholar]
- Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997 Nov 15;100(10):2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Perretti M., Szabó C., Thiemermann C. Effect of interleukin-4 and interleukin-10 on leucocyte migration and nitric oxide production in the mouse. Br J Pharmacol. 1995 Oct;116(4):2251–2257. doi: 10.1111/j.1476-5381.1995.tb15061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick D. G., Villarete L. Regulation of cytokine gene expression by reactive oxygen and reactive nitrogen intermediates. J Leukoc Biol. 1996 Apr;59(4):471–475. doi: 10.1002/jlb.59.4.471. [DOI] [PubMed] [Google Scholar]
- Rollins B. J. Chemokines. Blood. 1997 Aug 1;90(3):909–928. [PubMed] [Google Scholar]
- Setoguchi K., Takeya M., Akaike T., Suga M., Hattori R., Maeda H., Ando M., Takahashi K. Expression of inducible nitric oxide synthase and its involvement in pulmonary granulomatous inflammation in rats. Am J Pathol. 1996 Dec;149(6):2005–2022. [PMC free article] [PubMed] [Google Scholar]
- Southan G. J., Szabó C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996 Feb 23;51(4):383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- Southan G. J., Zingarelli B., O'Connor M., Salzman A. L., Szabó C. Spontaneous rearrangement of aminoalkylisothioureas into mercaptoalkylguanidines, a novel class of nitric oxide synthase inhibitors with selectivity towards the inducible isoform. Br J Pharmacol. 1996 Feb;117(4):619–632. doi: 10.1111/j.1476-5381.1996.tb15236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichtenoth D. O., Frölich J. C. Nitric oxide and inflammatory joint diseases. Br J Rheumatol. 1998 Mar;37(3):246–257. doi: 10.1093/rheumatology/37.3.246. [DOI] [PubMed] [Google Scholar]
- Szabó C., Cuzzocrea S., Zingarelli B., O'Connor M., Salzman A. L. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J Clin Invest. 1997 Aug 1;100(3):723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M. J., Buhrow L. T., Connors M. J., Kaneko F. T., Erzurum S. C., Kavuru M. S. Nitric oxide inhibits inflammatory cytokine production by human alveolar macrophages. Am J Respir Cell Mol Biol. 1997 Sep;17(3):279–283. doi: 10.1165/ajrcmb.17.3.2998m. [DOI] [PubMed] [Google Scholar]
- Tsao P. S., Wang B., Buitrago R., Shyy J. Y., Cooke J. P. Nitric oxide regulates monocyte chemotactic protein-1. Circulation. 1997 Aug 5;96(3):934–940. doi: 10.1161/01.cir.96.3.934. [DOI] [PubMed] [Google Scholar]
- Villa L. M., Salas E., Darley-Usmar V. M., Radomski M. W., Moncada S. Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12383–12387. doi: 10.1073/pnas.91.26.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Zeiher A. M., Fisslthaler B., Schray-Utz B., Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995 Jun;76(6):980–986. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]
- Zingarelli B., Virág L., Szabó A., Cuzzocrea S., Salzman A. L., Szabó C. Oxidation, tyrosine nitration and cytostasis induction in the absence of inducible nitric oxide synthase. Int J Mol Med. 1998 May;1(5):787–795. doi: 10.3892/ijmm.1.5.787. [DOI] [PubMed] [Google Scholar]
- van de Loo F. A., Arntz O. J., van Enckevort F. H., van Lent P. L., van den Berg W. B. Reduced cartilage proteoglycan loss during zymosan-induced gonarthritis in NOS2-deficient mice and in anti-interleukin-1-treated wild-type mice with unabated joint inflammation. Arthritis Rheum. 1998 Apr;41(4):634–646. doi: 10.1002/1529-0131(199804)41:4<634::AID-ART10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]


