Abstract
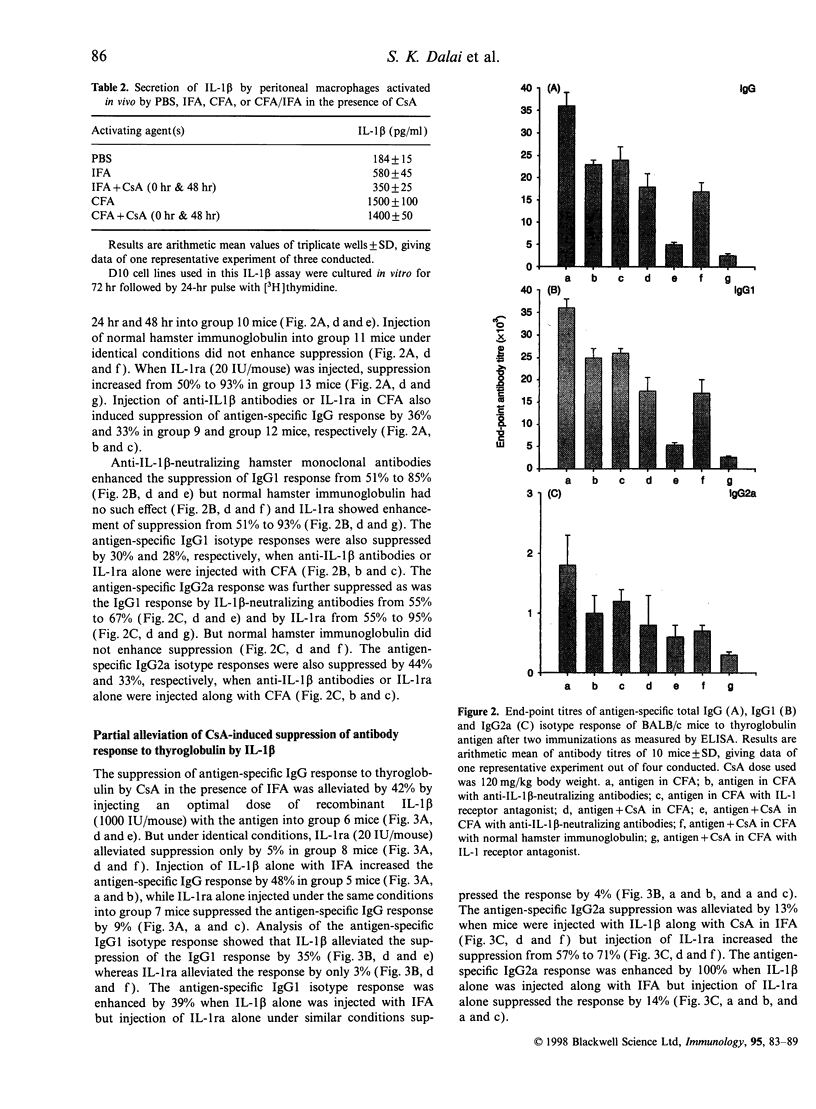
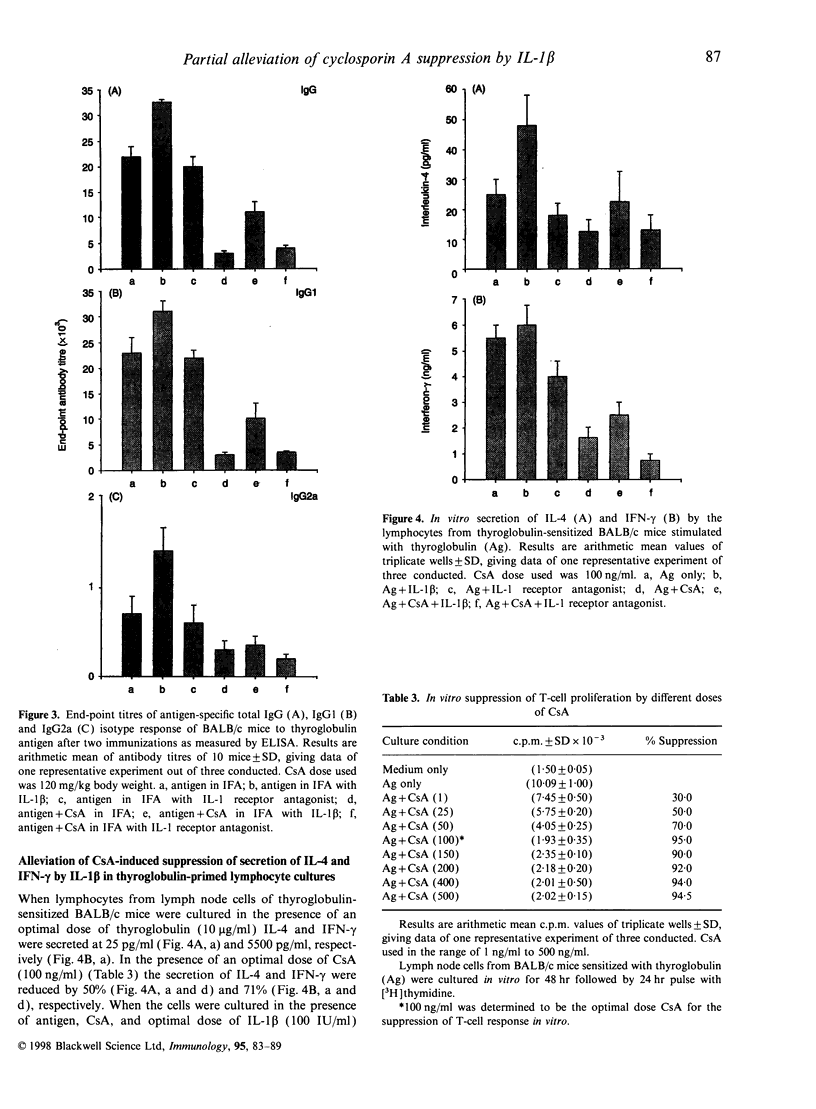
Cyclosporin A (CsA) at 120 mg/kg body weight when injected subcutaneously into BALB/c mice along with thyroglobulin emulsified in incomplete Freund's adjuvant (IFA) was found to suppress antigen-specific IgG titre by 86%. Isotyping revealed that both IgG1 and IgG2a titres were suppressed by 87% and 57%, respectively. But under identical conditions when complete Freund's adjuvant (CFA) was used, the suppression of antigen-specific IgG, IgG1 and IgG2a titres was 50%, 51% and 55%, respectively. Injection of anti-IL-1beta-neutralizing hamster monoclonal antibodies along with thyroglobulin and CsA emulsified in CFA increased the suppression of antigen-specific IgG titre. Under such conditions the IgG1 titre was suppressed more than the IgG2a titre. Recombinant human interleukin-1 receptor antagonist (rhuIL-1ra) also enhanced the suppression caused by CsA in the presence of CFA but control hamster immunoglobulin had no such effect. Recombinant human IL-1beta, when administered along with thyroglobulin and CsA emulsified in IFA, alleviated the suppression of antigen-specific IgG titre and the IgG1 titre was alleviated more than the IgG2a titre. Under identical conditions, rhuIL-1ra did not alleviate CsA-induced suppression. Lymphocytes from the lymph nodes of thyroglobulin-sensitized BALB/c mice when stimulated in vitro by thyroglobulin in the presence of CsA, secreted very little interferon-gamma (IFN-gamma) and IL-4, but on addition of an optimal dose of rhuIL-1beta, IFN-gamma and IL-4 secretion was partially restored.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz M., Fox B. S. Regulation and development of cytochrome c-specific IL-4-producing T cells. J Immunol. 1990 Aug 15;145(4):1046–1052. [PubMed] [Google Scholar]
- Bram R. J., Hung D. T., Martin P. K., Schreiber S. L., Crabtree G. R. Identification of the immunophilins capable of mediating inhibition of signal transduction by cyclosporin A and FK506: roles of calcineurin binding and cellular location. Mol Cell Biol. 1993 Aug;13(8):4760–4769. doi: 10.1128/mcb.13.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalath H. R., Titus R. G. Leishmania major-parasitized macrophages augment Th2-type T cell activation. J Immunol. 1994 Nov 15;153(10):4378–4387. [PubMed] [Google Scholar]
- Chang T. L., Shea C. M., Urioste S., Thompson R. C., Boom W. H., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. III. Responses of IL-2- and IL-4-producing (Th1 and Th2) clones to antigens presented by different accessory cells. J Immunol. 1990 Nov 1;145(9):2803–2808. [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Mosmann T. R., Vitetta E. S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J Exp Med. 1988 Aug 1;168(2):543–558. doi: 10.1084/jem.168.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman D. A., Klee C. B., Bierer B. E., Burakoff S. J. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3686–3690. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Hadjipetrou-Kourounakis L., Möller E. Adjuvants influence the immunoglobin subclass distribution of immune responses in vivo. Scand J Immunol. 1984 Mar;19(3):219–225. doi: 10.1111/j.1365-3083.1984.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Huber M., Rutherford A., Meister W., Weiss A., Röllinghoff M., Lohoff M. TCR- and IL-1-mediated co-stimulation reveals an IL-4-independent way of Th2 cell proliferation. Int Immunol. 1996 Aug;8(8):1257–1263. doi: 10.1093/intimm/8.8.1257. [DOI] [PubMed] [Google Scholar]
- Kincy-Cain T., Bost K. L. Substance P-induced IL-12 production by murine macrophages. J Immunol. 1997 Mar 1;158(5):2334–2339. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Zubiaga A. M., Merrow M., Sauter N. P., Huber B. T. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990 Jul 1;172(1):95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Togawa A., Chedid L., Mizel S. Components of mycobacteria and muramyl dipeptide with adjuvant activity induce lymphocyte activating factor. Cell Immunol. 1980 Mar 1;50(1):71–81. doi: 10.1016/0008-8749(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Schmid F. X. Prolyl isomerase: enzymatic catalysis of slow protein-folding reactions. Annu Rev Biophys Biomol Struct. 1993;22:123–142. doi: 10.1146/annurev.bb.22.060193.001011. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Fleissner S., Heimann-Weitschat I., Lindstaedt R., Pomberg B., Werner U., Szelenyi I. Effect of corticosteroids, cyclosporin A, and methotrexate on cytokine release from monocytes and T-cell subsets. Immunopharmacology. 1994 May-Jun;27(3):173–179. doi: 10.1016/0162-3109(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Le Gros G., Ben-Sasson S. Z., Urban J., Jr, Finkelman F. D., Paul W. E. Increased frequency of interleukin 4-producing T cells as a result of polyclonal priming. Use of a single-cell assay to detect interleukin 4-producing cells. Eur J Immunol. 1991 May;21(5):1241–1247. doi: 10.1002/eji.1830210522. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Svendsen Bollen L., Crowley A., Stodulski G., Hau J. Antibody production in rabbits and chickens immunized with human IgG. A comparison of titre and avidity development in rabbit serum, chicken serum and egg yolk using three different adjuvants. J Immunol Methods. 1996 May 27;191(2):113–120. doi: 10.1016/0022-1759(96)00010-5. [DOI] [PubMed] [Google Scholar]


