Abstract
In order to study the mechanism of interleukin-12 (IL-12) antitumour activity, RH7777 rat hepatoma cells were engineered to express mouse IL-12 (mIL-12) (RH7777/mIL-12) under the tight control of doxycycline (dox). The production of the mIL-12 protein was regulated by the concentration of dox that was present in the culture medium. RH7777/mIL-12 cells appeared to have the same tumorigenic activity as did parental RH7777 cells, when subcutaneously injected into syngeneic rat (BUF/N) in the absence of dox. However, the tumorigenicity of RH7777/mIL-12, but not RH7777, cells were significantly decreased when dox was administrated to the animals. In addition, established tumours of RH7777/mIL-12 cells gradually disappeared upon the induction of mIL-12 by dox. To elucidate the kinetic profile of immune cells involved in the mIL-12-induced tumour regression, both histological and immunohistochemical analyses were performed 1, 3 and 14 days after the dox treatment on rats bearing tumours that were approximately 0. 5 cm in diameter. Tumour-infiltrating macrophages began to appear at the tumour site one day after dox treatment. As time elapsed, the number of tumour infiltrates including CD4+, CD8+, natural killer (NK) cells and macrophages gradually increased. In particular, CD8+ and NK cells constituted the major population of the tumour-infiltrated cells. Furthermore, it was found that resting peritoneal macrophages (PM) from rats were chemoattracted in response to mIL-12. The effects of mIL-12 on PM chemotaxis were reproducibly observed in concentrations as low as 0.1 ng/ml. These findings suggest that IL-12 can directly recruit macrophages into tumour sites which, in turn, leads to a broad and intense immunological response against tumour.
Full text
PDF

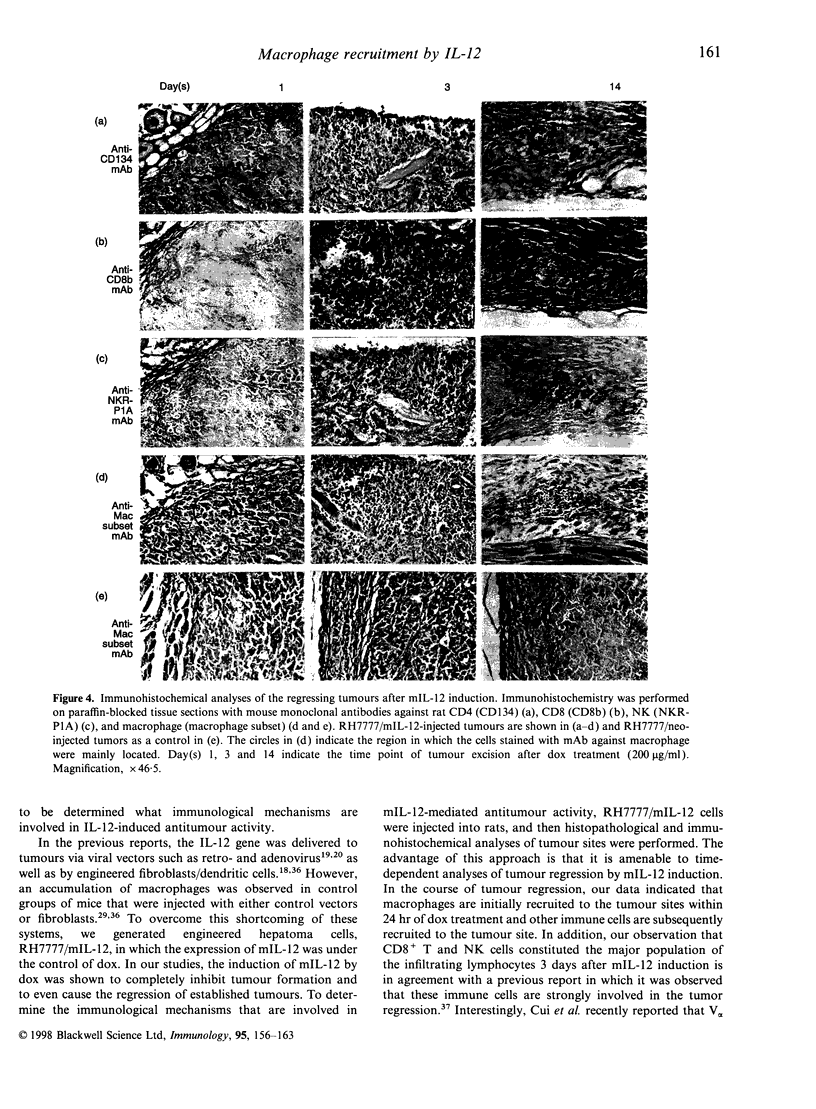
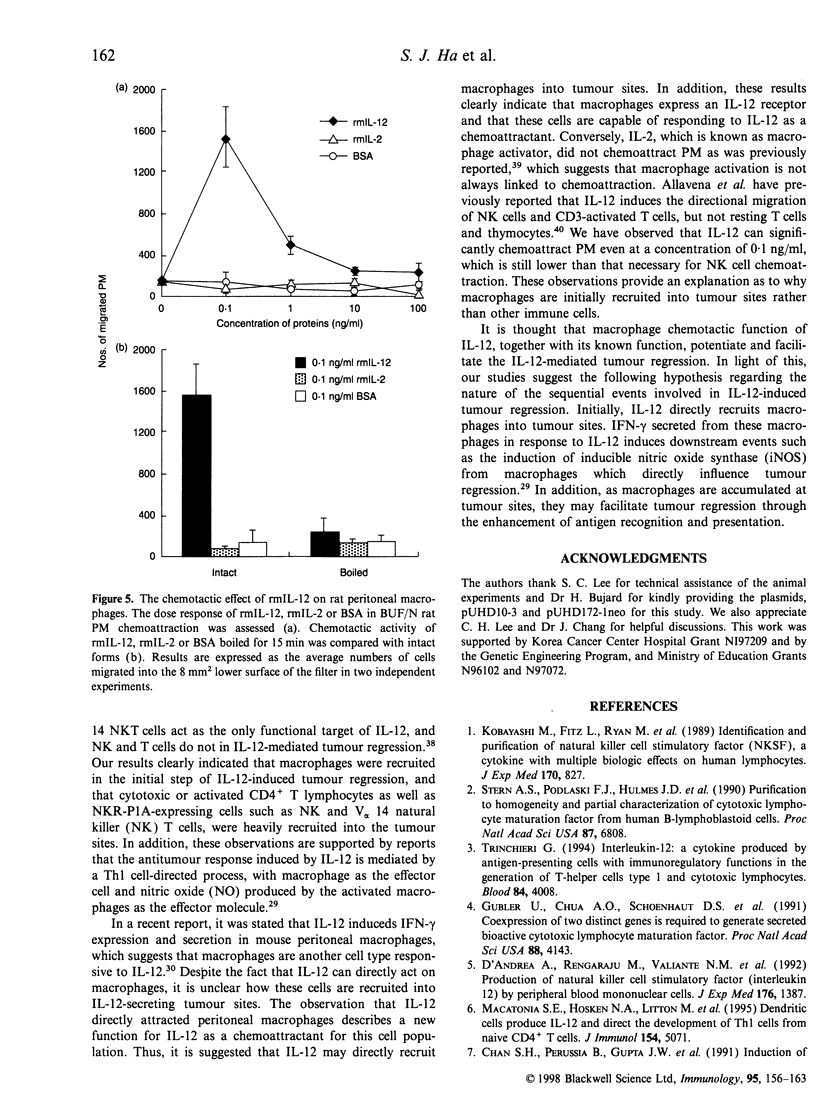

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allavena P., Paganin C., Zhou D., Bianchi G., Sozzani S., Mantovani A. Interleukin-12 is chemotactic for natural killer cells and stimulates their interaction with vascular endothelium. Blood. 1994 Oct 1;84(7):2261–2268. [PubMed] [Google Scholar]
- Bacon K. B., Camp R. D., Cunningham F. M., Woollard P. M. Contrasting in vitro lymphocyte chemotactic activity of the hydroxyl enantiomers of 12-hydroxy-5,8,10,14-eicosatetraenoic acid. Br J Pharmacol. 1988 Nov;95(3):966–974. doi: 10.1111/j.1476-5381.1988.tb11727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunda M. J., Luistro L., Warrier R. R., Wright R. B., Hubbard B. R., Murphy M., Wolf S. F., Gately M. K. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993 Oct 1;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso M., Pham-Nguyen K., Kwong Y. L., Xu B., Kosai K. I., Finegold M., Woo S. L., Chen S. H. Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11302–11306. doi: 10.1073/pnas.93.21.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo F., Signorelli P., Giovarelli M., Musiani P., Modesti A., Brunda M. J., Colombo M. P., Forni G. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997 Jul 16;89(14):1049–1058. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Chen D., Block E., O'Donnell M., Kufe D. W., Clinton S. K. Eradication of murine bladder carcinoma by intratumor injection of a bicistronic adenoviral vector carrying cDNAs for the IL-12 heterodimer and its inhibition by the IL-12 p40 subunit homodimer. J Immunol. 1997 Jul 1;159(1):351–359. [PubMed] [Google Scholar]
- Cui J., Shin T., Kawano T., Sato H., Kondo E., Toura I., Kaneko Y., Koseki H., Kanno M., Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997 Nov 28;278(5343):1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N. M., Chehimi J., Kubin M., Aste M., Chan S. H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992 Nov 1;176(5):1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gately M. K., Desai B. B., Wolitzky A. G., Quinn P. M., Dwyer C. M., Podlaski F. J., Familletti P. C., Sinigaglia F., Chizonnite R., Gubler U. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol. 1991 Aug 1;147(3):874–882. [PubMed] [Google Scholar]
- Gossen M., Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., Freundlieb S., Bender G., Müller G., Hillen W., Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995 Jun 23;268(5218):1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Gubler U., Chua A. O., Schoenhaut D. S., Dwyer C. M., McComas W., Motyka R., Nabavi N., Wolitzky A. G., Quinn P. M., Familletti P. C. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto W., Takeda K., Anzai R., Ogasawara K., Sakihara H., Sugiura K., Seki S., Kumagai K. Cytotoxic NK1.1 Ag+ alpha beta T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995 May 1;154(9):4333–4340. [PubMed] [Google Scholar]
- Hiester A. A., Metcalf D. R., Campbell P. A. Interleukin-4 is chemotactic for mouse macrophages. Cell Immunol. 1992 Jan;139(1):72–80. doi: 10.1016/0008-8749(92)90100-4. [DOI] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Kistner A., Gossen M., Zimmermann F., Jerecic J., Ullmer C., Lübbert H., Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Hosken N. A., Litton M., Vieira P., Hsieh C. S., Culpepper J. A., Wysocka M., Trinchieri G., Murphy K. M., O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995 May 15;154(10):5071–5079. [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabors G. S., Afonso L. C., Farrell J. P., Scott P. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and Pentostam. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3142–3146. doi: 10.1073/pnas.92.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastala C. L., Edington H. D., McKinney T. G., Tahara H., Nalesnik M. A., Brunda M. J., Gately M. K., Wolf S. F., Schreiber R. D., Storkus W. J. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994 Aug 15;153(4):1697–1706. [PubMed] [Google Scholar]
- Noguchi Y., Richards E. C., Chen Y. T., Old L. J. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2219–2223. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddu P., Fantuzzi L., Borghi P., Varano B., Rainaldi G., Guillemard E., Malorni W., Nicaise P., Wolf S. F., Belardelli F. IL-12 induces IFN-gamma expression and secretion in mouse peritoneal macrophages. J Immunol. 1997 Oct 1;159(7):3490–3497. [PubMed] [Google Scholar]
- Rakhmilevich A. L., Turner J., Ford M. J., McCabe D., Sun W. H., Sondel P. M., Grota K., Yang N. S. Gene gun-mediated skin transfection with interleukin 12 gene results in regression of established primary and metastatic murine tumors. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6291–6296. doi: 10.1073/pnas.93.13.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. S., Podlaski F. J., Hulmes J. D., Pan Y. C., Quinn P. M., Wolitzky A. G., Familletti P. C., Stremlo D. L., Truitt T., Chizzonite R. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara H., Lotze M. T. Antitumor effects of interleukin-12 (IL-12): applications for the immunotherapy and gene therapy of cancer. Gene Ther. 1995 Mar;2(2):96–106. [PubMed] [Google Scholar]
- Tahara H., Zeh H. J., 3rd, Storkus W. J., Pappo I., Watkins S. C., Gubler U., Wolf S. F., Robbins P. D., Lotze M. T. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 1994 Jan 1;54(1):182–189. [PubMed] [Google Scholar]
- Tahara H., Zitvogel L., Storkus W. J., Zeh H. J., 3rd, McKinney T. G., Schreiber R. D., Gubler U., Robbins P. D., Lotze M. T. Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector. J Immunol. 1995 Jun 15;154(12):6466–6474. [PubMed] [Google Scholar]
- Takeda K., Seki S., Ogasawara K., Anzai R., Hashimoto W., Sugiura K., Takahashi M., Satoh M., Kumagai K. Liver NK1.1+ CD4+ alpha beta T cells activated by IL-12 as a major effector in inhibition of experimental tumor metastasis. J Immunol. 1996 May 1;156(9):3366–3373. [PubMed] [Google Scholar]
- Tan J., Newton C. A., Djeu J. Y., Gutsch D. E., Chang A. E., Yang N. S., Klein T. W., Hua Y. Injection of complementary DNA encoding interleukin-12 inhibits tumor establishment at a distant site in a murine renal carcinoma model. Cancer Res. 1996 Aug 1;56(15):3399–3403. [PubMed] [Google Scholar]
- Tannenbaum C. S., Wicker N., Armstrong D., Tubbs R., Finke J., Bukowski R. M., Hamilton T. A. Cytokine and chemokine expression in tumors of mice receiving systemic therapy with IL-12. J Immunol. 1996 Jan 15;156(2):693–699. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993 Jul;14(7):335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994 Dec 15;84(12):4008–4027. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- Tsung K., Meko J. B., Peplinski G. R., Tsung Y. L., Norton J. A. IL-12 induces T helper 1-directed antitumor response. J Immunol. 1997 Apr 1;158(7):3359–3365. [PubMed] [Google Scholar]
- Zitvogel L., Couderc B., Mayordomo J. I., Robbins P. D., Lotze M. T., Storkus W. J. IL-12-engineered dendritic cells serve as effective tumor vaccine adjuvants in vivo. Ann N Y Acad Sci. 1996 Oct 31;795:284–293. doi: 10.1111/j.1749-6632.1996.tb52678.x. [DOI] [PubMed] [Google Scholar]
- Zitvogel L., Tahara H., Robbins P. D., Storkus W. J., Clarke M. R., Nalesnik M. A., Lotze M. T. Cancer immunotherapy of established tumors with IL-12. Effective delivery by genetically engineered fibroblasts. J Immunol. 1995 Aug 1;155(3):1393–1403. [PubMed] [Google Scholar]
- Zou J. P., Yamamoto N., Fujii T., Takenaka H., Kobayashi M., Herrmann S. H., Wolf S. F., Fujiwara H., Hamaoka T. Systemic administration of rIL-12 induces complete tumor regression and protective immunity: response is correlated with a striking reversal of suppressed IFN-gamma production by anti-tumor T cells. Int Immunol. 1995 Jul;7(7):1135–1145. doi: 10.1093/intimm/7.7.1135. [DOI] [PubMed] [Google Scholar]