Abstract
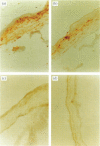
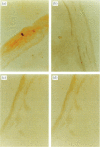
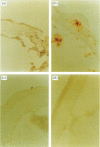
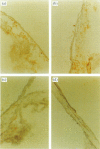
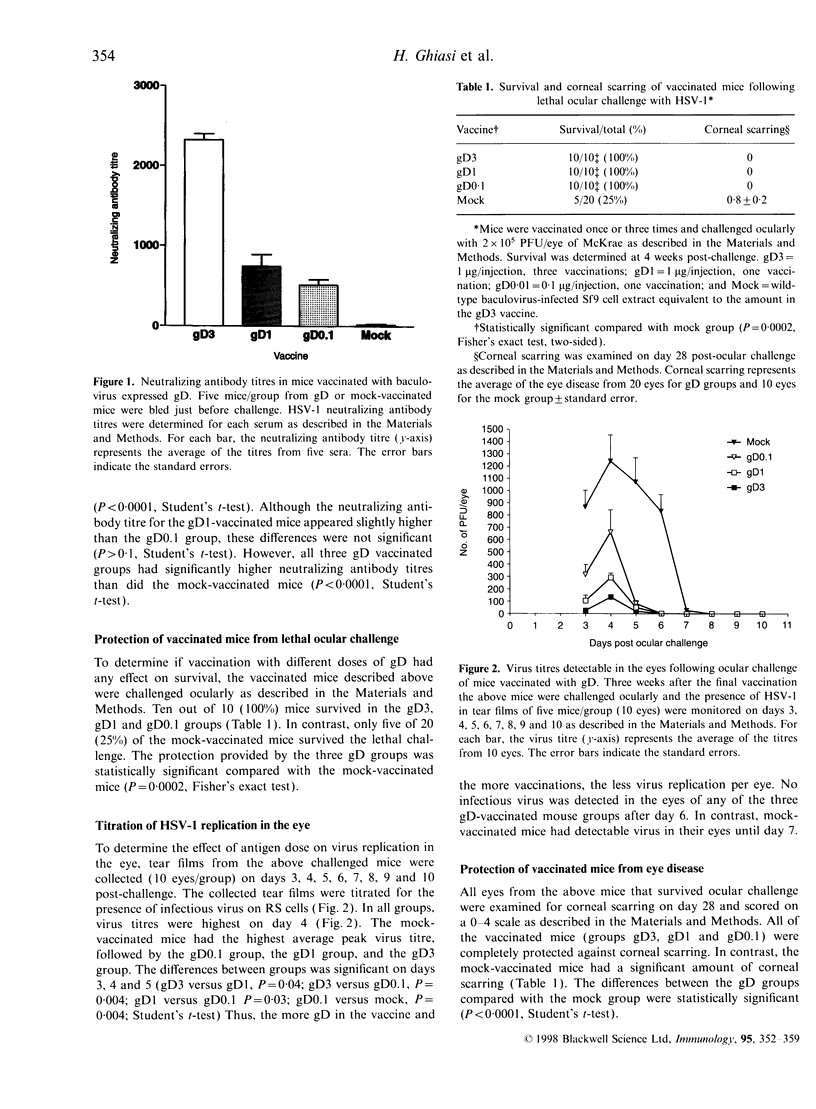
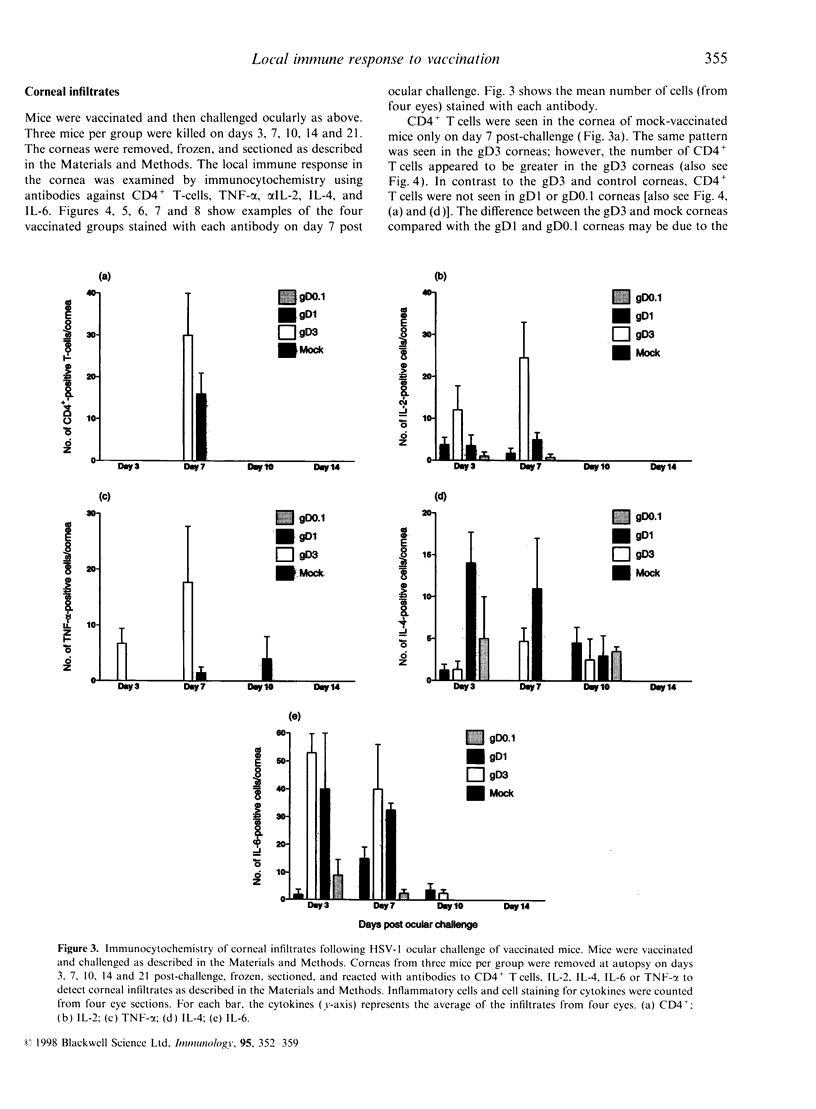
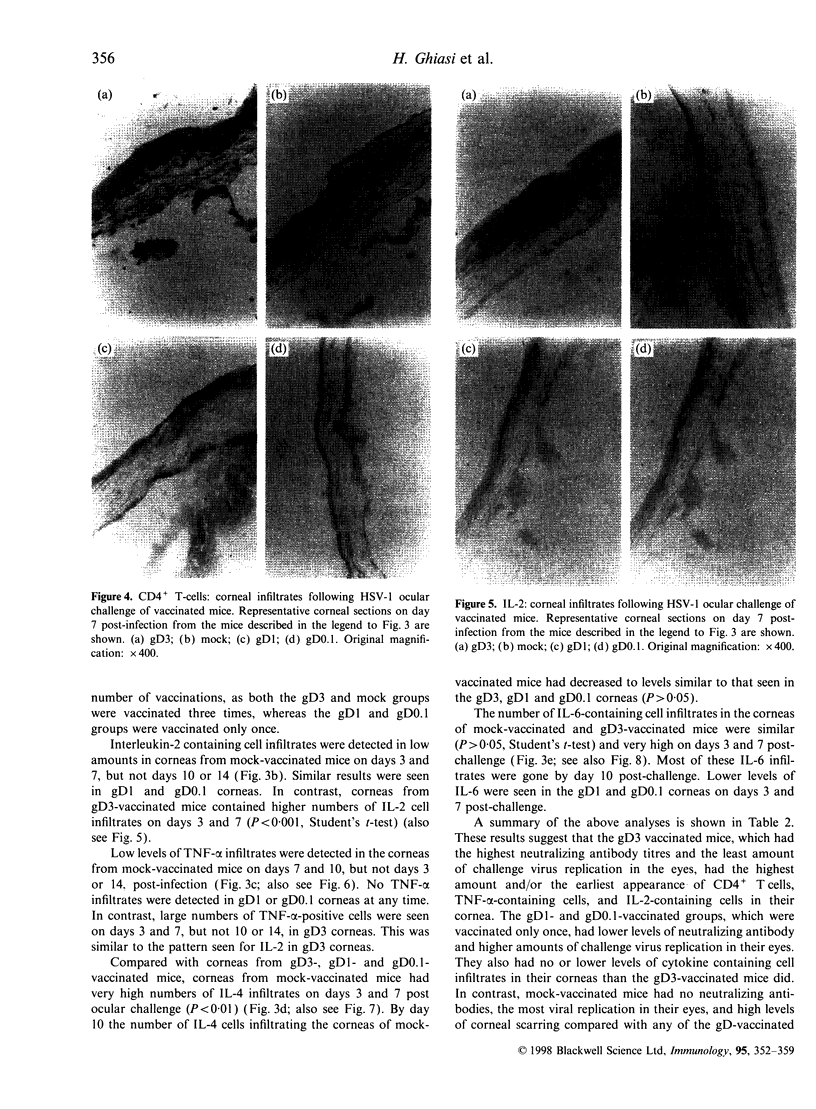
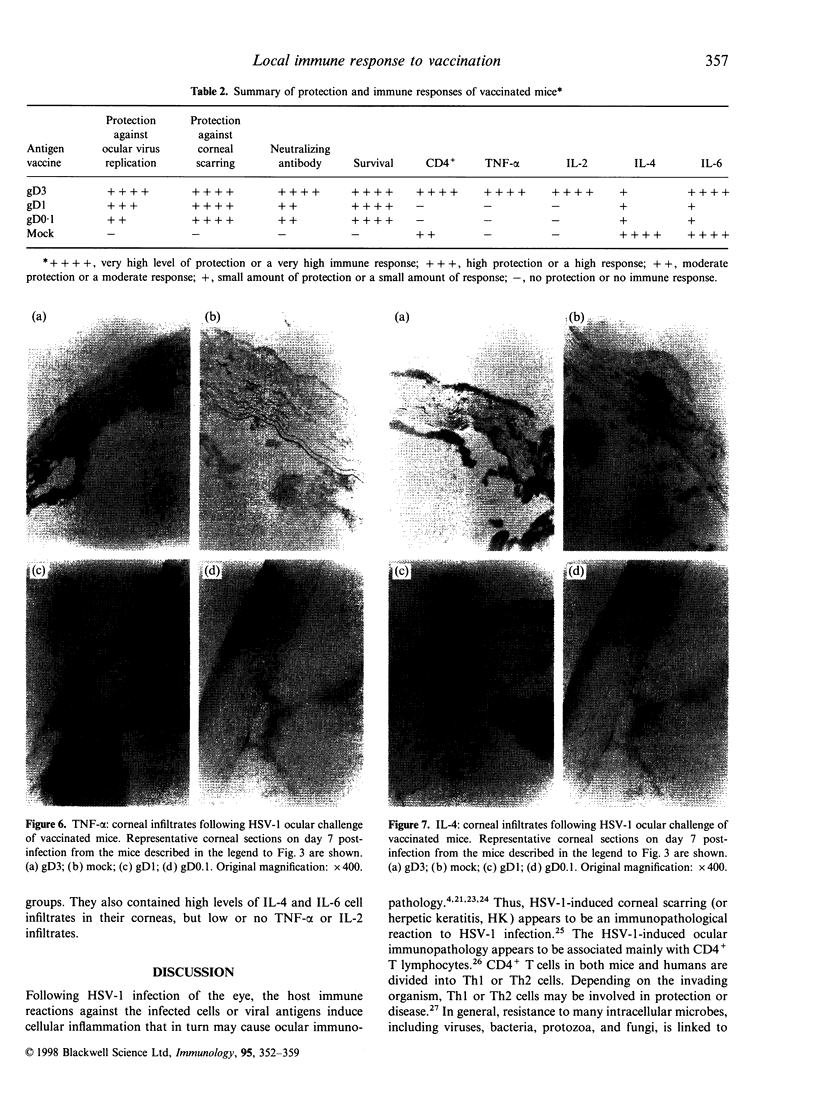
In order to determine the possible correlation of specific immune responses with protection against mortality and ocular disease following ocular herpes simplex virus type 1 (HSV-1) challenge, BALB/c mice were vaccinated with different doses and regimens of baculovirus-expressed gD. Neutralizing antibody, virus titres in the eyes, corneal scarring, and survival were measured. In addition, infiltration into the cornea of CD4+ T cells and cells containing the lymphokines interleukin-2 (IL-2), IL-4, IL-6 and tumour necrosis factor-alpha (TNF-alpha) were monitored on days 3, 7, 10, 14 and 21 post-challenge by immunocytochemistry. The vaccination regimens used induced varying degrees of immune responses and protection upon ocular challenge with HSV-1. Our results suggest that neutralizing antibody was the most important immune response in protecting mice against lethal ocular challenge and corneal scarring. TNF-alpha and IL-2 were not crucial in terms of survival and corneal scarring, since gD1 (one vaccination with 1 microg of gD) and gD0.1 (one vaccination with 0.1 microg of gD), both of which provided high levels of protection, showed no TNF-alpha or IL-2 expression. However, TNF-alpha and IL-2 were crucial in terms of virus clearance from the eyes, since gD3 (three vaccinations with 1 microg of gD), which had less virus in their eyes, had high numbers of TNF-alpha and IL-2 infiltrates. Finally, mock-vaccinated mice were not protected from death and corneal disease following HSV-1 challenge. Eyes of mock-vaccinated mice had little or no TNF-alpha or IL-2 responses and the strongest IL-4 and IL-6 responses.
Full text
PDF

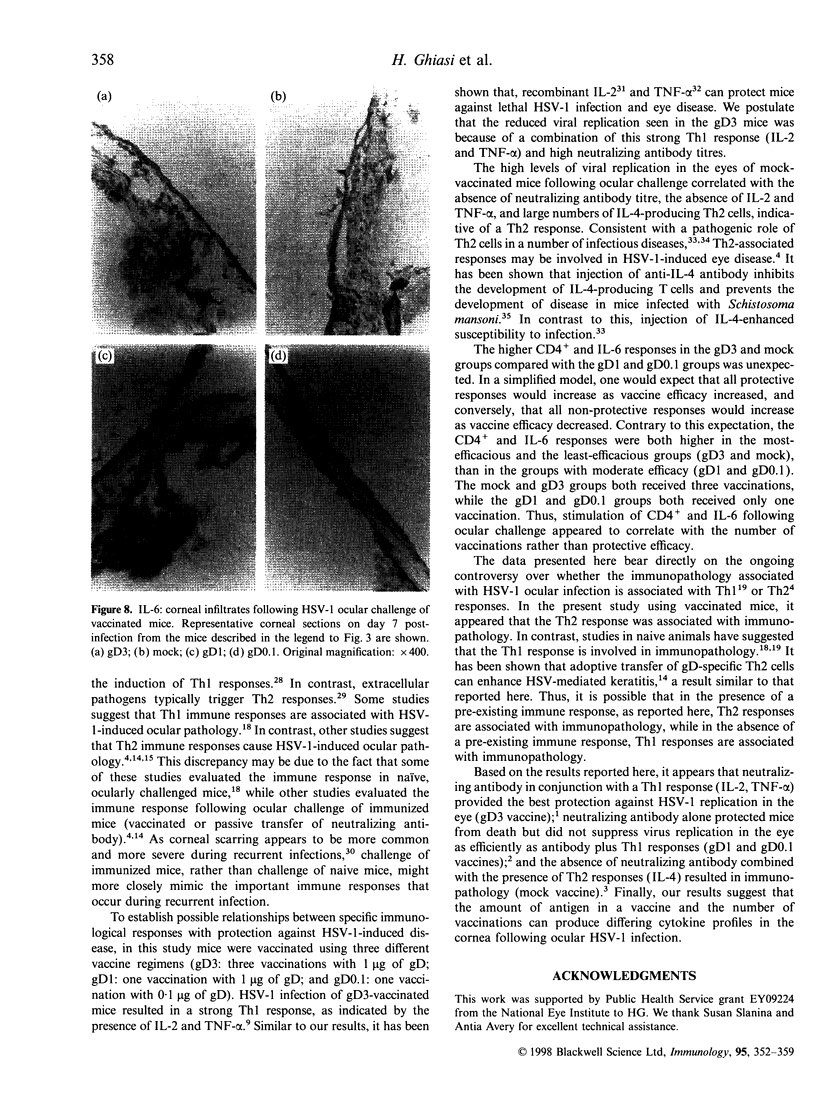

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Murphy K. M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996 Oct 31;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Babu J. S., Kanangat S., Rouse B. T. T cell cytokine mRNA expression during the course of the immunopathologic ocular disease herpetic stromal keratitis. J Immunol. 1995 May 1;154(9):4822–4829. [PubMed] [Google Scholar]
- Bass H., Mosmann T., Strober S. Evidence for mouse Th1- and Th2-like helper T cells in vivo. Selective reduction of Th1-like cells after total lymphoid irradiation. J Exp Med. 1989 Nov 1;170(5):1495–1511. doi: 10.1084/jem.170.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz C., Becker Y. Recombinant interleukin-1 alpha, interleukin-2 and M-CSF-1 enhance the survival of newborn C57BL/6 mice inoculated intraperitoneally with a lethal dose of herpes simplex virus-1. Arch Virol. 1992;124(1-2):83–93. doi: 10.1007/BF01314627. [DOI] [PubMed] [Google Scholar]
- Biron C. A. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol. 1994 Aug;6(4):530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Carmack M. A., Yasukawa L. L., Chang S. Y., Tran C., Saldana F., Arvin A. M., Prober C. G. T cell recognition and cytokine production elicited by common and type-specific glycoproteins of herpes simplex virus type 1 and type 2. J Infect Dis. 1996 Nov;174(5):899–906. doi: 10.1093/infdis/174.5.899. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Williams M. E., Wynn T. A., Finkelman F. D., Seder R. A., Cox T. M., Hieny S., Caspar P., Sher A. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol. 1994 Jul 15;153(2):753–759. [PubMed] [Google Scholar]
- Dawson C. R. Ocular herpes simplex virus infections. Clin Dermatol. 1984 Apr-Jun;2(2):56–66. doi: 10.1016/0738-081x(84)90066-x. [DOI] [PubMed] [Google Scholar]
- Dawson C. R., Togni B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv Ophthalmol. 1976 Sep-Oct;21(2):121–135. doi: 10.1016/0039-6257(76)90090-4. [DOI] [PubMed] [Google Scholar]
- Doymaz M. Z., Foster C. M., Destephano D., Rouse B. T. MHC II-restricted, CD4+ cytotoxic T lymphocytes specific for herpes simplex virus-1: implications for the development of herpetic stromal keratitis in mice. Clin Immunol Immunopathol. 1991 Dec;61(3):398–409. doi: 10.1016/s0090-1229(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Duchini A., Govindarajan S., Santucci M., Zampi G., Hofman F. M. Effects of tumor necrosis factor-alpha and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J Investig Med. 1996 Oct;44(8):474–482. [PubMed] [Google Scholar]
- Easty D. L., Shimeld C., Claoue C. M., Menage M. Herpes simplex virus isolation in chronic stromal keratitis: human and laboratory studies. Curr Eye Res. 1987 Jan;6(1):69–74. doi: 10.3109/02713688709020071. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Bahri S., Nesburn A. B., Wechsler S. L. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Invest Ophthalmol Vis Sci. 1995 Jun;36(7):1352–1360. [PubMed] [Google Scholar]
- Ghiasi H., Cai S., Nesburn A. B., Wechsler S. L. Vaccination with herpes simplex virus type 1 glycoprotein K impairs clearance of virus from the trigeminal ganglia resulting in chronic infection. Virology. 1996 Oct 1;224(1):330–333. doi: 10.1006/viro.1996.0537. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Kaiwar R., Nesburn A. B., Slanina S., Wechsler S. L. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J Virol. 1994 Apr;68(4):2118–2126. doi: 10.1128/jvi.68.4.2118-2126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi H., Wechsler S. L., Kaiwar R., Nesburn A. B., Hofman F. M. Local expression of tumor necrosis factor alpha and interleukin-2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J Virol. 1995 Jan;69(1):334–340. doi: 10.1128/jvi.69.1.334-340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. B., Braciale V. L., Braciale T. J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994 Oct 1;180(4):1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiligenhaus A., Jayaraman S., Soukiasian S., Dorf M., Foster C. S. Glykoprotein-D (5-23)-spezifische Th2-T-Zell-Linie induziert HSV-1-Keratitis. Ophthalmologe. 1995 Aug;92(4):484–491. [PubMed] [Google Scholar]
- Hendricks R. L., Tumpey T. M. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Invest Ophthalmol Vis Sci. 1990 Oct;31(10):1929–1939. [PubMed] [Google Scholar]
- Jayaraman S., Heiligenhaus A., Rodriguez A., Soukiasian S., Dorf M. E., Foster C. S. Exacerbation of murine herpes simplex virus-mediated stromal keratitis by Th2 type T cells. J Immunol. 1993 Nov 15;151(10):5777–5789. [PubMed] [Google Scholar]
- Manickan E., Kanangat S., Rouse R. J., Yu Z., Rouse B. T. Enhancement of immune response to naked DNA vaccine by immunization with transfected dendritic cells. J Leukoc Biol. 1997 Feb;61(2):125–132. doi: 10.1002/jlb.61.2.125. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Schödel F., Iino S., Koike K., Yasuda K., Peterson D., Milich D. R. Distinguishing between acute and symptomatic chronic hepatitis B virus infection. Gastroenterology. 1994 Apr;106(4):1006–1015. doi: 10.1016/0016-5085(94)90761-7. [DOI] [PubMed] [Google Scholar]
- Metcalf J. F., Kaufman H. E. Herpetic stromal keratitis-evidence for cell-mediated immunopathogenesis. Am J Ophthalmol. 1976 Dec;82(6):827–834. doi: 10.1016/0002-9394(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Peterson D. L., Schödel F., Jones J. E., Hughes J. L. Preferential recognition of hepatitis B nucleocapsid antigens by Th1 or Th2 cells is epitope and major histocompatibility complex dependent. J Virol. 1995 May;69(5):2776–2785. doi: 10.1128/jvi.69.5.2776-2785.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran T. M., Isobe H., Fernandez-Sesma A., Schulman J. L. Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J Virol. 1996 Aug;70(8):5230–5235. doi: 10.1128/jvi.70.8.5230-5235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nesburn A. B. Recurrence in ocular herpes simplex infection. Int Ophthalmol Clin. 1975 Winter;15(4):101–110. doi: 10.1097/00004397-197501540-00009. [DOI] [PubMed] [Google Scholar]
- Niemialtowski M. G., Rouse B. T. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992 Nov 1;149(9):3035–3039. [PubMed] [Google Scholar]
- Pearlman E., Lass J. H., Bardenstein D. S., Kopf M., Hazlett F. E., Jr, Diaconu E., Kazura J. W. Interleukin 4 and T helper type 2 cells are required for development of experimental onchocercal keratitis (river blindness). J Exp Med. 1995 Oct 1;182(4):931–940. doi: 10.1084/jem.182.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol-Voth R., Rossol S., Schütt K. H., Corridori S., de Cian W., Falke D. In vivo protective effect of tumour necrosis factor alpha against experimental infection with herpes simplex virus type 1. J Gen Virol. 1991 Jan;72(Pt 1):143–147. doi: 10.1099/0022-1317-72-1-143. [DOI] [PubMed] [Google Scholar]
- Rouse B. T. Virus-induced immunopathology. Adv Virus Res. 1996;47:353–376. doi: 10.1016/S0065-3527(08)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld C., Hill T. J., Blyth W. A., Easty D. L. Passive immunization protects the mouse eye from damage after herpes simplex virus infection by limiting spread of virus in the nervous system. J Gen Virol. 1990 Mar;71(Pt 3):681–687. doi: 10.1099/0022-1317-71-3-681. [DOI] [PubMed] [Google Scholar]
- Shimeld C., Whiteland J. L., Nicholls S. M., Easty D. L., Hill T. J. Immune cell infiltration in corneas of mice with recurrent herpes simplex virus disease. J Gen Virol. 1996 May;77(Pt 5):977–985. doi: 10.1099/0022-1317-77-5-977. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Nesburn A. B., Watson R., Slanina S., Ghiasi H. Fine mapping of the major latency-related RNA of herpes simplex virus type 1 in humans. J Gen Virol. 1988 Dec;69(Pt 12):3101–3106. doi: 10.1099/0022-1317-69-12-3101. [DOI] [PubMed] [Google Scholar]
- Wynn T. A., Cheever A. W. Cytokine regulation of granuloma formation in schistosomiasis. Curr Opin Immunol. 1995 Aug;7(4):505–511. doi: 10.1016/0952-7915(95)80095-6. [DOI] [PubMed] [Google Scholar]