Abstract
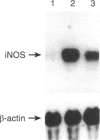
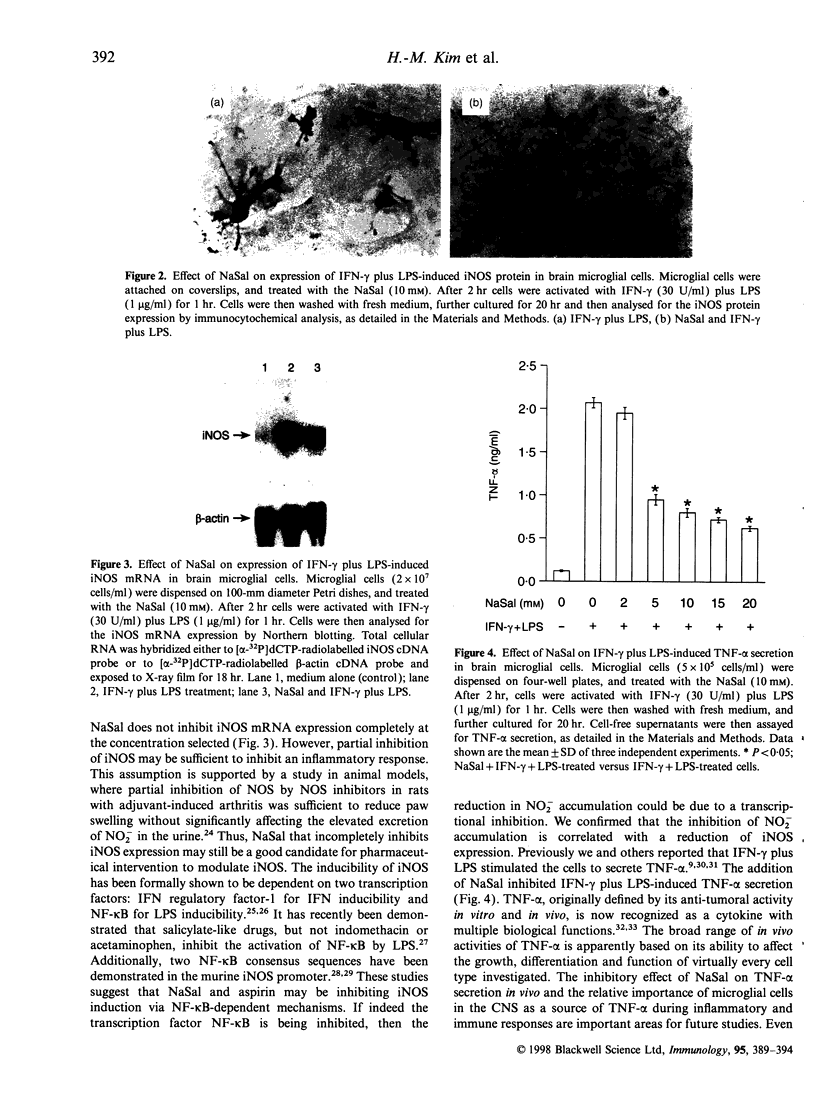
The induction of the inducible nitric oxide synthase (iNOS) has been proposed to play a role in a variety of inflammatory diseases. Sodium salicylate (NaSal) is the most commonly used anti-inflammatory agent. We investigated whether NaSal can diminish the induction of iNOS in murine brain microglial cells. In primary cultures, interferon-gamma (IFN-gamma) or lipopolysaccharide (LPS) separately did not stimulate nitric oxide (NO) production, whereas IFN-gamma combined with LPS synergistically induced iNOS. NaSal inhibited both the production of NO and expression of iNOS in microglial cells. Synergy between IFN-gamma and LPS was mainly dependent on tumour necrosis factor-alpha (TNF-alpha) secretion as the increase of the induction of the iNOS by IFN-gamma plus LPS was associated with the increase of TNF-alpha secretion and IFN-gamma plus LPS-induced TNF-alpha secretion by microglial cells was decreased by the treatment with NaSal. These results suggest a possible use of NaSal in managing inflammation of the central nervous system through inhibition of the iNOS induction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeberhard E. E., Henderson S. A., Arabolos N. S., Griscavage J. M., Castro F. E., Barrett C. T., Ignarro L. J. Nonsteroidal anti-inflammatory drugs inhibit expression of the inducible nitric oxide synthase gene. Biochem Biophys Res Commun. 1995 Mar 28;208(3):1053–1059. doi: 10.1006/bbrc.1995.1441. [DOI] [PubMed] [Google Scholar]
- Amin A. R., Vyas P., Attur M., Leszczynska-Piziak J., Patel I. R., Weissmann G., Abramson S. B. The mode of action of aspirin-like drugs: effect on inducible nitric oxide synthase. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7926–7930. doi: 10.1073/pnas.92.17.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouet I., Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995 Jan 17;206(2):533–540. doi: 10.1006/bbrc.1995.1076. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Hu S., Molitor T. W., Shaskan E. G., Peterson P. K. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992 Oct 15;149(8):2736–2741. [PubMed] [Google Scholar]
- Clancy R. M., Levartovsky D., Leszczynska-Piziak J., Yegudin J., Abramson S. B. Nitric oxide reacts with intracellular glutathione and activates the hexose monophosphate shunt in human neutrophils: evidence for S-nitrosoglutathione as a bioactive intermediary. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3680–3684. doi: 10.1073/pnas.91.9.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Frei K., Bodmer S., Schwerdel C., Fontana A. Astrocyte-derived interleukin 3 as a growth factor for microglia cells and peritoneal macrophages. J Immunol. 1986 Dec 1;137(11):3521–3527. [PubMed] [Google Scholar]
- Grilli M., Pizzi M., Memo M., Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science. 1996 Nov 22;274(5291):1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- Hassan N. F., Rifat S., Campbell D. E., McCawley L. J., Douglas S. D. Isolation and flow cytometric characterization of newborn mouse brain-derived microglia maintained in vitro. J Leukoc Biol. 1991 Jul;50(1):86–92. doi: 10.1002/jlb.50.1.86. [DOI] [PubMed] [Google Scholar]
- Jun C. D., Choi B. M., Kim H. M., Chung H. T. Involvement of protein kinase C during taxol-induced activation of murine peritoneal macrophages. J Immunol. 1995 Jun 15;154(12):6541–6547. [PubMed] [Google Scholar]
- Jun C. D., Hoon-Ryu, Um J. Y., Kim T. Y., Kim J. M., Kang S. S., Kim H. M., Chung H. T. Involvement of protein kinase C in the inhibition of nitric oxide production from murine microglial cells by glucocorticoid. Biochem Biophys Res Commun. 1994 Mar 15;199(2):633–638. doi: 10.1006/bbrc.1994.1275. [DOI] [PubMed] [Google Scholar]
- Jun C. D., Kim S. H., Soh C. T., Kang S. S., Chung H. T. Nitric oxide mediates the toxoplasmastatic activity of murine microglial cells in vitro. Immunol Invest. 1993 Dec;22(8):487–501. doi: 10.3109/08820139309084178. [DOI] [PubMed] [Google Scholar]
- Kim H. M., Hirota S., Chung H. T., Ohno S., Osada S., Shin T., Ko K. I., Kim J. B., Kitamura Y., Nomura S. Differential expression of protein kinase C genes in cultured mast cells derived from normal and mast-cell-deficient mice and mast cell lines. Int Arch Allergy Immunol. 1994 Nov;105(3):258–263. doi: 10.1159/000236766. [DOI] [PubMed] [Google Scholar]
- Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994 Aug 12;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Activation of mouse peritoneal macrophages during infection with Salmonella typhimurium does not result in enhanced intracellular killing. J Immunol. 1990 Jun 1;144(11):4340–4346. [PubMed] [Google Scholar]
- Lowenstein C. J., Alley E. W., Raval P., Snowman A. M., Snyder S. H., Russell S. W., Murphy W. J. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994 Sep 23;78(6):927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumour necrosis factor. Polypeptide mediator network. 1987 Mar 26-Apr 1Nature. 326(6111):330–331. doi: 10.1038/326330a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pow D. V., Perry V. H., Morris J. F., Gordon S. Microglia in the neurohypophysis associate with and endocytose terminal portions of neurosecretory neurons. Neuroscience. 1989;33(3):567–578. doi: 10.1016/0306-4522(89)90409-0. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Walter U. NO at work. Cell. 1994 Sep 23;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Scuderi P., Sterling K. E., Lam K. S., Finley P. R., Ryan K. J., Ray C. G., Petersen E., Slymen D. J., Salmon S. E. Raised serum levels of tumour necrosis factor in parasitic infections. Lancet. 1986 Dec 13;2(8520):1364–1365. doi: 10.1016/s0140-6736(86)92007-6. [DOI] [PubMed] [Google Scholar]
- Stefanovic-Racic M., Meyers K., Meschter C., Coffey J. W., Hoffman R. A., Evans C. H. N-monomethyl arginine, an inhibitor of nitric oxide synthase, suppresses the development of adjuvant arthritis in rats. Arthritis Rheum. 1994 Jul;37(7):1062–1069. doi: 10.1002/art.1780370712. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Mitchell J. A., Appleton I., Tomlinson A., Bishop-Bailey D., Croxtall J., Willoughby D. A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994 Feb 18;269(7):4705–4708. [PubMed] [Google Scholar]
- Xie Q. W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993 Jun 1;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H. J., Jun C. D., Kim J. M., Rim G. N., Kim H. M., Chung H. T. Phorbol ester synergistically increases interferon-gamma-induced nitric oxide synthesis in murine microglial cells. Neuroimmunomodulation. 1994 Nov-Dec;1(6):377–382. doi: 10.1159/000097191. [DOI] [PubMed] [Google Scholar]