Abstract
Recombinant adeno-associated virus (rAAV) has become an attractive tool for gene therapy because of its ability to transduce both dividing and nondividing cells, elicit a limited immune response, and the capacity for imparting long-term transgene expression. Previous studies have utilized rAAV serotype 2 predominantly and found that transduction of vascular cells is relatively inefficient. The purpose of the present study was to evaluate the transduction efficiency of rAAV serotypes 1 through 5 in human and rat aortic endothelial cells (HAEC and RAEC). rAAV vectors with AAV2 inverted terminal repeats containing the human α1-antitrypsin (hAAT) gene were transcapsidated using helper plasmids to provide viral capsids for the AAV1 through 5 serotypes. True type rAAV2 and 5 vectors encoding β-galactosidase or green fluorescence protein were also studied. Infection with rAAV1 resulted in the most efficient transduction in both HAEC and RAEC compared to other serotypes (p < 0.001) at 7 days posttransduction. Interestingly, expression was increased in cells transduced with rAAV5 to levels surpassing rAAV1 by day 14 and 21. Transduction with rAAV1 was completely inhibited by removal of sialic acid with sialidase, while heparin had no effect. These studies are the first demonstration that sialic acid residues are required for rAAV1 transduction in endothelial cells. Transduction of rat aortic segments ex vivo and in vivo demonstrated significant transgene expression in endothelial and smooth muscle cells with rAAV1 and 5 serotype vectors, in comparison to rAAV2. These results suggest the unique potential of rAAV1 and rAAV5-based vectors for vascular-targeted gene-based therapeutic strategies.
OVERVIEW SUMMARY
Gene delivery to the vasculature has significant potential as a therapeutic strategy for several cardiovascular disorders including atherosclerosis, hypertension, angiogenesis, and chronic vascular rejection of transplanted organs. However, limited advances have been made in achieving successful vascular endothelial cell gene transfer. The results of the present study demonstrate the superior efficacy of recombinant adeno-associated virus (rAAV) serotype 1 and 5 vectors in comparison to the traditionally used rAAV serotype 2 in transduction of primary vascular endothelial and smooth muscle cells in vitro. Our results have identified sialic acid residues for rAAV1 transduction in endothelial cells, similar to rAAV5. Transduction of rat aortic segments demonstrated significant transgene expression in endothelial and smooth muscle cells with rAAV1 and 5 serotype vectors both ex vivo and in vivo, while rAAV2 showed no significant transduction. These results suggest significant advantages of using alternative rAAV serotypes 1 and 5 for vascular-targeted gene delivery.
INTRODUCTION
The vascular endothelium participates in many physiological and pathophysiologic processes including vascular permeability, angiogenesis, inflammation, tumor progression, and rejection of transplanted organs (Gonzalez and Selwyn, 2003). Damage to the endothelium has been implicated in the pathogenesis of many diseases including atherosclerosis, hypertension, ischemic heart disease, diabetic microangiopathy, vasculitis, thromboembolic disorders, and cancer. Thus, there is growing interest in targeting endothelial cells using gene therapy-based techniques (Katusic, 2002; Crook and Akyurek, 2003; Work et al., 2003). Products of transduced genes can not only affect the endothelium per se but also may influence the local and systemic milieu through paracrine or endocrine effects. However, limited progress has thus far been made in gene therapy strategies for endothelial cells. Transcytosis, regular replacement of endothelial cells and an abundance of extracellular matrix are but a few factors that limit the efficacy of transduction in endothelial cells (Pajusola et al., 2002; Pascariu et al., 2004).
Transient gene expression in endothelial cells has been achieved using cationic liposomes and adenoviral and retroviral vectors (Steg et al., 1994; Landau et al., 1995; Luo et al., 1999; Matsumura et al., 1999; Savontaus et al., 2002; Griese et al., 2003; Wang et al., 2003). In addition to transient expression, some of these modalities have often been associated with adverse host immune responses, particularly with the use of adenoviral vectors (Liu and Muruve, 2003). The unique properties of recombinant adeno-associated virus (rAAV) vectors including long-term stable expression (Flotte et al., 1993; Xiao et al., 1996; Song et al., 1998), the absence of viral coding sequences and a relatively low host immunogenic response makes rAAV an ideal candidate for vascular gene delivery. rAAV has been used to deliver genes of potential therapeutic relevance in several human clinical trials (Kay et al., 2000; Flotte et al., 2003). However, most applications for rAAV vectors have thus far been limited to tissues efficiently transduced by the vector, namely skeletal muscle, neurons, and hepatocytes (Kaplitt et al., 1994; Xiao et al., 1996; Peel et al., 1997; Snyder et al., 1997; Song et al., 1998).
There are eight known serotypes of AAV with the designations AAV1–8. Of these, AAV7 and AAV8 have only recently been described (Gao et al., 2002). Most previous studies of rAAV have utilized serotype 2-derived vectors and have reported relatively insufficient transduction of vascular endothelial cells (Gnatenko et al., 1997; Maeda et al., 1997; Richter et al., 2000; Nicklin et al., 2001; Dishart et al., 2003; Vassalli et al., 2003). A few studies have modified rAAV2 vectors with endothelial specific ligands and shown increased transduction in vascular cells (Nicklin et al., 2001; Muller et al., 2003; White et al., 2004). Recent reports have demonstrated that alternative serotypes of rAAV, particularly AAV1 and AAV5, have higher transduction efficiency in tissues such as skeletal muscle, retina, pancreatic islets, liver and neurons (Chiorini et al., 1999; Alisky et al., 2000; Chao et al., 2000; Davidson et al., 2000; Zabner et al., 2000; Flotte et al., 2001; Walters et al., 2001; Auricchio et al., 2002; Rabinowitz et al., 2002). Each AAV serotype interacts with specific receptors and may account for the differential tissue tropism (Summerford and Samulski, 1998; Qing et al., 1999; Kaludov et al., 2001; Walters et al., 2001; Hauck and Xiao, 2003). The purpose of the present study was to evaluate the transduction efficiency of rAAV serotypes for gene delivery in vascular endothelial and smooth muscle cells.
MATERIALS AND METHODS
Cell culture
Human aortic endothelial cells (HAEC) and smooth muscle cells (HASMC) were obtained from Clonetics (Walkersville, MD) and were grown in endothelial basal medium (EBM) supplemented with 10% fetal bovine serum, gentamicin, amphotericin B, hydrocortisone, human epidermal growth factor (EGF), and bovine brain extract (Clonetics). These cells stain positively for endothelial-specific markers including factor VIII-related von Willebrand factor and platelet endothelial cell adhesion molecule-1 (anti-rat CD31, Antigenix America, Huntington Station, NY) and are able to take up DiI-Ac-LDL (Biomedical Technologies Inc., Stoughton, MA). HASMC were grown in smooth muscle cell basal medium with supplements including human EGF, insulin, bovine serum albumin, dexamethasone and gentamicin/amphotericin-B (Clonetics). These cells stain positive for α-smooth muscle cell actin. For these studies, cells were used for up to 6–7 passages.
Isolation of rat aortic endothelial cells
Male Lewis rats (Harlan Sprague Dawley Inc., Indianapolis, IN) between 220–260 g were anesthetized with pentobarbital (30–40 mg/kg body weight, intraperitoneally). The aorta was isolated, cut into approximately 1-mm rings, and 15–20 rings placed on the bottom of 100-mm dishes and cultured in complete EBM at pH 7.45 without disturbance for 60 hr. The rings were then discarded and the cells maintained as regular endothelial cell culture. The rat aortic endothelial cells (RAEC) were confirmed as endothelial in origin by using DiI-Ac-LDL direct labeling and indirect immunofluorescence microscopy using anti-rat CD31 antibody staining. The cells were maintained in the same culture conditions as HAEC with medium containing 10% fetal bovine serum at 37°C, 5% CO2 and 95% room air. These cells were also used for up to 6–7 passages.
Construction and preparation of rAAV vectors
rAAV vectors expressing β-galactosidase (β-gal), green fluorescence protein (GFP), human α1-antitrypsin (hAAT) and luciferase-enhanced yellow fluorescent protein (Luc-EYFP), respectively, were generated and purified by previously described methods (Chiorini et al., 1999; Zolotukhin et al., 1999, 2002). rAAV2- and rAAV5-β-gal vectors were constructed using an expression cassette consisting of a Rous sarcoma virus (RSV) long-terminal repeat promoter and a nuclear targeted β-gal transgene, flanked by either AAV2-inverted terminal repeats (ITR) or AAV5-ITRs (kindly provided by Dr. Sandra Afione, Molecular Hematology Branch, National Institutes of Health, Bethesda, MD) (Chiorini et al., 1999). The true-type rAAV2 and 5-β-gal vectors were packaged by cotransfection of vector plasmid with the 5RepCapB helper plasmid into Ad5-infected COS cells and purified by cesium chloride ultracentrifugation as previously described (Chiorini et al., 1999). True-type rAAV2 and rAAV5-GFP vectors encoding a “humanized” GFP cDNA (Zolotukhin et al., 1996) driven by a cytomegaloviruschicken β-actin hybrid (CBA) promoter and flanked by either AAV2 or AAV5 ITRs were constructed and purified by iodixanol gradient centrifugation and anion-exchange (Q-Sepharose) chromatography as described previously (Zolotukhin et al., 2002). rAAV vectors containing hAAT or Luc-EYFP driven by the CBA promoter and flanked by AAV2-ITRs were constructed and packaged using serotype-specific helper plasmids as described previously (Zolotukhin et al., 2002). The physical titers of vector preparations were assessed by quantitative competitive polymerase chain reaction (PCR) and dot-blot analysis. The rAAV2 production was performed according to the previously described method of double transfection of a permissive human cell line (HEK293) (Zolotukhin et al., 1999). Plasmid pDG containing both the AAV2 rep and cap genes as well as a subset of Ad5 genes (E2a, E4, and VA-RNA; Grimm et al., 1998) were cotransfected along with rAAV2-GFP vector plasmid into HEK293 cells (Ela+, Elb+) grown in cell factories (Nalge Nunc, Rochester, NY). Cells were harvested and lysed by freeze-thaw methods to release virions. The virus-containing supernatant was subsequently purified by iodixanol gradient ultracentrifugation followed by heparin sepharose affinity chromatography. Purity of preparations was determined by silver stained sodium dodecyl sulfate (SDS) acrylamide gel electrophoresis. Infectious center assays were used to determine rAAV virus titer, with dot-blot analysis performed to quantify the titer of the rAAV physical particles and to determine the particle to infectivity ratio. The particle to infectivity ratio averaged approximately 10 in all the rAAV preparations. All rAAV2 preparations were tested in HEK293 cells prior to being used in the experiments.
Cellular transduction
HAEC, RAEC, and HASMC were cultured in 24-well plates for 2 days and transduced with each of the rAAV serotypes containing reporter genes (i.e., GFP, hAAT, β-gal, Luc-EYFP) at approximately 50% cellular confluency. Negative control wells included cells not infected with virus. The cells were washed twice with serum-free medium followed by the addition of 0.3 ml per well serum-free medium. The vectors were then added to the cells at a multiplicity of infection (MOI) as described below and incubated for 30 min at 37°C, 5% CO2 and 95% room air, after which 1 ml per well of complete medium was added. HAEC were transduced with rAAV5- or rAAV2-β-gal vectors at a MOI of 10, 100, and 1000 particles per cell. HAEC were transduced with rAAV-GFP vectors at a MOI of 1000 particles per cell (both rAAV2 and 5) and 100,000 particles per cell (rAAV2 only). Adenovirus (Ad5) coinfection at a MOI of 1 was used to accelerate leading strand synthesis and transgene expression was confirmed at day 3 after transduction.
rAAV serotype 1–5 vectors encoding hAAT were used to transduce HAEC, HASMC, and RAEC at a MOI of 1000. No Ad coinfection was used in these experiments. Medium was collected at 7 days after transduction for hAAT measurement. For RAEC, the media was collected at days 7, 14, and 21 after transduction. HAEC were transduced with rAAV1-Luc-EYFP serotype 1 at a MOI of 1000 particles per cell in the absence of Ad5 coinfection. Seven days after transduction, cells were lysed using reporter lysis buffer for luciferase activity, as per the manufacturer's protocol (Promega, Madison, WI).
Transduction of rat aortic segments ex vivo and in vivo
Male Lewis rats (Harlan Sprague Dawley, Inc.) between 220–260 g were anesthetized with pentobarbital (30–40 mg/kg body weight, intraperitoneally). The studies were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. For the ex vivo studies, the aorta was isolated, flushed with phosphate-buffered saline (PBS), cut into 1.5-cm segments and an atraumatic vascular clamp was placed on one end. After intraluminal instillation of 25 μl of rAAV-Luc-EYFP serotype 1, 2 or 5 (1 × 1013 viral particles per milliliter; n = 3 per serotype) the other end of the vascular segment was clamped. This titer was used following pilot studies, where lower titers of rAAV1 and rAAV5 (1 × 1010–1012 viral particles per milliliter) showed no significant transduction. No Ad5 coinfection was used. The tissue was placed in serum-free EBM in a 35-mm tissue culture dish and incubated for 30 min at 37°C, with 95% room air and 5% CO2. Complete EBM was added and the media was changed every 2 days. Tissues were collected at 7 days after transduction for analysis of transgene expression. Half the segment from each group was dounce homogenized in 2.5× passive lysis buffer (Promega) for measurement of luciferase activity, expressed per milligram of wet weight of tissue. The other half was fixed in 10% formalin and processed for immunohistochemistry.
For in vivo studies, the infrarenal segment of the abdominal aorta was exposed under anesthesia (2.5% isoflurane) via a midline incision, clamped just below the renal arteries, a vascular cannula introduced into the isolated vascular segment, and the aorta flushed with normal saline. The segment was transduced intraluminally with rAAV1, rAAV2, or rAAV5-Luc-EYFP (20 μl, 1 × 1013 viral particles per milliliter) or an equal volume of saline (n = 3 per group). No Ad5 coinfection was used. The vector was allowed to dwell in the aorta for 30 min before removal of the cannula and suturing of the incision. The rats were sacrificed at 3 weeks after transduction and analyzed for transgene expression by immunohistochemistry.
Cell surface modification with sialidase and heparin competition
To identify the potential receptor(s) involved in rAAV1-mediated transduction, endothelial cells were exposed to rAAV1, rAAV2, or rAAV5 in the presence of heparin or sialidase. HAEC were rinsed with fresh medium and incubated thereafter in medium containing 200 mU/ml of sialidase (neuraminidase III from Vibrio cholerae, Sigma, St. Louis, MO) for 2 hr at 37°C. The cells were washed again prior to infection with rAAV-Luc-EYFP serotype 1, 2, or 5 (MOI: 1000 viral particles per cell). In heparin competition studies, the vector was pretreated for 30 min in serum-free medium containing 20 μg/ml of soluble heparin from porcine intestinal mucosa (Sigma Chemical Co.). Transgene expression was evaluated at 7 days after transduction. The concentrations of sialidase and heparin were based on previously published studies in the literature (Walters et al., 2001).
Cell viability assays
To evaluate the cellular effects of exposure to sialidase, cell viability was determined in HAEC incubated with sialidase (0, 50, 100, 200, and 400 mU/ml). Cells were exposed to sialidase for 2 hr in serum-free medium, washed, and complete medium was added. Trypan blue staining was performed at 24 hr and showed that more than 98% of cells excluded the dye suggesting integrity of the cell membrane. A clonogenic assay was also performed to evaluate the ability of the cells to survive and proliferate following exposure to sialidase. In these experiments, equal number of HAEC were plated into 24-well dishes and incubated with sialidase as above. Twenty-four hours later, cells were washed with PBS, trypsinized, and seeded at a density of 1000 cells per 100-mm dish. Cells were maintained in culture for 5 days at 37°C. The dishes were then washed with PBS, fixed with cold methanol and stained with a 0.2% crystal violet solution. Colonies greater than 0.5 mm in size were counted using a dissecting microscope. The clonogenic capability of the cells is presented as the percentage of colonies formed in comparison to the control cells.
Measurement of reporter gene expression
The level of hAAT in the cell culture medium was determined by standard enzyme-linked immunosorbent assay (ELISA) as previously described (Song et al., 1998). Microtiter plates (Immoulon 4; Dynex Technologies, Chantilly, VA) were coated with 100 μl of goat anti-hAAT (diluted 1:200, Sigma) in Voller's buffer overnight at 4°C. Duplicate standard curves of hAAT and serially diluted unknown samples were incubated in the plate at 37°C for 1 hr. After blocking with 3% bovine serum albumin (BSA), rabbit anti-hAAT (diluted 1:1000, Roche Molecular Biochemicals, Indianapolis, IN) was reacted with the captured antigen at 37°C for 1 hr. A third antibody, goat anti-rabbit immunoglobulin G (IgG) conjugated with peroxidase (1:800 diluted; Roche Molecular Biochemicals), was incubated at 3°C for 1 hr. The plate was washed with PBS-Tween 20 between reactions. After reaction with the substrate (o-phenylenediamine, Sigma Immunochemical), plates were read at 490 nm on an MRX microplate reader (Dynex Technologies). β-Gal staining was performed as described previously (Flotte et al., 2001). GFP expression was monitored using an inverted fluorescent microscope (Olympus S × 70). For luciferase assay, cells/tissue samples were lysed in 2.5× passive lysis buffer (Promega) and luciferase activity was measured using a Luciferase assay kit (Promega).
Immunohistochemical analysis of luciferase expression
Immunohistochemistry was performed as previously described (Kapturczak et al., 2002). Briefly, tissue sections were pretreated with Trilogy antigen retrieval solution (Cell Marque, Austin, TX) at 95°C for 25 min then incubated with goat antiluciferase (1:1500, Abcam, Oshkosh, WI) for 1 hr at room temperature. Immunoreactivities were visualized by incubation with biotinylated horse anti-goat secondary antibody (1:400, Vector Laboratories, Burlingame, CA) followed by streptavidin-biotin-peroxidase complex technique using Vectastain Elite ABC kit (Vector Labsoratories) with diaminobenzidine as chromogen. Hematoxylin was used as counterstain. As a negative control, sections were reacted with normal goat IgG instead of the specific antibody. Images were captured using a Leica DMIRB microscope.
Statistical analysis
Results are derived from at least three independent experiments performed each time using 4–8 replicates. Student's t test, analysis of variance (ANOVA), and Student-Newman-Keuls test analysis were used for multiple comparisons and a p value of < 0.05 was considered significant.
RESULTS
Transduction of HAEC with rAAV2 and rAAV5 encoding μ-gal or GFP
Previous studies have demonstrated that it is difficult to achieve successful gene transfer into endothelial cells using nonviral or viral vectors including rAAV (Gnatenko et al., 1997; Nicklin et al., 2001; Vassalli et al., 2003). However, with regard to rAAV previously most investigations have been performed using rAAV serotype 2. Recent studies have demonstrated that transduction efficiency of alternative rAAV serotypes varies in different cells and tissues (Alisky et al., 2000; Chao et al., 2000; Davidson et al., 2000; Zabner et al., 2000; Flotte et al., 2001; Walters et al., 2001; Auricchio et al., 2002; Rabinowitz et al., 2002). In this experiment, we compared the efficacy of rAAV2 and 5 in the transduction of HAEC. We first transduced HAEC with rAAV2-β-gal and rAAV5-β-gal, driven by the RSV promoter, at a MOI of 1000, 100, and 10 viral particles per cell, respectively; all with Ad5 coinfection (MOI of 1). In HAEC transduced with rAAV5-β-gal, there was modest expression of β-gal at a MOI of 10 and 100 viral particles (4% and 9%, respectively) and significant expression at a MOI of 1000 viral particles (38%) at 72 hr posttransduction (Fig. 1A-1C). No expression was observed in cells transduced with rAAV2-β-gal at similar MOI (Fig. 1D-1F). We then transduced HAEC with the same serotype vectors carrying GFP as a reporter gene and driven by a CBA promoter. When HAEC were infected with rAAV5-GFP (MOI 1,000) with Ad5 co-infection, significant GFP expression (49%) was observed at 72 hr posttransduction (Fig. 1H). However, only very low GFP expression (less than 5%) was seen with rAAV2-GFP, even when the cells were infected with a 100-fold higher MOI (106 viral particles per cell) (Fig. 1G). Similar results were obtained with these vectors in the absence of Ad5 helper virus, though the expression was delayed (7–10 days). These results indicate that under our experimental conditions rAAV5 is superior to rAAV2 in transduction of HAEC.
FIG. 1.
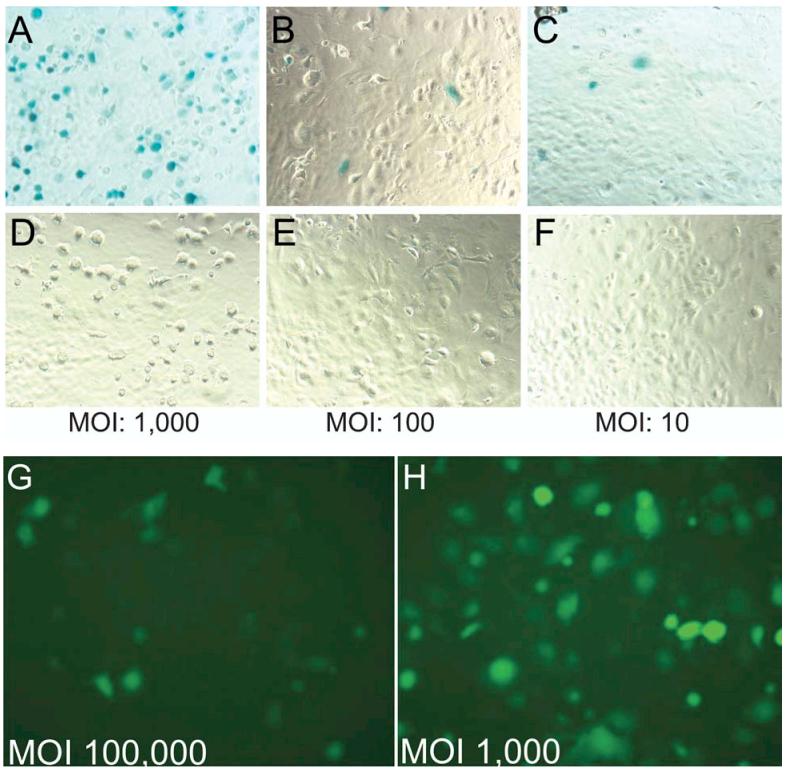
Transduction of human aortic endothelial cells (HAEC) with recombinant adeno-associated virus 5 (rAAV5) or rAAV2-β-galactosidase (β-gal) and green fluorescence protein (GFP). The expression cassette for rAAV-β-gal consisted of a Rous sarcoma virus (RSV) long-terminal repeat promoter, a poly A, sequence and a nuclear targeted β-gal transgene, flanked by either rAAV2-inverted terminal repeats (ITRs) or rAAV5-ITRs. Panels (A), (B), and (C) represent HAEC infected with rAAV5 at multiplicity of infection (MOI) of 1000, 100, and 10 viral particles per cell, respectively. Panels (D), (E), and (F) represent HAEC-infected rAAV2 with MOI of 1000, 100, and 10 viral particles per cell, respectively. Adenovirus (Ad) coinfection (MOI of 1) was used to accelerate leading strand synthesis and transgene expression was confirmed by β-gal staining (blue color) at day 3 after transduction. Panels (G) and (H) represent HAEC transduced with vector constructs for rAAV2 (MOI 100,000 viral particles per cell) and rAAV5 (MOI of 1000 viral particles per cell), respectively. The vector consisted of a cytomegalovirus (CMV)/chicken β actin (CBA) hybrid promoter, a chimeric intron, “humanized” GFP cDNA, a poly A sequence and flanked by either rAAV2-ITRs or rAAV5-ITRs. Ad coinfection (MOI of 1) was used to accelerate transgene expression which was confirmed by immunofluorescence for GFP.
HAEC, RAEC, and HASMC transduced with rAAV1-5 CBA-hAAT
To explore the transduction characteristics of rAAV serotypes, human vascular cells, HAEC and HASMC, were infected with rAAV1-5 CBA-hAAT. No Ad5 coinfection was used in these experiments. As shown in Figure 2, at a MOI of 1000 viral particles per cell, infection with rAAV serotype 1 resulted in the most efficient transduction as assessed by expression of hAAT protein (ng/ml) in HAEC and HASMC culture supernatants (p < 0.001) versus other serotypes. Seven days after infection, maximal hAAT levels (ng/ml) were seen with rAAV serotype 1 (Fig. 2B) in HAEC. In HASMC, significant levels of hAAT were observed with serotype 1, followed by serotype 3, 2, and 5 (Fig. 2C).
FIG. 2.
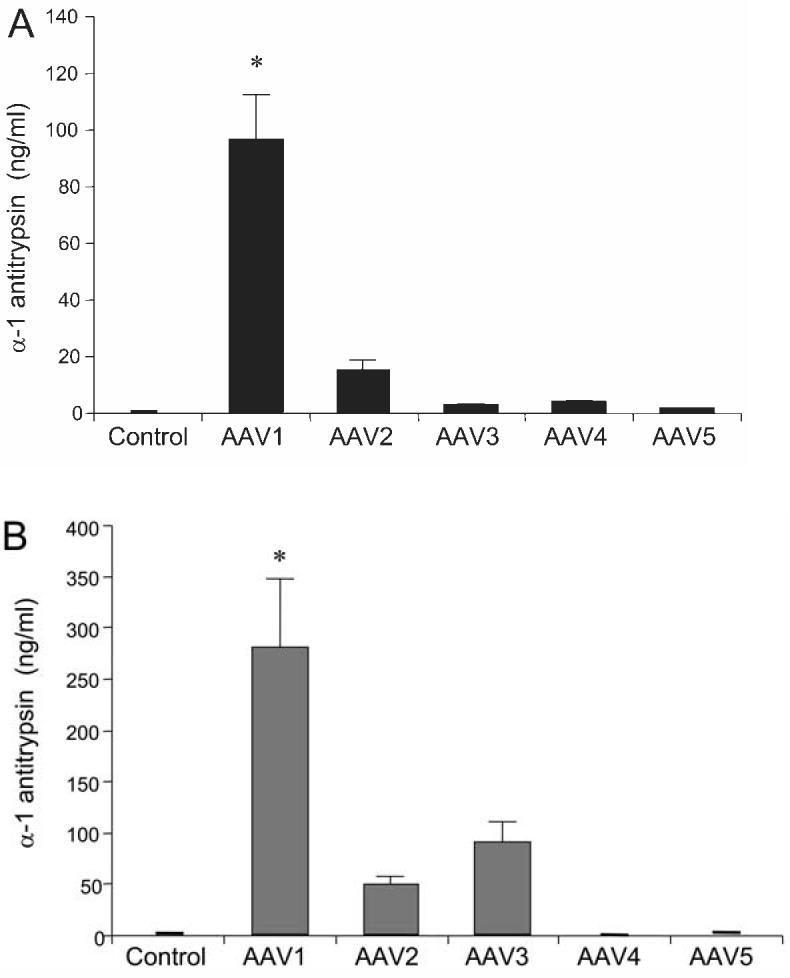
Transduction of human aortic endothelial cells (HAEC) and human aortic smooth muscle cells (HASMC) with recombinant adeno-associated virus (rAAV) serotypes. HAEC (A) and HASMC (B) were cultured to approximately 50% confluence and transduced with rAAV serotype vectors at a multiplicity of infection (MOI) of 1000 viral particles per cell. The rAAV vectors consisted of human α1-antitrypsin (hAAT), that were transcapsidated using helper plasmids to provide AAV1, 2, 3, 4, or 5 capsid proteins, respectively, but with rAAV2-inverted terminal repeats (ITRs) in all cases. No adenovirus (Ad) coinfection was used in these experiments. Media were collected at 7 days after transduction and hAAT levels (ng/ml) were measured by enzyme-linked immunosorbent assay (ELISA) as described in Materials and Methods. Values are expressed as mean ± standard error of the mean (SEM), n = 4; *represents p < 0.001 vs. other serotypes.
To examine if the cell-selective infection of rAAV serotype 1 was species-specific, RAEC instead of HAEC were incubated with the rAAV vectors. Similar serotype selective infection of RAEC was observed at 7 days after transduction. As shown in Figure 3, hAAT levels in the culture medium were highest in serotype 1, followed by rAAV5 in RAEC. Interestingly, the hAAT expression from rAAV1-transduced RAEC increased significantly at 14 days after transduction but remained stable at 21 days (Fig. 3). At the same time, hAAT expression from rAAV5-transduced RAEC increased at 14 and 21 days posttransduction. hAAT levels after rAAV1 or rAAV5 transduction were 714.8 ± 154.6 versus 27.54 ± 0.9 ng/ml (at day 7, p < 0.05), 6553.3 ± 510.3 versus 7243.1 ± 190.8 ng/ml (at day 14, p = 0.06) and 5551.1 ± 650.1 versus 8446.1 ± 257.6 ng/ml (at day 21, p < 0.001) (all values are mean ± standard error of the mean [SEM], n = 4). These results suggest that maximal expression of a transgene in rAAV5-transduced endothelial cells is delayed in comparison to other rAAV serotype vectors and surpasses the efficiency of rAAV1-mediated transduction at later time points.
FIG. 3.
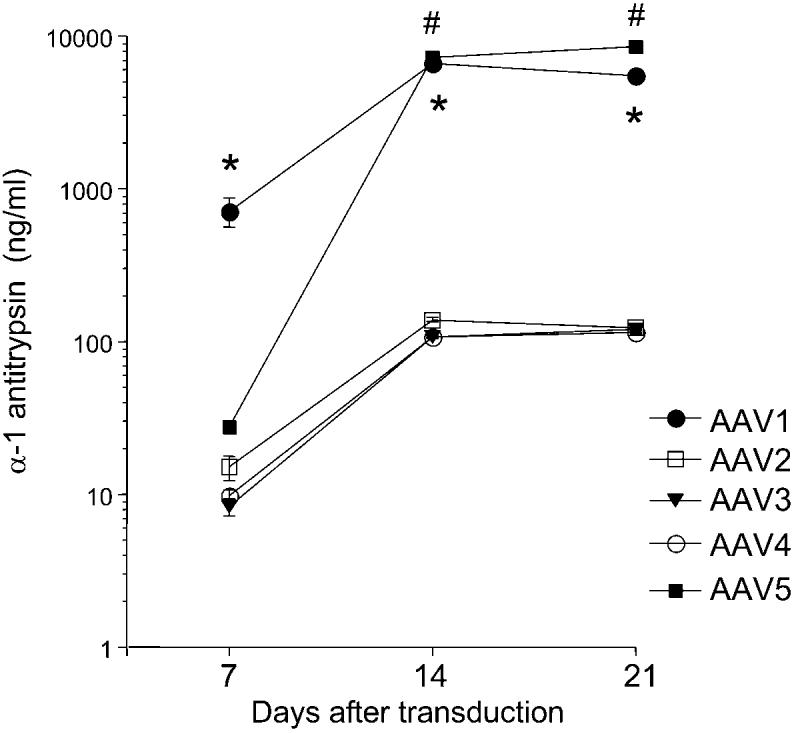
Transduction of rat aortic endothelial cells (RAEC) with recombinant adeno-associated virus (rAAV) serotypes. RAEC were cultured to approximately 50% confluence in 24-well plates and transduced with rAAV serotype vectors containing human α1-antitrypsin (hAAT) at a multiplicity of infection (MOI) of 1000 viral particles per cell. No adenovirus (Ad) coinfection was used in these experiments. Media were collected at days 7, 14, and 21 after transduction and hAAT levels were measured by enzyme-linked immunosorbent assay (ELISA) as described in Materials and Methods. Values are expressed as mean ± standard error of the mean (SEM), n = 4; *represents p < 0.05 vs. rAAV2, 3, 4, and 5 (at days 7 and 21); #represents p < 0.05 vs. rAAV2, 3, and 4.
rAAV1 transduction of endothelial cells requires sialic acid-containing receptors
Heparan sulfate proteoglycan (HSPG) and 2,3-linked sialic acid have been identified as high-affinity receptors for rAAV2 and rAAV5, respectively (Summerford and Samulski, 1998; Kaludov et al., 2001; Walters et al., 2001). However, the receptor for rAAV1 has not yet been identified. Considering the similarity of tropism of AAV1 and AAV5, they may enter cells by the same receptors. To test our hypothesis, HAEC were exposed to rAAV1-Luc-EYFP in the presence or absence of heparin or sialidase. Transduction with rAAV1 was completely inhibited by removal of sialic acid with sialidase, while heparin had no effect (Fig. 4). These results suggest that sialic acid residues may be potential receptors for rAAV1-mediated transduction of HAEC. To exclude the possibility of sialidase-induced cell injury, we performed cell viability studies in HAEC after exposure to 0, 50, 100, 200, and 400 mU/ml sialidase. No evidence of cell injury in the form of cell detachment, rounding, or vacuolation was observed by phase contrast microscopy. In addition, no differences in the growth potential of HAECs exposed to sialidase were observed by a clonogenic assay (Fig. 4, inset).
FIG. 4.
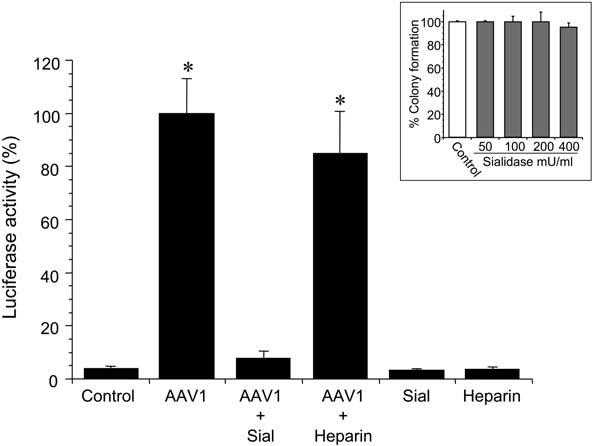
Effect of sialidase and heparin on recombinant adeno-associated virus 1 (rAAV1)-mediated transduction of human aortic endothelial cells (HAEC). HAEC were cultured to approximately 50% confluence in 24-well plates. Cells were treated with sialidase (Sial) or the vector was incubated with heparin as described in the Materials and Methods prior to transduction with rAAV1-CB-Luc-EYFP (multiplicity of infection [MOI] of 1000 viral particles per cell). Cells with the same treatment but without rAAV infection were used as controls. rAAV1-CB-Luc-EYFP containing a CBA promoter, a chimeric intron, a luciferase (Luc) fused with enhanced yellow fluorescent protein (EYFP) gene, woodchuck hepatitis posttranscriptional regulatory element (WPRE), a poly A sequence, and flanked by rAAV2-inverted terminal repeats (ITRs) was used in these experiments. Transduction efficiency was measured by l 6uciferase activity at day 7 after transduction. Values are expressed as mean ± standard error of the mean (SEM), n = 3, *represents p < 0.01 vs. control, AAV1 + Sial, Sial alone, or Heparin. Inset: HAEC viability measured by a clonogenic assay after exposure to sialidase. HAEC were treated with either control (phosphate-buffered saline [PBS]) or increasing concentrations of sialidase (50, 100, 200, and 400 mU/ml) as described in Materials and Methods. Twenty-four hours later, cells were washed with PBS, trypsinized, and seeded at a density of 1000 cells per 100-mm dish. After 5 days in culture colonies greater than 0.5 mm in size were counted. The clonogenic capability of the cells is presented as the percentage of colonies formed in comparison to the control cells. The results represent two independent experiments performed each time in triplicate and are depicted as mean ± standard error of the mean (SEM).
To confirm the specificity of our findings, HAEC were also transduced with rAAV2 and rAAV5-Luc-EYFP in the presence and absence of heparin or sialidase. Pretreatment with sialidase inhibited rAAV5-mediated transduction and heparin was without effect (Fig. 5). Although transduction of HAEC with rAAV2 was low, heparin blocked rAAV2-mediated transduction, but sialidase had no significant effect (Fig. 5). These findings are consistent with previous studies in other cell types, that report the requirement of sialic acid residues and HSPG for rAAV5 and rAAV2-mediated transduction, respectively (Summerford and Samulski, 1998; Kaludov et al., 2001; Walters et al., 2001).
FIG. 5.
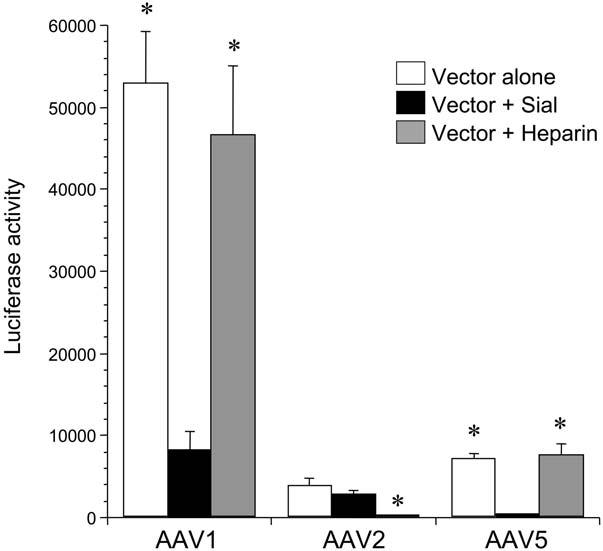
Effect of sialidase and heparin on recombinant adeno-associated virus 2 (rAAV2) and rAAV5-mediated transduction of human aortic endothelial cells (HAEC). HAEC were cultured to approximately 50% confluence in 24-well plates. Cells were treated with sialidase (Sial) or the vector was incubated with heparin as described in Materials and Methods prior to transduction with rAAV-CB-Luc-EYFP serotype 1, 2, or 5 (multiplicity of infection [MOI] of 1000 viral particles per cell) alone, or in the presence of sial or heparin. The vectors were flanked by rAAV2-inverted terminal repeats (ITRs) in these experiments. Transduction efficiency was measured by luciferase activity at day 7 after transduction. Values are expressed as mean ± standard error of the mean (SEM), n = 4, *represents p < 0.001 vs. Vector+Sial for rAAV1 and rAAV5; p < 0.05 vs. Vector alone and Vector + Sial for rAAV2.
Ex vivo transduction of rat aortic segments
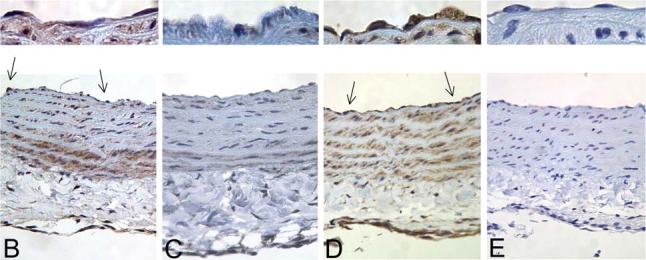
To evaluate the transduction efficiency of rAAV serotypes in intact vessels, isolated rat aortic segments were transduced with rAAV1, 2 and 5-Luc-EYFP. As shown in Figure 6A, significant transgene expression was observed in vascular segments transduced with rAAV1 and 5, compared to minimal expression with rAAV2. Immunohistochemical studies to localize the cell types transduced showed significant expression in endothelial and smooth muscle cells with rAAV1 and 5 (Fig. 6B and 6D, arrows and upper panels), compared to vascular segments transduced with rAAV2 (Fig. 6C).
FIG. 6.
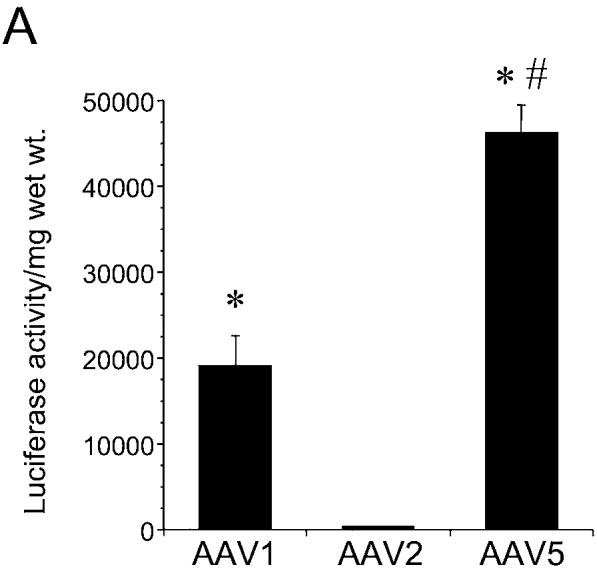
Transduction of rat aortic segments ex vivo with recombinant adeno-associated virus 1 (rAAV1), 2, and 5 serotype vectors. Freshly isolated rat aortic segments (1.5 cm) were transduced intraluminally with 25 μl of rAAV-Luc-EYFP serotype 1, 2, and 5 (1 × 1013 viral particles per milliliter) (n = 3/serotype), respectively, as described in Materials and Methods. A: Luciferase activity per milligram of wet weight in vascular segments transduced with rAAV1, 2, or 5. Values are mean ± standard error of the mean (SEM). *p < 0.05 vs. AAV2, #p < 0.01 vs. AAV1 and AAV2. B–E: Immunohistochemical detection for luciferase (brown) was performed using an antiluciferase antibody (1:1500, Abcam, Oshkosh, WI) in aortic segments transduced with rAAV1 (B), rAAV2 (C) and rAAV5 (D), respectively. The section in (E) represents a negative control of an aortic segment transduced with rAAV5 reacted with normal goat immunoglobulin G (IgG) instead of the primary antibody. Arrows indicate positive staining of endothelial cells, a higher magnification of which is depicted in the upper panels.
Transduction of rat aortic segments in vivo
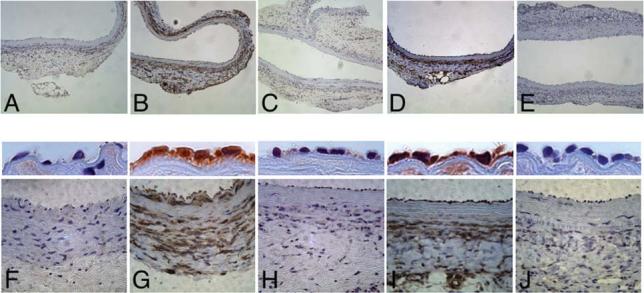
Given our encouraging results with rAAV1 and rAAV5-Luc-EYFP in rat aortic segments ex vivo, we performed transduction experiments in vivo. Significant transgene expression was evident in endothelial and smooth muscle cells in vascular segments transduced with rAAV1 (Fig. 7B and 7G) and rAAV5 (Fig. 7D and 7I), while no significant staining was observed in aortic segments transduced with rAAV2 (Fig. 7C and 7H) or saline controls (Fig. 7A and 7F). Occasional smooth muscle cells were positive in rAAV2-transduced vascular segments. A higher magnification of the endothelial layer is shown in the middle panels of Figure 7 and shows positive staining of endothelial cells in rAAV1- and rAAV5-transduced vascular segments in vivo.
FIG. 7.
Transduction of rat aortic segments in vivo with recombinant adeno-associated virus 1 (rAAV1), 2, and 5 serotype vectors. Rat aortic segments were transduced intraluminally for 30 min with saline (A and F), rAAV-Luc-EYFP serotype 1 (B and G), 2 (C and H), and 5 (D and I) (1 × 1013 viral particles per milliliter) (n = 3 per group), respectively, as described in Materials and Methods. Animals were sacrificed at 3 weeks after transduction. Representative images are shown. Immunohistochemical detection for luciferase (brown) was performed using an antiluciferase antibody (1:1500, Abcam, Oshkosh, WI). The sections in (E) and (J) represent a negative control of an aortic segment transduced with rAAV1 reacted with normal goat immunoglobulin G (IgG) instead of the primary antibody. Images in (A–E) and (F–J) were captured using a 10× and 40× objective, respectively. A higher power image (63× objective) of the endothelial cell layer is depicted in the middle panels.
DISCUSSION
Gene delivery to the vasculature has significant potential as a therapeutic strategy for atherosclerotic vascular disease and angiogenesis as well as chronic vascular rejection of transplanted organs (Crook and Akyurek, 2003; Gonzalez and Selwyn, 2003; Work et al., 2003). However, limited advances have been made in achieving successful vascular endothelial cell gene transfer. The results of the present study demonstrate the superior efficacy of rAAV serotype 1 and 5 vectors in comparison to the traditionally used rAAV serotype 2 in transduction of primary vascular endothelial and smooth muscle cells in vitro, ex vivo, and in vivo in rat aortic segments. We also observed that the kinetics of maximal transgene expression from rAAV5 vectors were delayed and significantly higher levels of transduction were achieved in comparison to rAAV1 vectors at days 14 and 21 at similar titers. Furthermore, our results identified the requirement of sialic acid residues for transduction of rAAV1 in endothelial cells, similar to the identified mechanism of uptake for rAAV5 vectors (Kaludov et al., 2001; Walters et al., 2001). These results suggest significant advantages of using alternative rAAV serotypes 1 and 5 for vascular-targeted gene delivery.
Previous studies have reported low and inefficient transduction of the vasculature using rAAV serotype 2 vectors (Gnatenko et al., 1997; Maeda et al., 1997; Richter et al., 2000; Nicklin et al., 2001; Dishart et al., 2003; Vassalli et al., 2003) and is consistent with our results. Chang et al. (2003) have used rAAV2 vectors containing vascular endothelial growth factor (VEGF) delivered intra-arterially in a rat model of hindlimb ischemia and reported significant increases in new collateral blood vessels and improved muscle oxygen tension. Transgene expression was limited to skeletal myocytes, even though the vector was delivered intra-arterially. While these studies represent significant advances with respect to the application of rAAV-mediated gene delivery to promote angiogenesis via secreted proteins, vascular cell transduction per se has not been efficient. For example, luminal instillation of rAAV2 in mouse carotid artery for 20 min resulted in prolonged transgene expression in only approximately 2% of arterial endothelial cells (Vassalli et al., 2003). Richter et al. (2000) were able to achieve higher transduction (34% of smooth muscle cells) of rabbit carotid arteries by increasing intraluminal pressure using rAAV2 vectors. Endosomal acidification and proteasomal activity may, at least in part, account for the limited transduction efficiency of rAAV2 vectors (Duan et al., 2000; Nicklin et al., 2001). However, the involvement of these processes in rAAV-mediated transduction of vascular cells is conflicting (Pajusola et al., 2002). Recent studies suggest that the extracellular matrix compartment of endothelial cells is a major barrier to efficient cellular transduction (Pajusola et al., 2002). Pajusola et al. (2002) analyzed the expression pattern of HSPG, the primary receptor for AAV2, and demonstrated significant deposition of HSPG in the extracellular matrix of endothelial cells. Transduction with rAAV2 resulted in binding of the vector to the extracellular matrix rather than the cell surface thereby contributing to the low efficiency. Removal of the matrix component by infecting cells in suspension increased rAAV2-mediated transduction by 11-fold, suggesting that matrix associated HSPGs are an important barrier to successful endothelial cell transduction (Pajusola et al., 2002).
Recent studies have incorporated peptides identified by phage display into rAAV serotype 2 vectors and have reported modestly improved transduction in vascular cells and isolated arteries (Nicklin et al., 2001; Muller et al., 2003; White et al., 2004; Work et al., 2004). Given our encouraging results with rAAV serotype 1 and 5 vectors, combining the use of endothelial-specific peptides/ligands in these alternative rAAV serotype vectors may further improve transduction of the otherwise resistant vasculature. Nicklin et al. (2001) engineered a retargeted rAAV vector (AAVsig) by the incorporation of SIGYPLP, a heptamer peptide isolated from a linear phage display, into position I-587 of the AAV serotype 2 capsid. Transduction with AAVsig increased gene expression about 5.9-fold in human umbilical vein endothelial cells and 28-fold in human saphenous vein endothelial cells, while no transduction was observed in human saphenous vein-derived smooth muscle cells (Nicklin et al., 2001). In separate studies these authors have developed rAAV2 vectors with a smooth muscle specific ligand (EYHHYNK) and have reported significantly higher transduction of venous and arterial smooth muscle cells, but not in endothelial cells (Work et al., 2004). Muller et al. (2003) screened AAV serotype 2 libraries on human coronary artery endothelial cells and identified several specific peptide motifs. Intravenous injection of a vector carrying one such motif, NSSRDLG, in the AAV capsid, resulted in a 5-fold higher selective transduction in the hearts of mice compared to animals injected with wild-type vector (Muller et al., 2003). Whether the coronary arteries were specifically transduced in vivo was not reported. White et al. (2004) incorporated human venous endothelial cell targeted peptides identified by phage display into AAV serotype 2 capsids after position 587 and reported higher transduction of venous endothelial cells, but not arterial endothelial cells.
Thus far, several cell lines and tissues including muscle, liver, lung, retina, pancreatic islets, and brain have been successfully transduced with rAAV1 and 5 with variable degrees of transduction efficiencies (Chiorini et al., 1999; Alisky et al., 2000; Chao et al., 2000; Davidson et al., 2000; Zabner et al., 2000; Flotte et al., 2001; Walters et al., 2001; Auricchio et al., 2002; Rabinowitz et al., 2002). Studies by Zabner et al. (2000) have shown that rAAV5 was more efficient in transducing both human and murine airway epithelia compared to rAAV2 or rAAV4 and utilized 2,3-linked sialic acid residues as potential receptors (Walters et al., 2001). In rat retina, serotypes 5 and 4 were shown to be the most efficient (Rabinowitz et al., 2002). We have also observed that in comparison to rAAV2, the transduction efficiency using rAAV1 and 5 is significantly higher in pancreatic islets (Flotte et al., 2001; Loiler et al., 2003) and, as noted in the present study, in vascular endothelial cells. Dishart et al. (2003) have compared the transduction efficiency of rAAV serotypes 2–6 in human umbilical and saphenous venous endothelial cells and reported that alternate serotypes provided lower levels of transduction compared to rAAV2 at day 5. However, rAAV1 was not evaluated in these studies. In addition, later time points were not examined in these studies. rAAV7-based vectors demonstrate transduction efficiencies similar to rAAV1 in skeletal muscle, and rAAV8 is far superior to the other serotypes in the liver (Gao et al., 2002). An evaluation of rAAV serotype 7 and 8 in vascular cell transduction would be of interest.
The differences in tissue tropism and transduction efficiencies of the alternative AAV serotype vectors suggest the requirement for specific receptors for virus uptake. AAV2 attaches to cells via a HSPG receptor (Summerford and Samulski, 1998). Once attached, AAV2 entry is dependent upon the presence of a coreceptor, which may consist of either the fibroblast growth factor receptor-1 or αv-β5 integrin (Qing et al., 1999; Summerford et al., 1999). Comparison of transduction efficiencies in a variety of cells, lack of inhibition by soluble heparin and cotransduction experiments suggested that AAV5 utilized a mechanism of uptake distinct from AAV2, leading to the identification of 2,3-linked sialic acid as a high affinity receptor for rAAV5 (Walters et al., 2001). This was confirmed by the observation that binding of AAV5 was blocked by genetic or enzymatic removal of sialic acid from the cell surface. Furthermore, lectins that specifically bound to 2,3-linked sialic acid successfully inhibited AAV5-mediated gene transfer in airway epithelial cells (Kaludov et al., 2001; Walters et al., 2001). Our studies also demonstrate the requirement of sialic acid residues in rAAV5-mediated transduction of endothelial cells. AAV4 was also reported to require a similar mechanism for uptake, however, resialylation experiments have demonstrated that AAV4 requires O-linked sialic acid, whereas AAV5 requires N-linked sialic acid (Kaludov et al., 2001; Walters et al., 2001). More recently, platelet-derived growth factor receptor has been reported as an additional receptor for AAV5 (Di Pasquale et al., 2003).
The high transduction efficiency of rAAV5 in endothelial cells prompted us to investigate the role of sialic acid residues in rAAV1-mediated uptake in endothelial cells. Our studies based on enzymatic removal of sialic acid with neuraminidase (sialidase) on endothelial cells, suggest that sialic acid residues may be part of the receptor complex required for rAAV1 uptake. Specifically, sialidase treatment blocked rAAV1-mediated transduction while heparin had no effect. These latter findings are consistent with the report of Hauck and Xiao (Hauck and Xiao, 2003) showing that the heparin binding domain is not required for AAV1-mediated transduction in muscle. Further studies using resialylation experiments in endothelial cells will be necessary to confirm these observations.
In summary, the results of our studies have demonstrated that rAAV1 and rAAV5-derived vectors are more efficient and represent a better serotype than rAAV2, 3, or 4, for gene transfer in primary human and rat vascular endothelial cells and in rat aortic segments. rAAV1 is also superior to the other serotypes in transduction of HASMC. The studies have also identified the requirement of sialic acid residues and not HSPGs for rAAV1-mediated transduction in endothelial cells. Alternative AAV serotype vectors, particularly AAV1 and AAV5, have unique potential for gene delivery to vascular tissues.
ACKNOWLEDGMENTS
This work was supported by grants from the Juvenile Diabetes Research Foundation Gene Therapy Center and National Institutes of Health (R21 DK067472 to A.A. and P01 HL066973 to R.J.S.). We gratefully acknowledge Tracy Clarke for her technical assistance.
REFERENCES
- ALISKY JM, HUGHES SM, SAUTER SL, JOLLY D, DUBENSKY TW, JR., STABER PD, CHIORINI JA, DAVIDSON BL. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport. 2000;11:2669–2673. doi: 10.1097/00001756-200008210-00013. [DOI] [PubMed] [Google Scholar]
- AURICCHIO A, O'CONNOR E, WEINER D, GAO GP, HILDINGER M, WANG L, CALCEDO R, WILSON JM. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Invest. 2002;110:499–504. doi: 10.1172/JCI15780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG DS, SU H, TANG GL, BREVETTI LS, SARKAR R, WANG R, KAN YW, MESSINA LM. Adeno-associated viral vector-mediated gene transfer of VEGF normalizes skeletal muscle oxygen tension and induces arteriogenesis in ischemic rat hindlimb. Mol. Ther. 2003;7:44–51. doi: 10.1016/s1525-0016(02)00035-7. [DOI] [PubMed] [Google Scholar]
- CHAO H, LIU Y, RABINOWITZ J, LI C, SAMULSKI RJ, WALSH CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- CHIORINI JA, KIM F, YANG L, KOTIN RM. Cloning and characterization of adeno-associated virus type 5. J. Virol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROOK MF, AKYUREK LM. Gene transfer strategies to inhibit neointima formation. Trends Cardiovasc. Med. 2003;13:102–106. doi: 10.1016/s1050-1738(02)00255-4. [DOI] [PubMed] [Google Scholar]
- DAVIDSON BL, STEIN CS, HETH JA, MARTINS I, KOTIN RM, DERKSEN TA, ZABNER J, GHODSI A, CHIORINI JA. Recombinant adeno-associated virus type 2, 4, and 5 vectors: Transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI PASQUALE G, DAVIDSON BL, STEIN CS, MARTINS I, SCUDIERO D, MONKS A, CHIORINI JA. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- DISHART KL, DENBY L, GEORGE SJ, NICKLIN SA, YENDLURI S, TUERK MJ, KELLEY MP, DONAHUE BA, NEWBY AC, HARDING T, BAKER AH. Third-generation lentivirus vectors efficiently transduce and phenotypically modify vascular cells: Implications for gene therapy. J. Mol. Cell. Cardiol. 2003;35:739–748. doi: 10.1016/s0022-2828(03)00136-6. [DOI] [PubMed] [Google Scholar]
- DUAN D, YUE Y, YAN Z, YANG J, ENGELHARDT JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOTTE TR, AFIONE SA, CONRAD C, MCGRATH SA, SOLOW R, OKA H, ZEITLIN PL, GUGGINO WB, CARTER BJ. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOTTE T, AGARWAL A, WANG J, SONG S, FENJVES ES, INVERARDI L, CHESNUT K, AFIONE S, LOILER S, WASSERFALL C, KAPTURCZAK M, ELLIS T, NICK H, ATKINSON M. Efficient ex vivo transduction of pancreatic islet cells with recombinant adeno-associated virus vectors. Diabetes. 2001;50:515–520. doi: 10.2337/diabetes.50.3.515. [DOI] [PubMed] [Google Scholar]
- FLOTTE TR, ZEITLIN PL, REYNOLDS TC, HEALD AE, PEDERSEN P, BECK S, CONRAD CK, BRASS-ERNST L, HUMPHRIES M, SULLIVAN K, WETZEL R, TAYLOR G, CARTER BJ, GUGGINO WB. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: A two-part clinical study. Hum. Gene Ther. 2003;14:1079–1088. doi: 10.1089/104303403322124792. [DOI] [PubMed] [Google Scholar]
- GAO GP, ALVIRA MR, WANG L, CALCEDO R, JOHNSTON J, WILSON JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GNATENKO D, ARNOLD TE, ZOLOTUKHIN S, NUOVO GJ, MUZYCZKA N, BAHOU WF. Characterization of recombinant adeno-associated virus-2 as a vehicle for gene delivery and expression into vascular cells. J. Invest. Med. 1997;45:87–98. [PubMed] [Google Scholar]
- GONZALEZ MA, SELWYN AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am. J. Med. 2003;115(Suppl 8A):99S–106S. doi: 10.1016/j.amjmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- GRIESE DP, ACHATZ S, BATZLSPERGER CA, STRAUCH UG, GRUMBECK B, WEIL J, RIEGGER GA. Vascular gene delivery of anticoagulants by transplantation of retrovirally-transduced endothelial progenitor cells. Cardiovasc. Res. 2003;58:469–477. doi: 10.1016/s0008-6363(03)00266-9. [DOI] [PubMed] [Google Scholar]
- GRIMM D, KERN A, RITTNER K, KLEINSCHMIDT JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- HAUCK B, XIAO W. Characterization of tissue tropism determinants of adeno-associated virus type 1. J. Virol. 2003;77:2768–2774. doi: 10.1128/JVI.77.4.2768-2774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALUDOV N, BROWN KE, WALTERS RW, ZABNER J, CHIORINI JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLITT MG, LEONE P, SAMULSKI RJ, XIAO X, PFAFF DW, O'MALLEY KL, DURING MJ. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- KAPTURCZAK M, ZOLOTUKHIN S, CROSS J, PILEGGI A, MOLANO RD, JORGENSEN M, BYRNE B, FLOTTE TR, ELLIS T, INVERARDI L, RICORDI C, NICK H, ATKINSON M, AGARWAL A. Transduction of human and mouse pancreatic islet cells using a bicistronic recombinant adeno-associated viral vector. Mol. Ther. 2002;5:154–160. doi: 10.1006/mthe.2002.0522. [DOI] [PubMed] [Google Scholar]
- KATUSIC ZS. Therapeutic angiogenesis: new indication for endothelial NO synthase gene transfer. Arterioscler. Thromb. Vasc. Biol. 2002;22:1254–1255. doi: 10.1161/01.atv.0000026860.08501.0f. [DOI] [PubMed] [Google Scholar]
- KAY MA, MANNO CS, RAGNI MV, LARSON PJ, COUTO LB, MCCLELLAND A, GLADER B, CHEW AJ, TAI SJ, HERZOG RW, ARRUDA V, JOHNSON F, SCALLAN C, SKARSGARD E, FLAKE AW, HIGH KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- LANDAU C, PIRWITZ MJ, WILLARD MA, GERARD RD, MEIDELL RS, WILLARD SE. Adenoviral mediated gene transfer to atherosclerotic arteries after balloon angioplasty. Am. Heart J. 1995;129:1051–1057. doi: 10.1016/0002-8703(95)90383-6. [DOI] [PubMed] [Google Scholar]
- LIU Q, MURUVE DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–940. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- LOILER SA, CONLON TJ, SONG S, TANG Q, WARRINGTON KH, AGARWAL A, KAPTURCZAK M, LI C, RICORDI C, ATKINSON MA, MUZYCZKA N, FLOTTE TR. Targeting recombinant adeno-associated virus vectors to enhance gene transfer to pancreatic islets and liver. Gene Ther. 2003;10:1551–1558. doi: 10.1038/sj.gt.3302046. [DOI] [PubMed] [Google Scholar]
- LUO Z, SATA M, NGUYEN T, KAPLAN JM, AKITA GY, WALSH K. Adenovirus-mediated delivery of fas ligand inhibits intimal hyperplasia after balloon injury in immunologically primed animals. Circulation. 1999;99:1776–1779. doi: 10.1161/01.cir.99.14.1776. [DOI] [PubMed] [Google Scholar]
- MAEDA Y, IKEDA U, OGASAWARA Y, URABE M, TAKIZAWA T, SAITO T, COLOSI P, KURTZMAN G, SHIMADA K, OZAWA K. Gene transfer into vascular cells using adeno-associated virus (AAV) vectors. Cardiovasc. Res. 1997;35:514–521. doi: 10.1016/s0008-6363(97)00163-6. [DOI] [PubMed] [Google Scholar]
- MATSUMURA JS, KIM R, SHIVELY VP, MACDONALD RC, PEARCE WH. Characterization of vascular gene transfer using a novel cationic lipid. J. Surg. Res. 1999;85:339–345. doi: 10.1006/jsre.1999.5678. [DOI] [PubMed] [Google Scholar]
- MULLER OJ, KAUL F, WEITZMAN MD, PASQUALINI R, ARAP W, KLEINSCHMIDT JA, TREPEL M. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat. Biotechnol. 2003;21:1040–1046. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- NICKLIN SA, BUENING H, DISHART KL, DE ALWIS M, GIROD A, HACKER U, THRASHER AJ, ALI RR, HALLEK M, BAKER AH. Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol. Ther. 2001;4:174–181. doi: 10.1006/mthe.2001.0424. [DOI] [PubMed] [Google Scholar]
- PAJUSOLA K, GRUCHALA M, JOCH H, LUSCHER TF, YLAHERTTUALA S, BUELER H. Cell-type-specific characteristics modulate the transduction efficiency of adeno-associated virus type 2 and restrain infection of endothelial cells. J. Virol. 2002;76:11530–11540. doi: 10.1128/JVI.76.22.11530-11540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASCARIU M, BENDAYAN M, GHITESCU L. Correlated endothelial caveolin overexpression and increased transcytosis in experimental diabetes. J. Histochem. Cytochem. 2004;52:65–76. doi: 10.1177/002215540405200107. [DOI] [PubMed] [Google Scholar]
- PEEL AL, ZOLOTUKHIN S, SCHRIMSHER GW, MUZYCZKA N, REIER PJ. Efficient transduction of green fluorescent protein in spinal cord neurons using adeno-associated virus vectors containing cell type-specific promoters. Gene Ther. 1997;4:16–24. doi: 10.1038/sj.gt.3300358. [DOI] [PubMed] [Google Scholar]
- QING K, MAH C, HANSEN J, ZHOU S, DWARKI V, SRIVASTAVA A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ JE, ROLLING F, LI C, CONRATH H, XIAO W, XIAO X, SAMULSKI RJ. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER M, IWATA A, NYHUIS J, NITTA Y, MILLER AD, HALBERT CL, ALLEN MD. Adeno-associated virus vector transduction of vascular smooth muscle cells in vivo. Physiol. Genomics. 2000;2:117–127. doi: 10.1152/physiolgenomics.2000.2.3.117. [DOI] [PubMed] [Google Scholar]
- SAVONTAUS MJ, SAUTER BV, HUANG TG, WOO SL. Transcriptional targeting of conditionally replicating adenovirus to dividing endothelial cells. Gene Ther. 2002;9:972–979. doi: 10.1038/sj.gt.3301747. [DOI] [PubMed] [Google Scholar]
- SNYDER RO, MIAO CH, PATIJN GA, SPRATT SK, DANOS O, NAGY D, GOWN AM, WINTHER B, MEUSE L, COHEN LK, THOMPSON AR, KAY MA. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- SONG S, MORGAN M, ELLIS T, POIRIER A, CHESNUT K, WANG J, BRANTLY M, MUZYCZKA N, BYRNE BJ, ATKINSON M, FLOTTE TR. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEG PG, FELDMAN LJ, SCOAZEC JY, TAHLIL O, BARRY JJ, BOULECHFAR S, RAGOT T, ISNER JM, PERRICAUDET M. Arterial gene transfer to rabbit endothelial and smooth muscle cells using percutaneous delivery of an adenoviral vector. Circulation. 1994;90:1648–1656. doi: 10.1161/01.cir.90.4.1648. [DOI] [PubMed] [Google Scholar]
- SUMMERFORD C, SAMULSKI RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMMERFORD C, BARTLETT JS, SAMULSKI RJ. AlphaVbeta5 integrin: A co-receptor for adeno-associated virus type 2 infection. Nat. Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- VASSALLI G, BUELER H, DUDLER J, VON SEGESSER LK, KAPPENBERGER L. Adeno-associated virus (AAV) vectors achieve prolonged transgene expression in mouse myocardium and arteries in vivo: A comparative study with adenovirus vectors. Int. J. Cardiol. 2003;90:229–238. doi: 10.1016/s0167-5273(02)00554-5. [DOI] [PubMed] [Google Scholar]
- WALTERS RW, YI SM, KESHAVJEE S, BROWN KE, WELSH MJ, CHIORINI JA, ZABNER J. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 2001;276:20610–20616. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- WANG K, KESSLER PD, ZHOU Z, PENN MS, FORUDI F, ZHOU X, TARAKJI K, KIBBE M, KOVESDI I, BROUGH DE, TOPOL EJ, LINCOFF AM. Local adenoviral-mediated inducible nitric oxide synthase gene transfer inhibits neointimal formation in the porcine coronary stented model. Mol. Ther. 2003;7:597–603. doi: 10.1016/s1525-0016(03)00061-3. [DOI] [PubMed] [Google Scholar]
- WHITE SJ, NICKLIN SA, BUNING H, BROSNAN MJ, LEIKE K, PAPADAKIS ED, HALLEK M, BAKER AH. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation. 2004;109:513–519. doi: 10.1161/01.CIR.0000109697.68832.5D. [DOI] [PubMed] [Google Scholar]
- WORK LM, NICKLIN SA, BAKER AH. Targeting gene therapy vectors to the vascular endothelium. Curr. Atheroscler. Rep. 2003;5:163–170. doi: 10.1007/s11883-003-0019-9. [DOI] [PubMed] [Google Scholar]
- WORK LM, NICKLIN SA, BRAIN NJ, DISHART KL, VON SEGGERN DJ, HALLEK M, BUNING H, BAKER AH. Development of efficient viral vectors selective for vascular smooth muscle cells. Mol. Ther. 2004;9:198–208. doi: 10.1016/j.ymthe.2003.11.006. [DOI] [PubMed] [Google Scholar]
- XIAO X, LI J, SAMULSKI RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZABNER J, SEILER M, WALTERS R, KOTIN RM, FULGERAS W, DAVIDSON BL, CHIORINI JA. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOLOTUKHIN S, POTTER M, HAUSWIRTH WW, GUY J, MUZYCZKA N. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J. Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOLOTUKHIN S, BYRNE BJ, MASON E, ZOLOTUKHIN I, POTTER M, CHESNUT K, SUMMERFORD C, SAMULSKI RJ, MUZYCZKA N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- ZOLOTUKHIN S, POTTER M, ZOLOTUKHIN I, SAKAI Y, LOILER S, FRAITES TJ, JR., CHIODO VA, PHILLIPSBERG T, MUZYCZKA N, HAUSWIRTH WW, FLOTTE TR, BYRNE BJ, SNYDER RO. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]