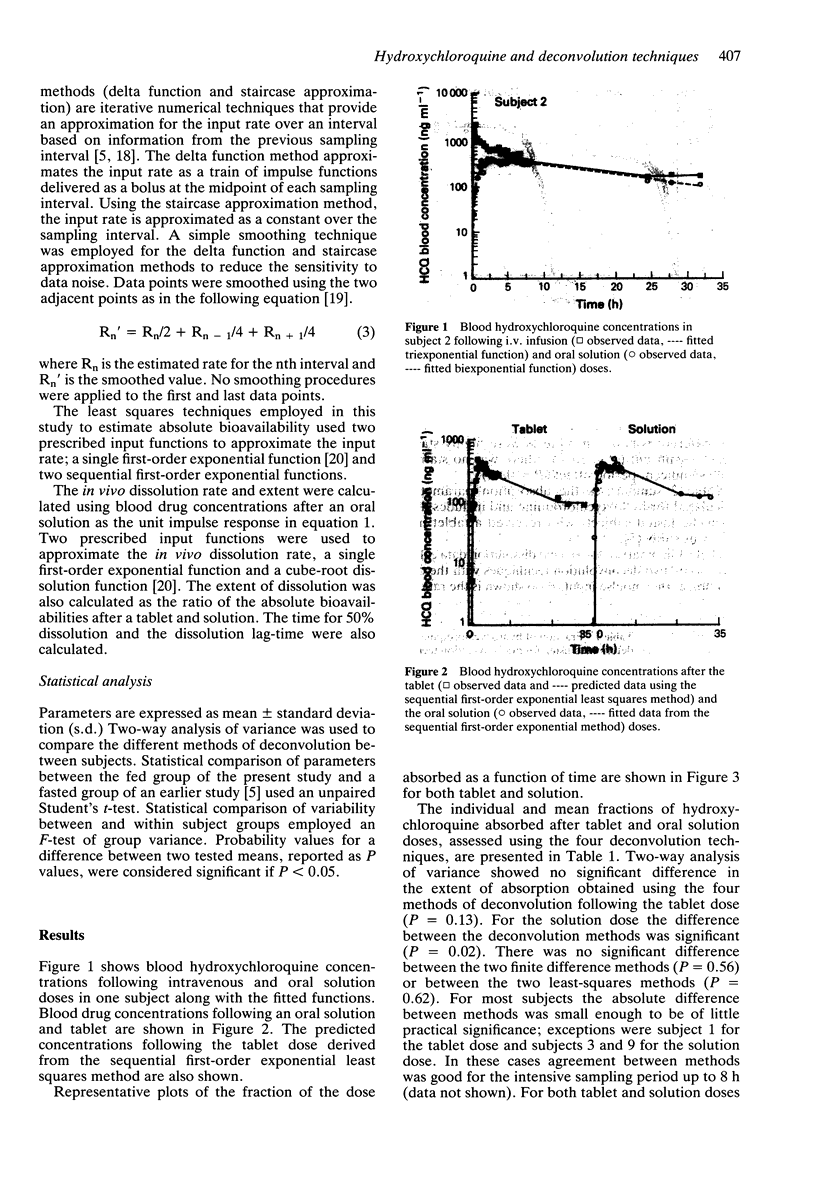
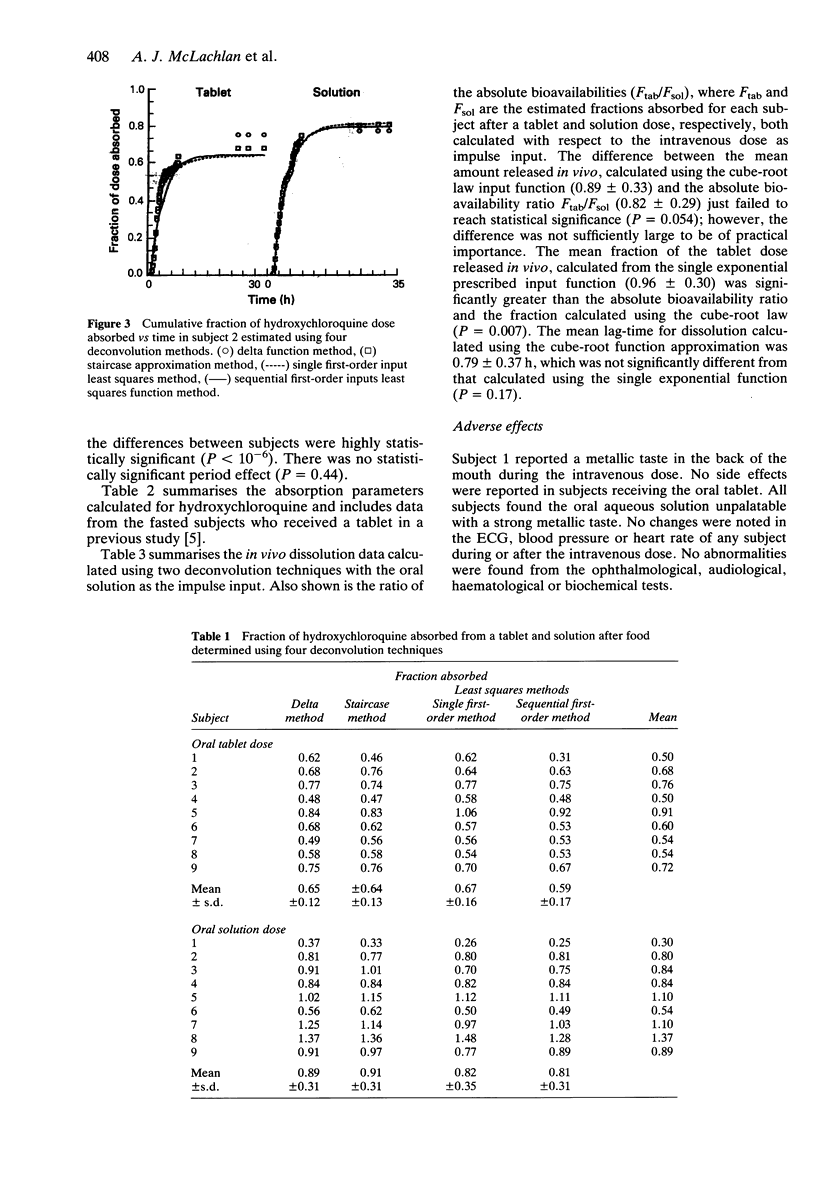
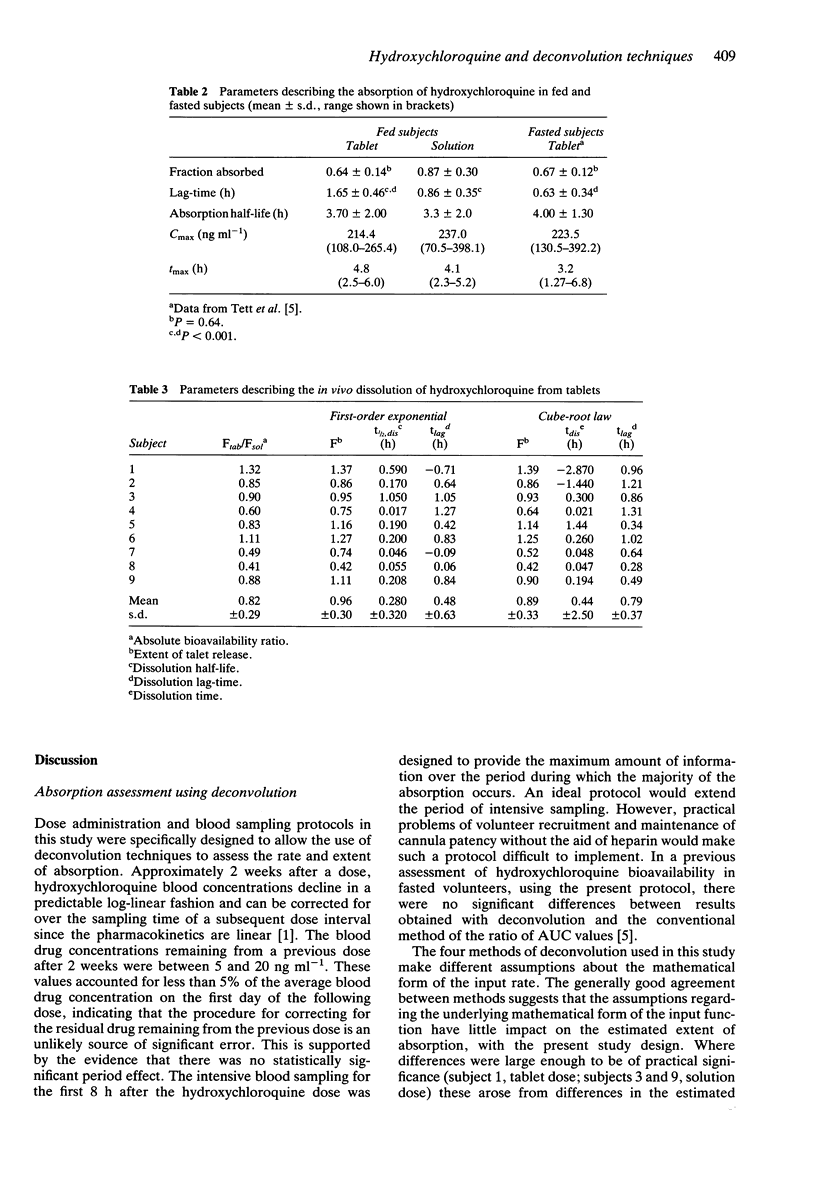
Abstract
1. Nine healthy subjects each received three doses of 155 mg rac-hydroxychloroquine, as a tablet, an oral solution and by intravenous infusion, in a randomised cross-over design study, 30 min after a standard high fat breakfast. 2. Four methods of deconvolution were used to assess the absolute bioavailability of the tablet and oral solution doses. These were the delta function method, the staircase approximation method, and two least squares methods using a single first-order input and a sequential first-order input. The mean (+/- s.d.) fraction absorbed estimated by the four methods was 0.64 +/- 0.14 after the tablet and 0.87 +/- 0.30 after the oral solution. Wide intersubject variability was observed (0.50-0.91 for the tablet; 0.30-1.37 for the solution). 3. The mean (+/- s.d.) absorption half-life was 3.7 +/- 2.0 h for the tablet and 3.3 +/- 1.6 h for the solution, suggesting that absorption following the tablet dose was not rate-limited by dissolution. 4. The in vivo dissolution rate, extent of release and lag-time were determined using cube-root law and first-order input functions. Dissolution was found to be rapid, after a significant lag-time, but incomplete in some subjects. 5. The rate and extent of absorption was similar to that reported previously for fasted subjects. The lag-time before absorption commenced in fed subjects (1.65 +/- 0.46 h) showed a significant three-fold increase over that reported previously in fasting subjects (0.63 +/- 0.33 h), but this difference is not likely to be of clinical significance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cutler D. J. Numerical deconvolution by least squares: use of prescribed input functions. J Pharmacokinet Biopharm. 1978 Jun;6(3):227–241. doi: 10.1007/BF01312264. [DOI] [PubMed] [Google Scholar]
- Cutler D. Assessment of rate and extent of drug absorption. Pharmacol Ther. 1981;14(2):123–160. doi: 10.1016/0163-7258(81)90058-9. [DOI] [PubMed] [Google Scholar]
- Day R. O., Miners J., Birkett D. J., Graham G. G., Whitehead A. Relationship between plasma oxipurinol concentrations and xanthine oxidase activity in volunteers dosed with allopurinol. Br J Clin Pharmacol. 1988 Oct;26(4):429–434. doi: 10.1111/j.1365-2125.1988.tb03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie W. R., Veng-Pedersen P. A polyexponential deconvolution method. Evaluation of the "gastrointestinal bioavailability" and mean in vivo dissolution time of some ibuprofen dosage forms. J Pharmacokinet Biopharm. 1985 Jun;13(3):289–307. doi: 10.1007/BF01065657. [DOI] [PubMed] [Google Scholar]
- Gillespie W. R., Veng-Pedersen P. Gastrointestinal bioavailability: determination of in vivo release profiles of solid oral dosage forms by deconvolution. Biopharm Drug Dispos. 1985 Jul-Sep;6(3):351–355. doi: 10.1002/bdd.2510060311. [DOI] [PubMed] [Google Scholar]
- Imbimbo B. P., Martinelli P., Rocchetti M., Ferrari G., Bassotti G., Imbimbo E. Efficiency of different criteria for selecting pharmacokinetic multiexponential equations. Biopharm Drug Dispos. 1991 Mar;12(2):139–147. doi: 10.1002/bdd.2510120207. [DOI] [PubMed] [Google Scholar]
- Karlsson M. O., Lindberg-Freijs A. Comparison of methods to calculate cyclosporine A bioavailability from consecutive oral and intravenous doses. J Pharmacokinet Biopharm. 1990 Aug;18(4):293–311. doi: 10.1007/BF01062270. [DOI] [PubMed] [Google Scholar]
- Melander A. Influence of food on the bioavailability of drugs. Clin Pharmacokinet. 1978 Sep-Oct;3(5):337–351. doi: 10.2165/00003088-197803050-00001. [DOI] [PubMed] [Google Scholar]
- Neuvonen P. J., Kivistö K. T. The clinical significance of food-drug interactions: a review. Med J Aust. 1989 Jan 2;150(1):36–40. doi: 10.5694/j.1326-5377.1989.tb136321.x. [DOI] [PubMed] [Google Scholar]
- Nicklasson M., Ellström K., Sjöqvist R., Sjövall J. Linear systems analysis and moment analysis in the evaluation of bacampicillin bioavailability from microcapsule suspensions. J Pharmacokinet Biopharm. 1984 Oct;12(5):467–478. doi: 10.1007/BF01060126. [DOI] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J. Apparent dose-dependence of chloroquine pharmacokinetics due to limited assay sensitivity and short sampling times. Eur J Clin Pharmacol. 1987;31(6):729–731. doi: 10.1007/BF00541305. [DOI] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J., Brown K. F. High-performance liquid chromatographic assay for hydroxychloroquine and metabolites in blood and plasma, using a stationary phase of poly(styrene divinylbenzene) and a mobile phase at pH 11, with fluorimetric detection. J Chromatogr. 1985 Nov 8;344:241–248. doi: 10.1016/s0378-4347(00)82024-1. [DOI] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J., Day R. O. Bioavailability of hydroxychloroquine tablets assessed with deconvolution techniques. J Pharm Sci. 1992 Feb;81(2):155–159. doi: 10.1002/jps.2600810211. [DOI] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J., Day R. O., Brown K. F. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1988 Sep;26(3):303–313. doi: 10.1111/j.1365-2125.1988.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J., Day R. O., Brown K. F. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989 Jun;27(6):771–779. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker G. T., Jackson P. R., Storey G. C., Holt D. W. Bioavailability of amiodarone. Eur J Clin Pharmacol. 1984;26(4):533–534. doi: 10.1007/BF00542153. [DOI] [PubMed] [Google Scholar]
- Urso R., Aarons L. Bioavailability of drugs with long elimination half-lives. Eur J Clin Pharmacol. 1983;25(5):689–693. doi: 10.1007/BF00542360. [DOI] [PubMed] [Google Scholar]
- Veng-Pedersen P., Miller R. Deconvolution at steady state: determination of gastrointestinal bioavailability of sustained release theophylline. Int J Clin Pharmacol Ther Toxicol. 1987 May;25(5):233–237. [PubMed] [Google Scholar]


