Abstract
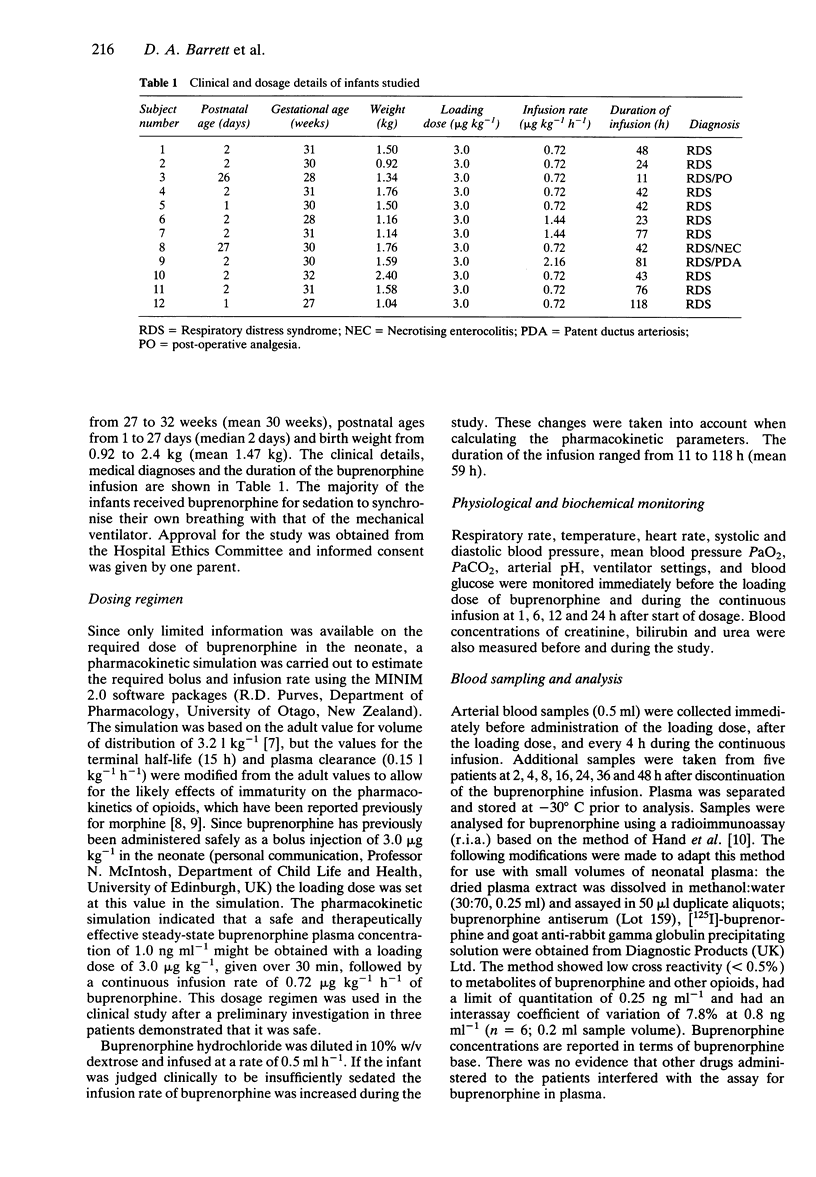
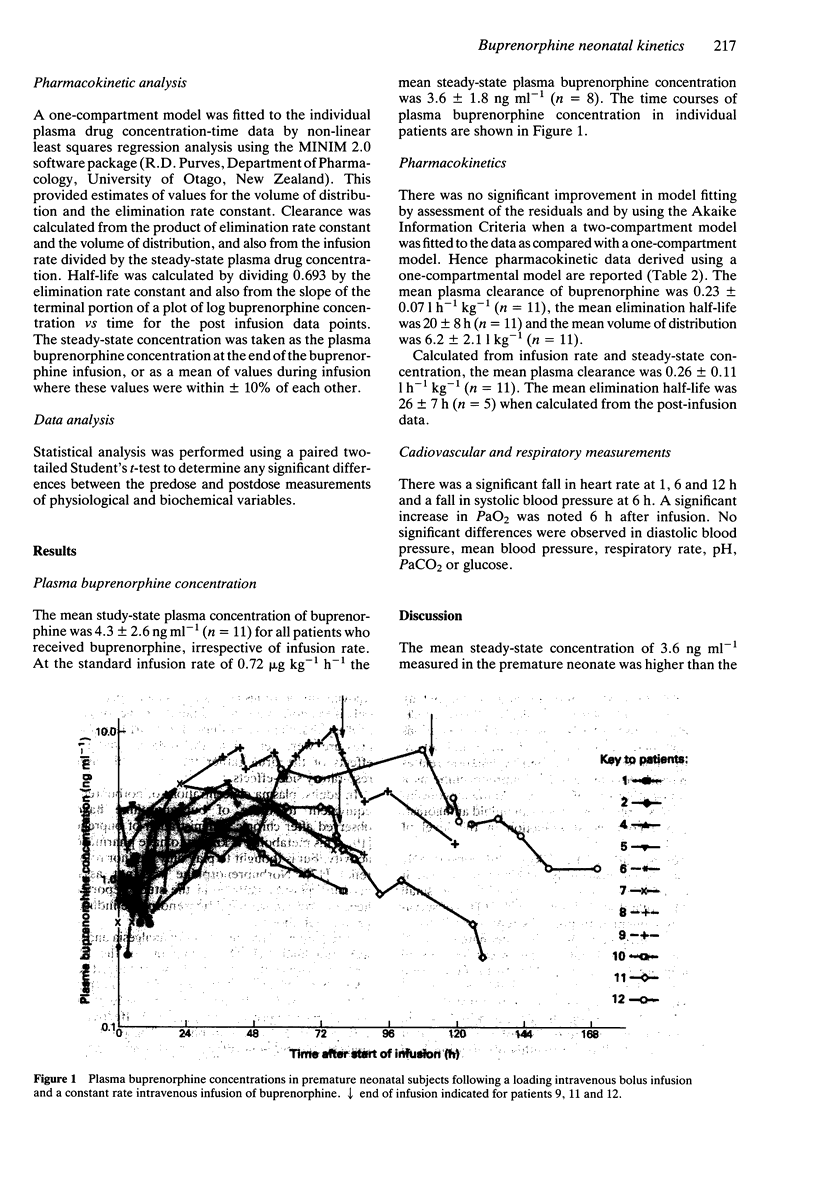
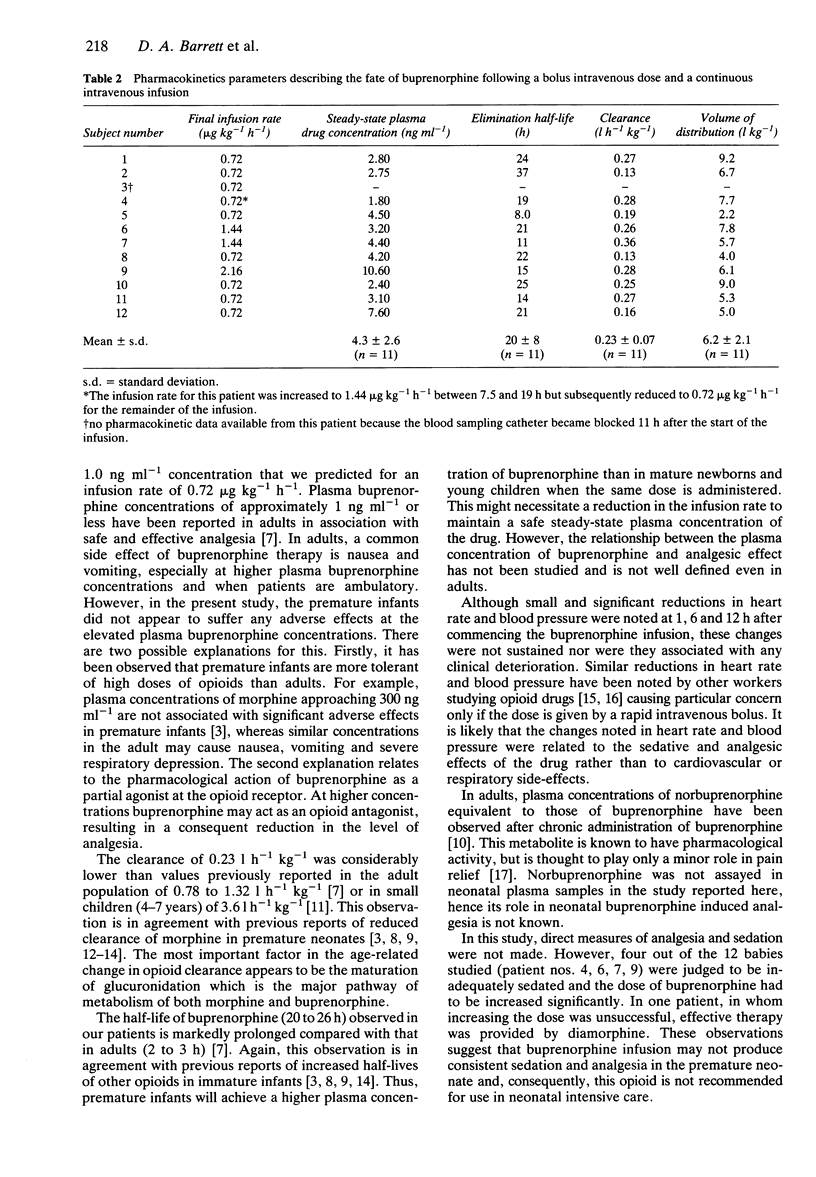
1. The pharmacokinetics and physiological effects of buprenorphine were studied in 12 newborn premature neonates (27 to 32 weeks gestational age) who were given a loading dose of 3.0 micrograms kg-1 of buprenorphine followed by an intravenous infusion of 0.72 micrograms kg-1 h-1 of buprenorphine. Plasma concentrations of buprenorphine were measured during the infusion, at steady-state and for 24 h after the cessation of the buprenorphine infusion. 2. The mean steady-state plasma buprenorphine concentration (+/- s.d.) for an infusion rate of 0.72 micrograms kg-1 h-1 was 4.3 +/- 2.6 ng ml-1. 3. Buprenorphine clearance was 0.23 +/- 0.07 l h-1 kg-1, the elimination half-life was 20 +/- 8 h and the volume of distribution was 6.2 +/- 2.11 l kg-1. 4. Small but significant falls were noted in systolic blood pressure at 6 h and heart rate at 1, 6 and 12 h after the administration of buprenorphine, but these did not appear to cause any clinical deterioration. 5. Four of the 12 subjects studied required an increase in the infusion rate of buprenorphine to achieve adequate sedation. 6. The results suggest that this dosing regimen of buprenorphine is safe but may not be as effective as other opioids in producing sedation and analgesia in premature newborns.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand K. J., Carr D. B. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am. 1989 Aug;36(4):795–822. doi: 10.1016/s0031-3955(16)36722-0. [DOI] [PubMed] [Google Scholar]
- Barrett D. A., Elias-Jones A. C., Rutter N., Shaw P. N., Davis S. S. Morphine kinetics after diamorphine infusion in premature neonates. Br J Clin Pharmacol. 1991 Jul;32(1):31–37. doi: 10.1111/j.1365-2125.1991.tb05609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt-Mehta V., Rosen D. A. Management of acute pain in children. Clin Pharm. 1991 Sep;10(9):667–685. [PubMed] [Google Scholar]
- Chay P. C., Duffy B. J., Walker J. S. Pharmacokinetic-pharmacodynamic relationships of morphine in neonates. Clin Pharmacol Ther. 1992 Mar;51(3):334–342. doi: 10.1038/clpt.1992.30. [DOI] [PubMed] [Google Scholar]
- Choonara I. A., McKay P., Hain R., Rane A. Morphine metabolism in children. Br J Clin Pharmacol. 1989 Nov;28(5):599–604. doi: 10.1111/j.1365-2125.1989.tb03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias-Jones A. C., Barrett D. A., Rutter N., Shaw P. N., Davis S. S. Diamorphine infusion in the preterm neonate. Arch Dis Child. 1991 Oct;66(10 Spec No):1155–1157. doi: 10.1136/adc.66.10_spec_no.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand C. W., Baldwin D., Moore R. A., Allen M. C., McQuay H. J. Radioimmunoassay of buprenorphine with iodine label: analysis of buprenorphine and metabolites in human plasma. Ann Clin Biochem. 1986 Jan;23(Pt 1):47–53. doi: 10.1177/000456328602300105. [DOI] [PubMed] [Google Scholar]
- Hand C. W., Sear J. W., Uppington J., Ball M. J., McQuay H. J., Moore R. A. Buprenorphine disposition in patients with renal impairment: single and continuous dosing, with special reference to metabolites. Br J Anaesth. 1990 Mar;64(3):276–282. doi: 10.1093/bja/64.3.276. [DOI] [PubMed] [Google Scholar]
- Lynn A. M., Slattery J. T. Morphine pharmacokinetics in early infancy. Anesthesiology. 1987 Feb;66(2):136–139. doi: 10.1097/00000542-198702000-00005. [DOI] [PubMed] [Google Scholar]
- Marlow N., Weindling A. M., Cooke R. W. Hazards of analgesia for newborn infants. Arch Dis Child. 1988 Oct;63(10):1293–1293. doi: 10.1136/adc.63.10.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunuksela E. L., Korpela R., Olkkola K. T. Double-blind, multiple-dose comparison of buprenorphine and morphine in postoperative pain of children. Br J Anaesth. 1988 Jan;60(1):48–55. doi: 10.1093/bja/60.1.48. [DOI] [PubMed] [Google Scholar]
- McRorie T. I., Lynn A. M., Nespeca M. K., Opheim K. E., Slattery J. T. The maturation of morphine clearance and metabolism. Am J Dis Child. 1992 Aug;146(8):972–976. doi: 10.1001/archpedi.1992.02160200094036. [DOI] [PubMed] [Google Scholar]
- Miall-Allen V. M., Whitelaw A. G. Effect of pancuronium and pethidine on heart rate and blood pressure in ventilated infants. Arch Dis Child. 1987 Nov;62(11):1179–1180. doi: 10.1136/adc.62.11.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkola K. T., Maunuksela E. L., Korpela R. Pharmacokinetics of intravenous buprenorphine in children. Br J Clin Pharmacol. 1989 Aug;28(2):202–204. doi: 10.1111/j.1365-2125.1989.tb05418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


