Abstract
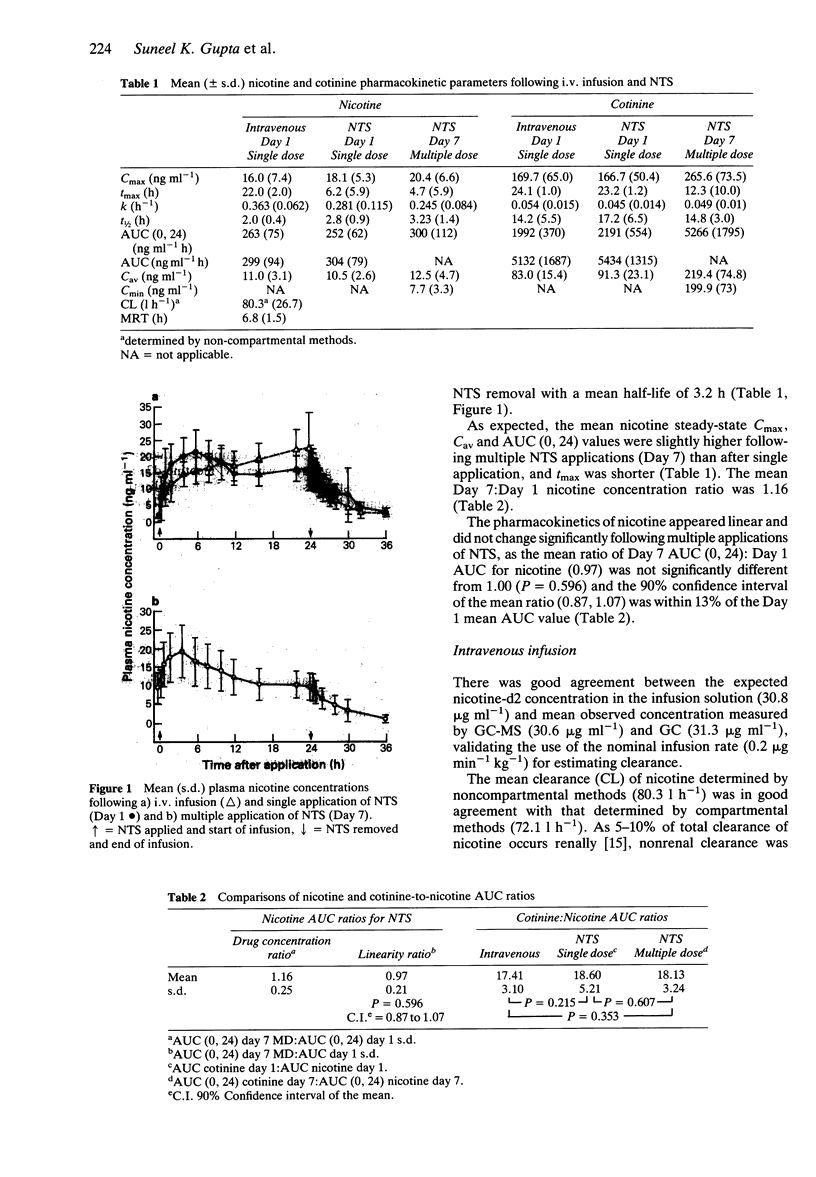
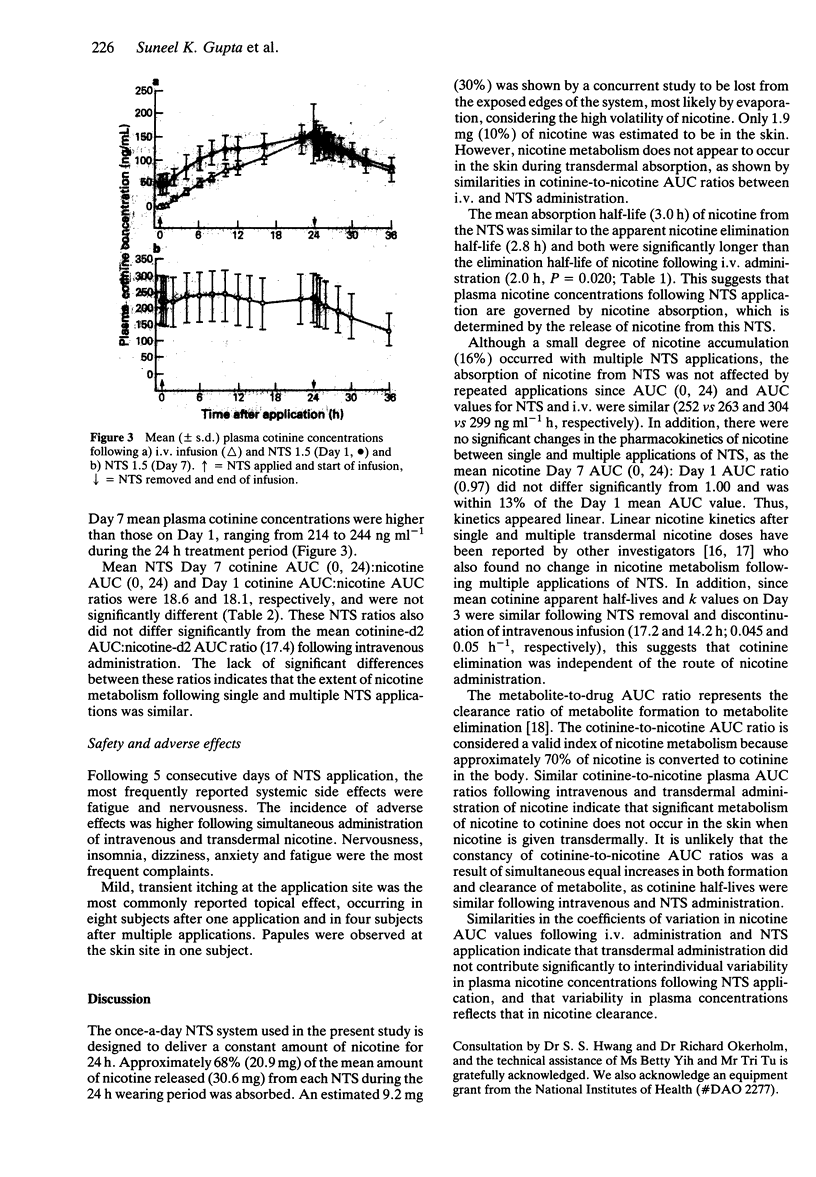
1. The absolute bioavailability and absorption kinetics of nicotine were investigated in 13 healthy adult male smokers following single and multiple applications of a nicotine transdermal system (NTS), designed to release nicotine at an approximate rate of 1.5 mg h-1 over 24 h. The absorption of nicotine from the single NTS application was calculated with reference to a simultaneous intravenous infusion (i.v.) of deuterium-labelled nicotine. 2. The mean input time (MIT) and mean absorption time (MAT) for nicotine following application of NTS for 24 h were 7.7 and 4.2 h, respectively. 3. Following NTS removal, the mean apparent nicotine elimination half-life was 2.8 h, compared with 2.0 h following i.v. nicotine, reflecting continued absorption of nicotine following NTS removal. 4. The mean amount of nicotine absorbed from the NTS after the 24 h application was 20.9 mg, which represents about 68% of the amount released from the system; the remaining 32% was lost from the system during daily activities. 5. The ratio of AUC values for the metabolite cotinine relative to nicotine was similar whether nicotine was administered transdermally or intravenously. 6. Following i.v. administration, the mean nicotine clearance was 72 l h-1 (coefficient of variation 29%). Since coefficients of variation in AUC values following NTS and i.v. treatments were similar, transdermal administration of nicotine was not associated with increased interindividual variability in plasma nicotine concentrations. 7. No significant changes were seen in the pharmacokinetics of nicotine between single and multiple applications of NTS. 8. As expected from the higher total plasma nicotine concentrations, the incidence of adverse effects was higher following simultaneous intravenous and transdermal administration of nicotine. The most frequently reported systemic side effects were nervousness and headache: mild itching was the most frequent topical effect.
Full text
PDF


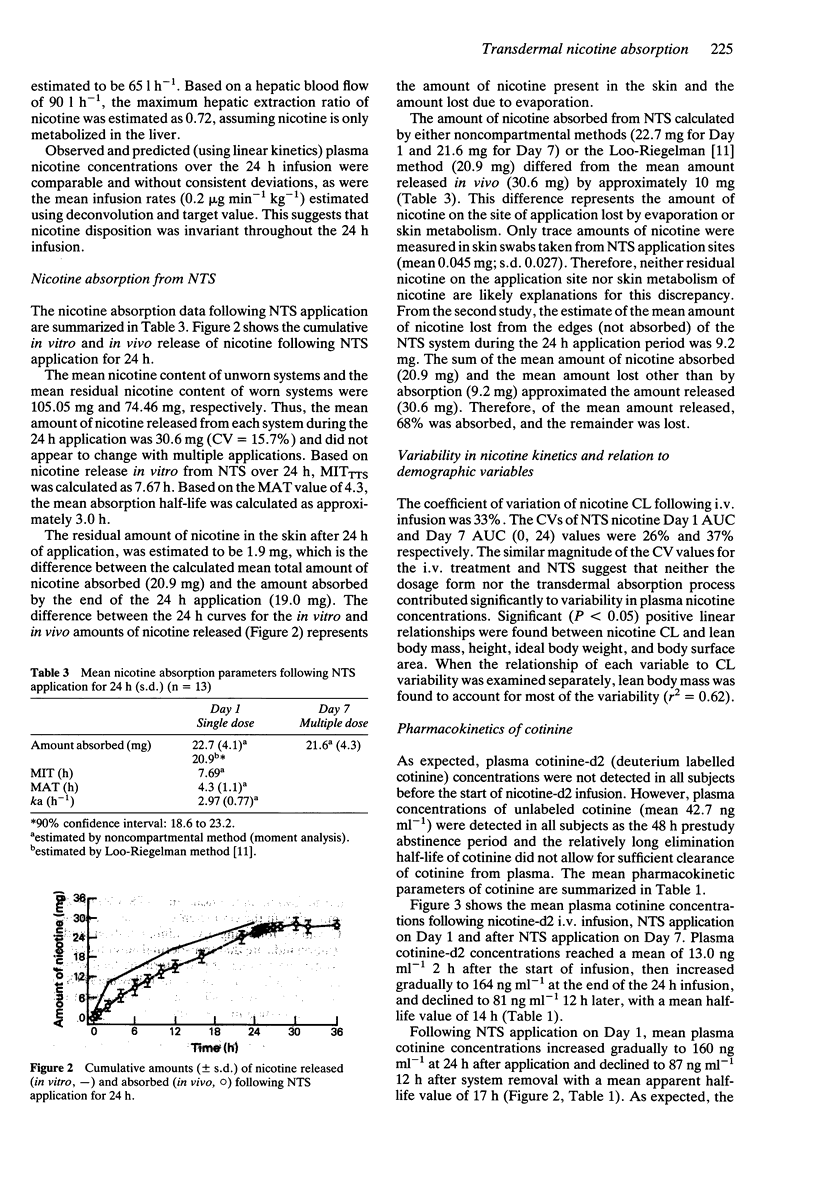

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benowitz N. L., Chan K., Denaro C. P., Jacob P., 3rd Stable isotope method for studying transdermal drug absorption: the nicotine patch. Clin Pharmacol Ther. 1991 Sep;50(3):286–293. doi: 10.1038/clpt.1991.138. [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Jacob P., 3rd, Jones R. T., Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982 May;221(2):368–372. [PubMed] [Google Scholar]
- Fagerström K. O. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3-4):235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Gorsline J., Gupta S. K., Dye D., Rolf C. N. Steady-state pharmacokinetics and dose relationship of nicotine delivered from Nicoderm (Nicotine Transdermal System). J Clin Pharmacol. 1993 Feb;33(2):161–168. doi: 10.1002/j.1552-4604.1993.tb03938.x. [DOI] [PubMed] [Google Scholar]
- Gorsline J., Okerholm R. A., Rolf C. N., Moos C. D., Hwang S. S. Comparison of plasma nicotine concentrations after application of nicoderm (nicotine transdermal system) to different skin sites. J Clin Pharmacol. 1992 Jun;32(6):576–581. doi: 10.1177/009127009203200615. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Okerholm R. A., Coen P., Prather R. D., Gorsline J. Single- and multiple-dose pharmacokinetics of Nicoderm (Nicotine Transdermal System). J Clin Pharmacol. 1993 Feb;33(2):169–174. doi: 10.1002/j.1552-4604.1993.tb03939.x. [DOI] [PubMed] [Google Scholar]
- Jacob P., 3rd, Yu L., Wilson M., Benowitz N. L. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3',3'-d2 in humans. Biol Mass Spectrom. 1991 May;20(5):247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- Lee B. L., Benowitz N. L., Jacob P., 3rd Influence of tobacco abstinence on the disposition kinetics and effects of nicotine. Clin Pharmacol Ther. 1987 Apr;41(4):474–479. doi: 10.1038/clpt.1987.59. [DOI] [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. New method for calculating the intrinsic absorption rate of drugs. J Pharm Sci. 1968 Jun;57(6):918–928. doi: 10.1002/jps.2600570602. [DOI] [PubMed] [Google Scholar]
- Ross H. D., Chan K. K., Piraino A. J., John V. A. Pharmacokinetics of multiple daily transdermal doses of nicotine in healthy smokers. Pharm Res. 1991 Mar;8(3):385–388. doi: 10.1023/a:1015810019076. [DOI] [PubMed] [Google Scholar]
- Rowland M., Gupta S. K. Cyclosporin-phenytoin interaction: re-evaluation using metabolite data. Br J Clin Pharmacol. 1987 Sep;24(3):329–334. doi: 10.1111/j.1365-2125.1987.tb03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. G. Drug accumulation. J Clin Pharmacol J New Drugs. 1967 Mar-Apr;7(2):84–88. doi: 10.1002/j.1552-4604.1967.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Watari N., Benet L. Z. Determination of mean input time, mean residence time, and steady-state volume of distribution with multiple drug inputs. J Pharmacokinet Biopharm. 1989 Oct;17(5):593–599. doi: 10.1007/BF01071351. [DOI] [PubMed] [Google Scholar]
- Watson P. E., Watson I. D., Batt R. D. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980 Jan;33(1):27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]


